Abstract
Experimental evidence suggests that metabotropic glutamate 2/3 (mGlu2/3) receptor antagonists affect cognitive function, although contradictory findings have been reported. To clarify the role of mGlu2/3 receptor antagonists in one aspect of cognition, the present study investigated the effects of a broad range of doses of the mGlu2/3 receptor antagonist LY341495 on post-training recognition memory components (storage and/or retrieval) in rats. The efficacy of LY341495 in antagonizing the extinction of recognition memory was also investigated. The novel object recognition test was used as the memory test. The highest LY341495 doses administered (0.3, 1, and 3 mg/kg) disrupted performance in this recognition memory procedure in rats at all delay conditions tested, whereas administration of lower doses (0.05 and 0.1 mg/kg) did not impair recognition memory. Moreover, administration of the low LY341495 doses (0.05 and 0.1 mg/kg) counteracted the extinction of recognition memory. The present results indicate that administration of the mGlu2/3 receptor antagonist LY341495 can either impair or enhance recognition memory in rats, depending on the dose of the compound and delay period used. Thus, together with previously reported findings, the present data suggest complex effects of this compound on cognitive function, particularly recognition memory.
Keywords: metabotropic glutamate (mGlu)2/3 receptor, LY341495, recognition memory, rat
1. Introduction
Glutamate is the major excitatory neurotransmitter in the mammalian brain and activates ionotropic AMPA, kainate, and N-methyl-d-aspartate (NMDA) receptors and a family of G-protein-coupled metabotropic glutamate (mGlu) receptors. Eight subtypes of mGlu receptors have been identified and classified into three Groups (I, II, and III) based on sequence homology, signal transduction pathways, and pharmacological selectivity [8]. The Group II subtypes (mGlu2/3 receptors) are located primarily presynaptically and on glia cells and couple to Gi/o proteins to negatively regulate adenylyl cyclase activity and regulate neurotransmitter release [6]. These receptors are highly expressed in brain areas associated with cognitive function, such as the hippocampus, amygdala, and prefrontal cortex [18,19]. Evidence suggests that the intracellular or extracellular accumulation of soluble amyloid β (Aβ) oligomers disrupts neuronal plasticity [22], and this accumulation of soluble Aβ is counteracted by the mGlu2/3 receptor antagonist LY341495 [15]. In addition, the mGlu2 receptor positive allosteric modulator LY566332 has been reported recently to amplify Aβ-induced neurodegeneration, and this effect was prevented by LY341495 administration [5]. Collectively, these findings suggest a role for mGlu2/3 receptor antagonists in the treatment of cognitive disorders. Presently, however, there is little evidence about the involvement of this class of compounds in learning and memory. Specifically, administration of LY341495 disrupted performance in the passive avoidance task and habituation test in mice when the compound was administered before training [3,21]. In contrast, LY341495 administration did not impair performance in the delayed alternation T-maze test in rats again when the compound was administered before training [12], and even facilitated performance in working and spatial memory tasks in rats [13].
Recognition memory stems from a series of neural processes by which a subject becomes aware that a stimulus has been previously experienced, with recognition as the behavioral outcome of these processes. Recognition memory requires that the perceived characteristics of the events are discriminated, identified, and compared with the memory of the characteristics of previously experienced events [23]. Various procedures are used to assess recognition memory in rodents, ranging from maze procedures to operantly controlled tasks and tasks that employ spatial stimuli. Other tasks are based on non-spatial, complex, visual, auditory, or olfactory information [23]. The novel object recognition task is a procedure that assesses recognition memory in rodents. It is a non-rewarded paradigm that is based on the spontaneous exploratory behavior of rodents [10,11].
The precise role of mGlu2/3 receptor antagonists on recognition memory has not yet been clarified. Administration of LY341495 alone did not impair performance in the novel object recognition task in rats when the compound was administered pretraining, but posttraining administration of LY341495 combined with administration of the Group I (mGlu5 receptor) antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) enhanced the amnestic effect of MPEP on recognition memory [1]. Moreover, LY341495 counteracted the ameliorative effect of clozapine in the same behavioral procedure [14].
The above studies [1,3,12-14,21] demonstrate the disparate effects of LY341495 on various cognitive functions. To our knowledge, no studies have addressed the effects of LY341495 on the posttraining components of recognition memory (i.e., the storage and retrieval of information) or examined the effects of a broad range of LY341495 doses on memory ability in rats.
The first aim of the present study was to evaluate the effects of posttraining administration of a range of LY341495 doses on recognition memory in rats using the novel object recognition test. Based on the findings from this first experiment that demonstrated that animals treated with low LY341495 doses (0.05 and 0.1 mg/kg) displayed similar recognition memory as their vehicle-treated counterparts, the second experiment aimed to investigate the efficacy of low LY341495 doses in antagonizing the extinction of recognition memory that occurs with a long delay interval between the initial exposure and test phase in this test, to further probe the role of mGlu2/3 receptor antagonists in recognition memory. The third aim was to determine whether the opposite effects of different LY341495 doses on recognition memory observed in the first two experiments (see Results below) could be attributable to potential general/nonspecific effects of LY341495 or specific actions on memory. The novel object recognition test was used for all of the experiments in the present study [10], and LY341495 was administered immediately after the sample phase of the novel object recognition test.
2. Materials and methods
2.1. Subjects
Male 3-month-old Wistar rats (Hellenic Pasteur Institute, Athens, Greece) that weighed 250-300 g were used. The animals were housed in Makrolon cages (47.5 cm length × 20.5 cm height × 27 cm width), three per cage, in a regulated environment (21 ± 1°C; 50-55% relative humidity; 12 h/12 h light/dark cycle, lights on at 7:00 AM) with free access to food and water. The experiments were conducted between 10:00 AM and 2:00 PM in a room where only these animals where housed. Behavior was video-recorded, and the behavioral observations were performed by experimenters who were unaware of the pharmacological treatments.
The procedures that involved animals and their care were in accordance with international guidelines and national and international laws and policies (EEC Council Directive 86/609, JL 358, 1, December 12, 1987; NIH Guide for Care and Use of Laboratory Animals, NIH publication no. 85-23, 1985).
2.2. Novel object recognition test
The test apparatus consisted of a dark open box made of Plexiglas (80 cm length × 50 cm height × 60 cm width) that was illuminated by a 60 W light suspended 60 cm above the box. The light intensity was equal in the different parts of the apparatus. The objects to be discriminated were made of glass, plastic, or metal, had three different shapes (i.e., cube, pyramid, and cylinder), were 7 cm high, and could not be displaced by the rats.
The novel object recognition test was performed as described previously [4,10]. Briefly, during the week before the test, the animals were handled twice daily for 3 consecutive days. Before testing, the rats were allowed to explore the apparatus for 2 min for 3 consecutive days. During testing, a session that consisted of two trials was conducted. During the “sample” trial (T1), two identical objects were placed in two opposite corners of the apparatus, 10 cm from the side wall. A rat was placed in the middle of the apparatus and allowed to explore these two identical objects. After T1, the rat was returned to its home cage, and an intertrial interval (ITI) followed. Subsequently, the “choice” trial (T2) was performed. During T2, a new object (N) replaced one of the objects presented in T1. Accordingly, the rats were reexposed to two objects: the familiar (F) and the new (N). All combinations and locations of the objects were counterbalanced to reduce potential bias caused by preference for particular locations or objects. To avoid the presence of olfactory cues, the apparatus and objects were thoroughly cleaned after each trial.
Exploration was defined as the following: directing the nose toward the object at a distance of 2 cm or less or touching the object with the nose. Turning around or sitting on the object was not considered exploratory behavior. The time spent by the rats exploring each object during T1 and T2 was manually recorded with a stopwatch. Based on this measure, a series of variables was then calculated: the total time spent exploring the two identical objects in T1 and the time spent exploring the two different objects (i.e., F and N) in T2. The discrimination between F and N during T2 was measured by comparing the time spent exploring object F with the time spent exploring object N. Because this time may be biased by differences in the overall level of exploration [7], a discrimination index (D) was calculated: D = N - F/N + F. D is the discrimination ratio, represents the difference in exploration time, and is expressed as a proportion of the total time spent exploring the two objects in T2 [7]. In addition, locomotor activity, expressed as the total number of steps during each trial, was recorded.
2.3. Drugs
LY341495 (2S-2-amino-2-[(1S,2S)-2-carboxycyclopropan-1-yl]-3-[xanth-9-yl]propionic acid) was purchased from Tocris Cookson (Ellisville, MO, USA). LY341495 was dissolved in 0.1 M NaOH to a final volume of 5 ml, with saline (0.9% NaCl) solution. The drug solution was adjusted to pH 7.4-7.8 with 1 M HCl before intraperitoneal (i.p.) administration in a volume of 1 ml/kg. Control animals received isovolumetric amounts of the vehicle (0.9% NaCl).
2.4. Experimental protocol
2.4.1. Experiment 1: Effects of posttraining administration of different LY341495 doses on performance in the novel object recognition task assessed at a delay condition of 1 h (ITI 1 h)
The rats were randomly divided into six experimental groups (10 rats per group): vehicle and 0.05, 0.1, 0.3, 1, and 3 mg/kg LY341495. The LY341495 doses were selected on the basis of results from previous published studies that evaluated the effects of this compound on cognition [1,3,12-14,21]. The rats were subjected to a training session that consisted of two 2-min trials. The animals received either vehicle or LY341495 immediately after T1. Using the 2-min trial duration, an ITI of 1 h was used because recognition memory is still intact in untreated control rats under these experimental conditions [2,4].
2.4.2. Experiment 2: Effects of posttraining administration of different LY341495 doses on antagonism of the extinction of recognition memory in the novel object recognition task
The rats were randomly divided into three experimental groups (10 rats per group): vehicle and 0.05 and 0.1 mg/kg LY341495. These doses of LY341495 were selected based on the results of Experiment 1, in which the animals treated with these LY341495 doses acquired the novel object recognition task similarly to their vehicle-treated counterparts (see Results below). The rats were subjected to a training session that consisted of two 2-min trials. For this experiment, the animals received either vehicle or LY341495 immediately after T1. Using the 2-min trial duration, an ITI of 24 h followed because recognition memory dissipates in control rats under these experimental conditions [2,4].
2.4.3. Experiment 3: Effects of posttraining administration of different LY341495 doses on performance in the novel object recognition task assessed at a delay condition of 24 h (ITI 24 h)
This last experiment sought to determine whether the opposite effects of different LY341495 doses on recognition memory observed in Experiments 1 and 2 were attributable to the potential general/nonspecific effects of LY341495 on retention memory. To rule out pharmacokinetic or other non-memory-related issues, we studied the effects of LY341495 on recognition memory at a delay condition of 24 h. Therefore, a different experimental protocol was used in Experiment 3 than that used in Experiments 1 and 2 to attempt to enhance the performance of control rats under this delay condition. The duration of the sample trial (T1) was increased from to 2 to 5 min, whereas the duration of the choice trial (T2) remained unchanged (2 min). The animals were randomly divided into six experimental groups (10 rats per group): vehicle and 0.05, 0.1, 0.3, 1, and 3 mg/kg LY341495. The rats were subjected to a session that consisted of two trials. Vehicle or LY341495 were administered to the animals immediately after T1.
2.5. Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA). Post hoc comparisons between groups were made with Tukey's t-test. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Experiment 1: Effects of posttraining administration of different LY341495 doses on performance in the novel object recognition task assessed at a delay condition of 1 h (ITI 1 h)
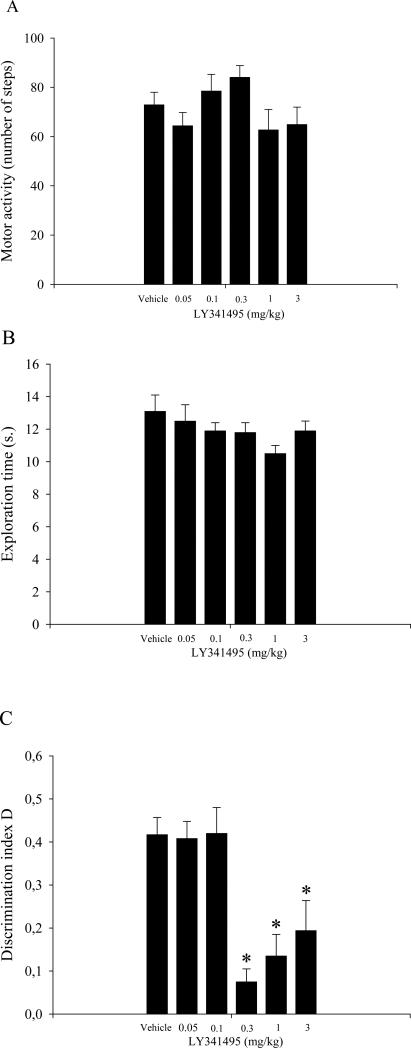
The statistical analyses of locomotor activity and exploration time data did not reveal any effect of the drug treatment on locomotor activity or exploration time (Fig. 1A and B, respectively). Importantly, the analysis of the D index (Fig. 1C) revealed a significant effect of treatment (F5,59 = 9.86, p < 0.01). The post-hoc comparisons showed that rats treated with 0.3, 1, and 3 mg/kg LY341495 displayed a lower level of discrimination compared with all other experimental groups (p < 0.05).
Figure 1.
Results from the novel object recognition test that involved a session that consisted of two 2-min trials and an 1 h ITI (see text for details). Vehicle and LY341495 were injected intraperitoneally immediately after T1. The data are expressed as the mean ± SEM of 10 rats per treatment group. (A) Total locomotor activity in different groups of rats during T2. (B) Total exploration time in different groups of rats during T2. (C) Discrimination index (D) in different groups of rats during T2. *p < 0.05, compared with the vehicle-treated group, and 0.05 and 0.1 mg/kg LY3414495-treated groups.
3.2. Experiment 2: Effects of posttraining administration of different LY341495 doses on antagonism of the extinction of recognition memory in the novel object recognition task
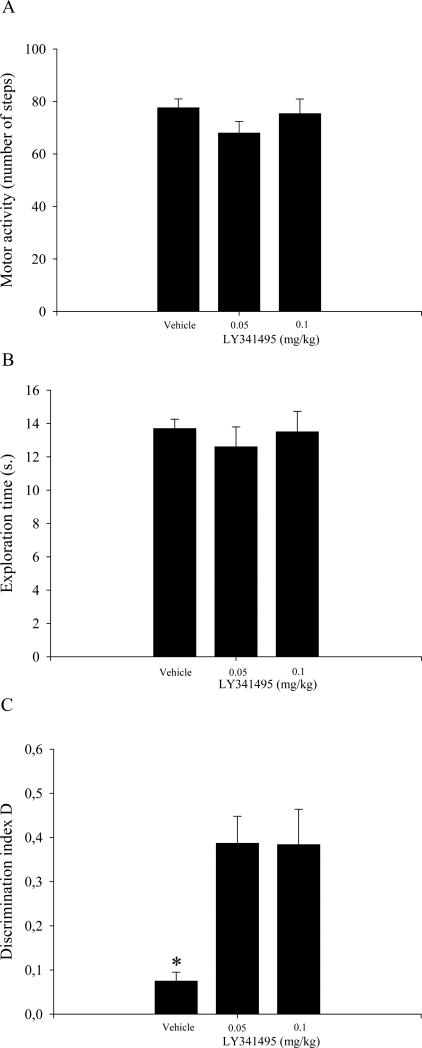
The analysis of locomotor activity and total exploration time did not reveal any significant effect of LY341495 (Fig. 2A and B, respectively). The analysis of the D index showed a main effect of treatment (F2,29 = 8.68, p < 0.01; Fig. 2C). The post-hoc comparisons indicated that rats treated with 0.05 and 0.1 mg/kg LY341495 expressed a higher level of discrimination, revealed by the D index, compared with their vehicle-treated counterparts (p < 0.05).
Figure 2.
Results from the novel object recognition test that involved a session that consisted of two 2-min trials and a 24 h ITI (see text for details). Vehicle and LY341495 were injected intraperitoneally immediately after T1. The data are expressed as the mean ± SEM of 10 rats per treatment group. (A) Total locomotor activity in different groups of rats during T2. (B) Total exploration time in different groups of rats during T2. (C) Discrimination index (D) in different groups of rats during T2. *p < 0.05, compared with 0.05 and 0.1 mg/kg LY3414495-treated groups.
3.3. Experiment 3: Effects of posttraining administration of different LY341495 doses on performance in the novel object recognition task assessed at a delay condition of 24 h (ITI 24 h)
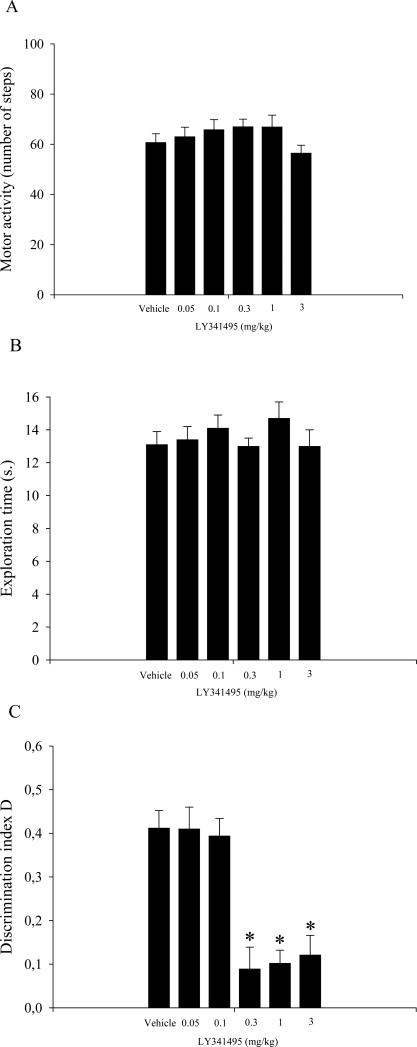
The analysis of locomotor activity and total exploration time did not reveal any significant effect of LY341495 (Fig. 3A and B, respectively). The analysis of the D index revealed a significant effect of treatment (F5,59 = 14.24, p < 0.01; Fig. 3C). The post-hoc comparisons showed that animals treated with 0.3, 1, and 3 mg/kg LY341495 did not exhibit significant discrimination between the novel and familiar objects compared with all other experimental groups (p < 0.05).
Figure 3.
Results from the novel object recognition test that consisted of one 5-min trial, one 2-min trial, and a 24 h ITI (see text for details). Vehicle and LY341495 were injected intraperitoneally immediately after T1. The data are expressed as the mean ± SEM of 10 rats per treatment group. (A) Total locomotor activity in different groups of rats during T2. (B) Total exploration time in different groups of rats during T2. (C) Discrimination index (D) in different groups of rats during T2. *p < 0.05, compared with the vehicle-treated group, and the 0.05 and 0.1 mg/kg LY3414495-treated groups.
4. Discussion
Consistent with previous findings [2,4], our results demonstrated that recognition memory ability in young vehicle-treated rats remained intact at a delay condition of 1 h but this recognition memory dissipated with a 24 h interval between initial exposure to the objects and the testing phase. Under these experimental conditions the effects of a broad range of LY341495 doses were investigated in this memory task called the novel object recognition test. The results of the present study showed that the mGlu2/3 receptor antagonist LY341495 differentially affected recognition memory in rats, depending on the time interval between drug administration and testing, and drug dose. Posttraining administration of low LY341495 doses (0.05 and 0.1 mg/kg) did not disrupt performance in the novel object recognition task when the animals were tested 1 h after the initial exposure to the objects and drug administration. Conversely, administration of the highest doses tested (0.3, 1 and 3 mg/kg) severely impaired recognition memory assessed in the same behavioral procedure when there was an 1 h intertrial interval. Moreover, administration of the low LY341495 doses (0.05 and 0.1 mg/kg) immediately after the sample trial counteracted the extinction of recognition memory that occurred in the vehicle-treated animals when an intertrial interval of 24 h was used between the sample and the choice phase of the novel object recognition task.
To clarify whether the amnesia produced by the higher LY341495 doses was related to nonspecific effects (e.g., pharmacokinetics or motivational or sensorimotor effects), we conducted an additional experiment using a different protocol than the one utilized in the first two experiments. To avoid the aforementioned potential nonspecific effects of LY341495, retention ability was assessed 24 h after treatment, and a longer duration of the sample trial (5 min) was used to enhance the performance of control rats during retention. Experiment 3 replicated the results of Experiment 1, in which a shorter ITI was used (1 h). Furthermore under experimentation conditions in which a longer ITI was utilized (24 h), recognition memory abilities of rats treated with low LY341495 doses (0.05 and 0.1 mg/kg) were not different than those displayed by vehicle-treated animals. In contrast, posttraining administration of the highest LY341495 doses (0.3, 1, and 3 mg/kg) severely impaired the animals’ performance in this recognition memory task compared to performance of rats treated with vehicle or low LY341495 doses (0.05 and 0.1 mg/kg).
In addition, in all of the experiments reported here, locomotor activity and exploration were not different among the various experimental groups, demonstrating that LY341495 did not induce nonspecific increases or decreases in activity. Importantly, the half-life of LY341495 is 44 min in rats after intraperitoneal administration [20], suggesting that it would have been unlikely that the compound affected performance 24 h later, although it could still have affected posttraining memory components (i.e., the storage or retrieval of information) because LY341495 was administered immediately after the sample trial. Altogether, the present results suggest that nonspecific factors did not influence performance. Moreover, the present results are consistent with a previous report in which LY341495 alone did not affect exploratory activity in the novel object recognition task in rats [1].
Our results with the low LY341495 doses are consistent with a previous study, in which this compound antagonized the memory deficits induced by the mGlu2/3 receptor agonist LY354740 in the delayed non-match-to-position task and improved performance in the Morris water maze [13]. Importantly, the pro-cognitive dose of LY341495 (1 mg/kg) used in the aforementioned study [13] exerted amnestic effects under our experimental conditions when the test-retest interval was only 1 h. These discrepant findings with regard to the effects of LY341495 may be attributable to differences in the experimental paradigms, including the type of behavior studied, which may reflect different aspects of memory. Specifically, the novel object recognition test assesses recognition memory. The delayed non-match-to-position task assesses long-term and working memory, while the Morris water maze assesses spatial memory. Additionally, the delayed non-match-to-position task test and Morris water maze are behavioral procedures based on reinforcement. Conversely, the novel object recognition test does not involve reward or punishment, and thus the behavioral outcome is not influenced by reinforcement/response interactions [9]. Therefore, this paradigm is quite similar to procedures used in humans and should therefore have a significant level of predictive validity [10,11].
The impairment in recognition memory induced by the higher LY341495 doses used in the present study is consistent with previous results in which LY341495 disrupted performance in mice in a negatively reinforced test (i.e., the passive avoidance procedure) and in an habituation task that assesses working memory in mice [3,21]. In addition, LY341495 counteracted the ameliorative effect of clozapine on recognition memory evaluated in the novel object recognition memory task [14]. Nevertheless, the present data appear to be in contrast with the results of a previous study, in which similar doses of this compound (1 and 3 mg/kg) did not disrupt recognition memory in rats unless the compound was co-administered with the mGlu5 receptor negative allosteric modulator [6-methyl-2-(phenylethynyl)pyridine] MPEP [1]. Again, the above discrepant findings may reflect the different experimental procedures between the two studies, likely reflecting different cognitive processes.
The mechanism of this biphasic effect of LY341495 on recognition memory is unclear. LY341495 is predominantly selective for mGlu2/3 receptors, but it also interacts with other mGlu receptors at high doses, including antagonism of mGlu8 receptors at submicromolar doses [16]. One possible hypothesis to explain the present results is that LY341495 acts through different mechanisms at different doses. For example, one cannot exclude the possibility that the effects of the low dose range of LY341495 are attributable to mGlu2/3 receptor blockade, whereas interactions with other mGlu receptors may underlie the effects of the high dose range of LY341495 on recognition memory observed in the present study.
In addition, mGlu2 receptors are located on presynaptic glutamatergic terminals, where they act as inhibitory autoreceptors to suppress the release of glutamate [6]. In contrast, mGlu3 receptors are mainly located postsynaptically on neurons but are also expressed in glia, where their functional role is unclear [6]. Thus, an alternative explanation may be that the effects of low LY341495 doses are attributable to blockade of presynaptic mGlu2 receptors, whereas higher doses act predominantly on postsynaptic mGlu3 receptors.
Another explanation may be that the differential and opposite delay-dependent effects of LY341495 on memory are attributable to different endogenous glutamate tone under the two delay conditions, because mGlu2 receptors modulate glutamate release based on endogenous tone [8]. Further research with additional compounds is required to elucidate the precise factors that contribute to the differential effects of high and low LY341495 doses.
Little evidence indicates which brain structures may mediate the effects of LY341495 on memory. Pretest microinjections of LY341495 into the prefrontal and perirhinal cortices, two brain areas involved in recognition memory [9,17,23], did not influence performance in the delayed-alternation T-maze task [12] or the novel object recognition task [1] in rodents, respectively. The lack of effect observed after selective local administration of LY341495 may be related to the narrow dose range of this compound used (one or two doses) in both studies [1,12].
mGlu2/3 receptors are highly expressed in the medial temporal lobe system, including the hippocampal formation (i.e., entorhinal cortex, dentate gyrus, CA1-CA4 subregions, and subiculum), amygdala, and parahippocampal cortices [18,19]. Evidence indicates that these brain structures are involved in recognition memory, although mixed results have been obtained [9]. Therefore, these areas may represent potential sites of action of LY341495.
In summary, the present results demonstrated that the mGlu2/3 receptor antagonist LY341495 differentially affected posttraining recognition memory components in rats. Specifically, the data showed that the effects of this compound on memory were dose-dependent, with low doses enhancing memory retention when a long delay interval was used that made memory dissipate in control rats, while high doses adversely affected storage and/or retrieval. Finally, when a longer delay interval was used resulting in dissipation of memory in control vehicle-treated rats, LY341495 at low doses antagonized this memory dissipation. Thus, the effects of this compound on memory are also dependent on delay between the sample and the choice phase of this recognition memory task.
Research Highlights.
The (mGlu)2/3 receptor antagonist LY341495 produced a dual effect on recognition memory>Low doses of LY341495 counteracted delay-dependent recognition memory deficits>The effects of it on memory are dose and delay-dependent.
Acknowledgements
This work was supported by a grant from the Research Committee of the University of Thessaly (no. 3689) to N.P., and National Institutes of Health grant R01MH087989 to A.M. The authors would like to thank Mr. Michael Arends for outstanding editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr. Markou has received contract research support from Lundbeck Research USA, Inc., Bristol-Myers Squibb Co., F. Hoffman-La Roche Ind., Pfizer, and Astra-Zeneca and honoraria/consulting fees from Abbott GmbH and Company, AstraZeneca, and Pfizer during the past 3 years. Dr. Markou has a patent application on the use of metabotropic glutamate compounds for the treatment of nicotine dependence. The other authors report no financial conflicts of interest.
References
- 1.Barker GRI, Bashir ZI, Brown MW, Warburton EC. A temporally distinct role for the group I and group II metabotropic glutamate receptors in object recognition memory. Learn Mem. 2006;13:178–86. doi: 10.1101/lm.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartolini L, Casamenti F, Pepeu G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol Biochem Behav. 1996;53:277–83. doi: 10.1016/0091-3057(95)02021-7. [DOI] [PubMed] [Google Scholar]
- 3.Bespalov A, Jongen-Relo AL, van Gaalen M, Harich S, Schoemaker H, Gross G. Habituation deficits induced by metabotropic glutamate receptors 2/3 blockade in mice: reversal by antipsychotic drugs. J Pharmacol Exp Ther. 2007;320:944–50. doi: 10.1124/jpet.106.110684. [DOI] [PubMed] [Google Scholar]
- 4.Boultadakis A, Georgiadou G, Pitsikas N. Effects of the nitric oxide synthase inhibitor L-NAME on different memory components as assessed in the object recognition task in the rat. Behav Brain Res. 2010;207:208–14. doi: 10.1016/j.bbr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Caraci F, Molinaro G, Battaglia G, Giuffrida ML, Riozzi B, Traficante A, Bruno V, Cannella M, Merlo S, Wang X, Heinz BA, Nisenbaum ES, Britton TC, Drago F, Sortino MA, Copani A, Nicoletti F. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer's disease: selective activation of mGlu2 receptor amplifies β-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol Pharmacol. 2011;79:618–26. doi: 10.1124/mol.110.067488. [DOI] [PubMed] [Google Scholar]
- 6.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 7.Cavoy A, Delacour J. Spatial but not object recognition is impaired by aging in rats. Physiol Behav. 1993;53:527–30. doi: 10.1016/0031-9384(93)90148-9. [DOI] [PubMed] [Google Scholar]
- 8.Conn PJ, Pin JP. Pharmacology and function of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats: 1. Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 11.Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–54. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 12.Gregory ML, Stech NE, Owens RW, Kalivas PW. Prefrontal group II metabotropic glutamate receptor activation decreases performance on a working memory task. Ann N Y Acad Sci. 2003;1003:405–9. doi: 10.1196/annals.1300.037. [DOI] [PubMed] [Google Scholar]
- 13.Higgins GA, Ballard TM, Kew JNC, Richards JG, Kemp JA, Adams G, Woltering T, Nakanishi S, Mutel V. Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 2004;46:907–17. doi: 10.1016/j.neuropharm.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Horiguchi M, Huang M, Meltzer HY. Interaction of mGlu2/3 agonism with clozapine and lurasidone to restore novel object recognition in subchronic phencyclidine-treated rats. Psychopharmacology. 2011;217:13–24. doi: 10.1007/s00213-011-2251-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Fraser PE, Westaway D, St. George-Hyslop PH, Ehrlich ME, Gandy S. Group II metabotropic glutamate receptor stimulation triggers production and release of Alzheimer's amyloid β42 from isolated intact nerve terminals. J Neurosci. 2010;30:3870–5. doi: 10.1523/JNEUROSCI.4717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingston AE, Omstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaie R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 17.Mumby DG, Glenn MJ, Nesbitt C, Kyriazis DA. Dissociation in retrograde memory for object discriminations and object recognition in rats with perirhinal cortex damage. Behav Brain Res. 2002;132:215–26. doi: 10.1016/s0166-4328(01)00444-2. [DOI] [PubMed] [Google Scholar]
- 18.Ohishi H, Shigemoto R, Nakanishi S, Misuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53:1009–18. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- 19.Ohishi H, Shigemoto R, Nakanishi S, Misuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–66. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 20.Ornstein PL, Bleisch TJ, Arnold MB, Kennedy JH, Wright RA, Johnson BG, Tizzano JP, Helton DR, Kallman MJ, Schoepp DD, Herin M. 2-substituted (2SR)-2-amino-2-((1SR,2SR)-2-carboxycycloprop-1-yl)glycines as potent and selective antagonists of group II metabotropic glutamate receptors: 2. Effects of aromatic substitution, pharmacological characterization, and bioavailability. J Med Chem. 1998;41:358–78. doi: 10.1021/jm970498o. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Inhibitory effects of group II mGluR-related drugs on memory performance in mice. Physiol Behav. 2004;80:747–58. doi: 10.1016/j.physbeh.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Sheardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steckler T, Drinkenburg WHIM, Sahgal A, Aggleton JP. Recognition memory in rats: I. Concepts and classification. Prog Neurobiol. 1998;54:289–311. doi: 10.1016/s0301-0082(97)00060-9. [DOI] [PubMed] [Google Scholar]