Abstract
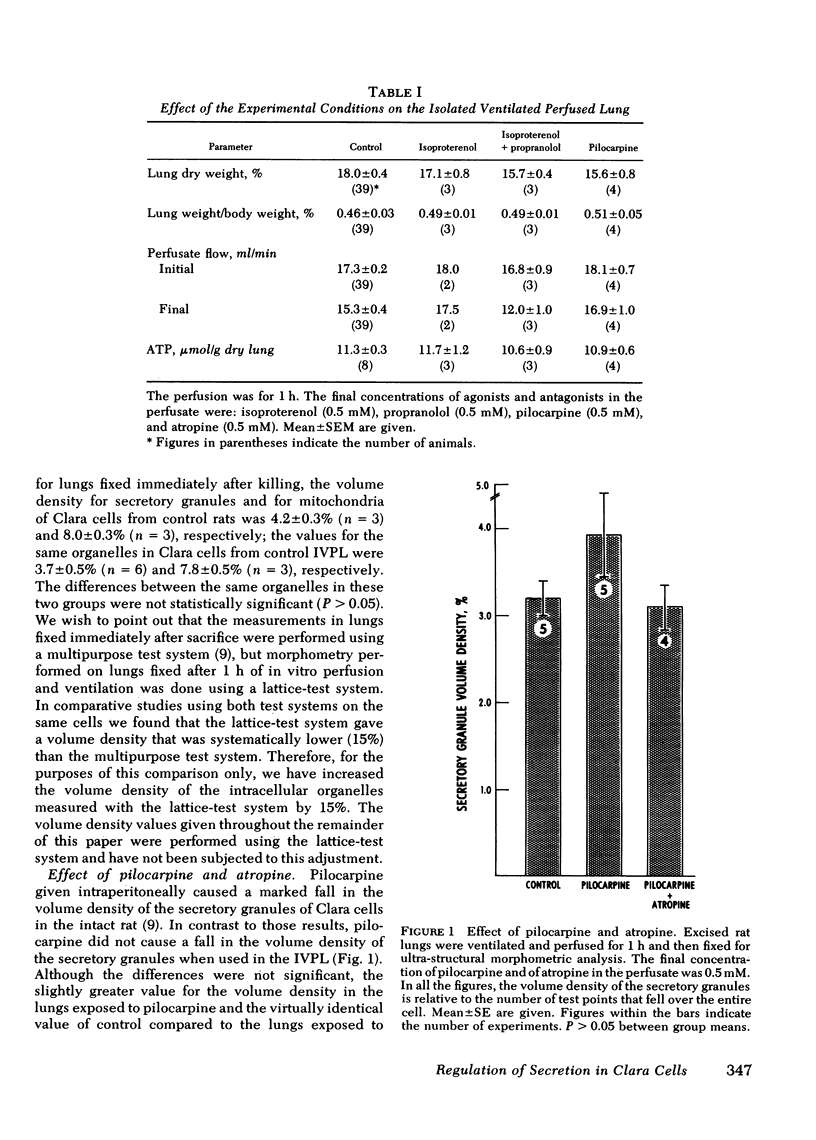
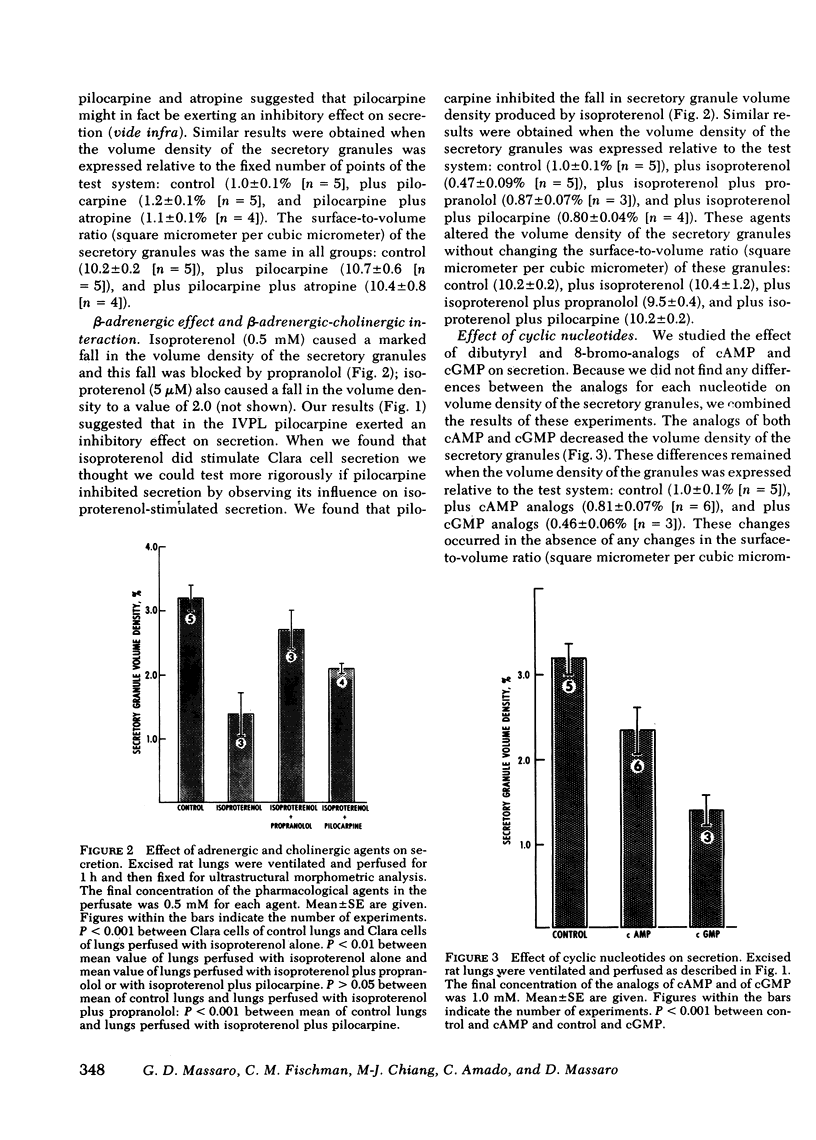
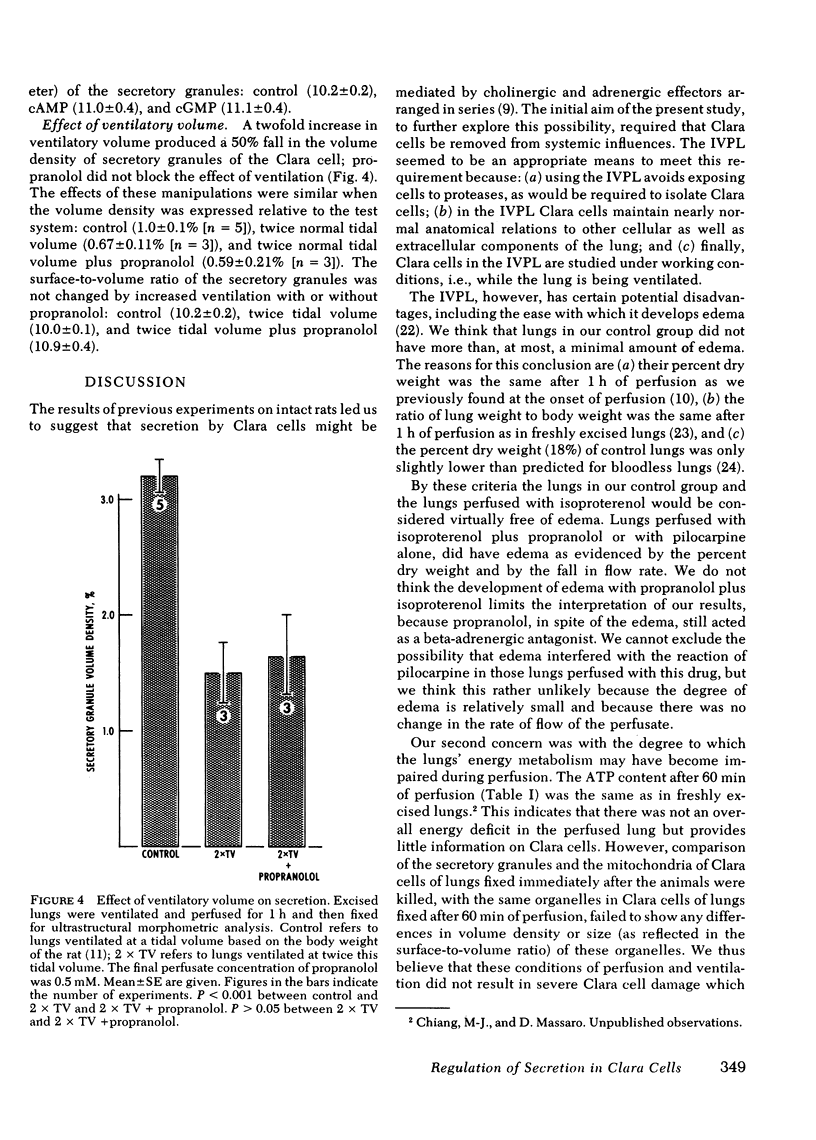
Previous studies from our laboratory indicated that both beta-adrenergic and cholinergic agents stimulate in vivo secretion by rat bronchiolar Clara cells. Those studies also provided support for an in-series beta-adrenergic-cholinergic stimulation of secretion. To further explore the regulation of secretion in Clara cells, and to do it in the absence of systemic influences, we have used the isolated ventilated perfused rat lung. We have again used morphometry and electron microscopy to assess secretion by measuring the volume density (fraction of cell volume) of the secretory granules of bronchiolar Clara cells. We found that in the isolated perfused lung, as in the intact animal, isoproterenol stimulated secretion in Clara cells and that this effect was blocked by the beta-adrenergic antagonist propranolol. Pilocarpine, unlike its action in the intact animal, did not stimulate secretion in the isolated lung; rather it inhibited the secretory effect of isoproterenol. Increased tidal-volume ventilation stimulated secretion; propranolol did not block this effect. Analogs of cyclic (c)AMP and of cGMP also stimulated secretion by Clara cells. These findings indicate that there are at least two mechanisms by which Clara cells can be stimulated to secrete. One seems to be beta-adrenergic-cAMP mediated but the triggering event is unknown. The other is initiated by increased tidal volume and cGMP may be involved in the intracellular mediation of this stimulatory event. Finally, we found evidence of beta-adrenergic (stimulatory) -cholinergic (inhibitory antagonism in the regulation of secretion in Clara cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain J. D., Frank N. R. The relation of age to the numbers of lung free cells, lung weight, and body weight in rats. J Gerontol. 1968 Jan;23(1):58–62. doi: 10.1093/geronj/23.1.58. [DOI] [PubMed] [Google Scholar]
- Brown J. H. Cholinergic inhibition of catecholamine-stimulable cyclic AMP accumulation in murine atria. J Cyclic Nucleotide Res. 1979 Dec;5(6):423–433. [PubMed] [Google Scholar]
- Chiang M. J., Whitney P., Jr, Massaro D. Protein metabolism in lung: use of isolated perfused lung to study protein degradation. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):72–78. doi: 10.1152/jappl.1979.47.1.72. [DOI] [PubMed] [Google Scholar]
- Collins M., Palmer G. C., Baca G., Scott H. R. Stimulation of cyclic AMP in the isolated perfused rat lung. Res Commun Chem Pathol Pharmacol. 1973 Nov;6(3):805–812. [PubMed] [Google Scholar]
- Dobbs L. G., Mason R. J. Pulmonary alveolar type II cells isolated from rats. Release of phosphatidylcholine in response to beta-adrenergic stimulation. J Clin Invest. 1979 Mar;63(3):378–387. doi: 10.1172/JCI109313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Horner K. A., Hayashi K., Tsuruhara T., Conn P. M., Catt K. J. Actions of choleragen and gonadotropin in isolated Leydig cells. Functional compartmentalization of the hormone-activated cyclic AMP response. J Biol Chem. 1978 May 25;253(10):3721–3729. [PubMed] [Google Scholar]
- Faridy E. E. Effect of ventilation on movement of surfactant in airways. Respir Physiol. 1976 Sep;27(3):323–334. doi: 10.1016/0034-5687(76)90061-x. [DOI] [PubMed] [Google Scholar]
- Freeman G., Juhos L. T., Furiosi N. J., Mussenden R., Stephens R. J., Evans M. J. Pathology of pulmonary disease from exposure to interdependent ambient gases (nitrogen dioxide and ozone). Arch Environ Health. 1974 Oct;29(4):203–210. doi: 10.1080/00039896.1974.10666569. [DOI] [PubMed] [Google Scholar]
- Hogg J. C., Macklem P. T., Thurlbeck W. M. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968 Jun 20;278(25):1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- KLEINMAN L. I., RADFORD E. P., Jr VENTILATION STANDARDS FOR SMALL MAMMALS. J Appl Physiol. 1964 Mar;19:360–362. doi: 10.1152/jappl.1964.19.2.360. [DOI] [PubMed] [Google Scholar]
- Klass D. J. Lung tissue guanosine 3',5'-monophosphate: effects of ventilation and anesthesia. J Appl Physiol Respir Environ Exerc Physiol. 1978 Oct;45(4):487–494. doi: 10.1152/jappl.1978.45.4.487. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Callaway L. A., Askin F. B. The formation of granules in the bronchiolar Clara cells of the rat. 1. Electron microscopy,. J Ultrastruct Res. 1974 Dec;49(3):387–400. doi: 10.1016/s0022-5320(74)90052-5. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Kuo W. N. Regulation by beta-adrenergic receptor and muscarinic cholinergic receptor activation of intracellular cyclic AMP and cyclic GMP levels in rat lung slices. Biochem Biophys Res Commun. 1973 Dec 10;55(3):660–665. doi: 10.1016/0006-291x(73)91195-9. [DOI] [PubMed] [Google Scholar]
- Massaro D., Kelleher K., Massaro G., Yeager H., Jr Alveolar macrophages: depression of protein synthesis during phagocytosis. Am J Physiol. 1970 Jun;218(6):1533–1539. doi: 10.1152/ajplegacy.1970.218.6.1533. [DOI] [PubMed] [Google Scholar]
- Massaro G. D., Massaro D. Granular pneumocytes. Electron microscopic radioautographic evidence of intracellular protein transport. Am Rev Respir Dis. 1972 Jun;105(6):927–931. doi: 10.1164/arrd.1972.105.6.927. [DOI] [PubMed] [Google Scholar]
- Massaro G. D., Paris M., Thet L. A. In vivo regulation of secretion of bronchiolar Clara cells in rats. J Clin Invest. 1979 Feb;63(2):167–172. doi: 10.1172/JCI109285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Oyarzún M. J., Clements J. A., Baritussio A. Ventilation enhances pulmonary alveolar clearance of radioactive dipalmitoyl phosphatidylcholine in liposomes. Am Rev Respir Dis. 1980 Apr;121(4):709–721. doi: 10.1164/arrd.1980.121.4.709. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Piper P., Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971 Apr 30;180:363–385. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- Reid L. M. Secretory cells. Fed Proc. 1977 Dec;36(13):2703–2707. [PubMed] [Google Scholar]
- Staub N. C. Pulmonary edema. Physiol Rev. 1974 Jul;54(3):678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W. Studies on the mechanism of hormone action. Science. 1972 Aug 4;177(4047):401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]


