Abstract
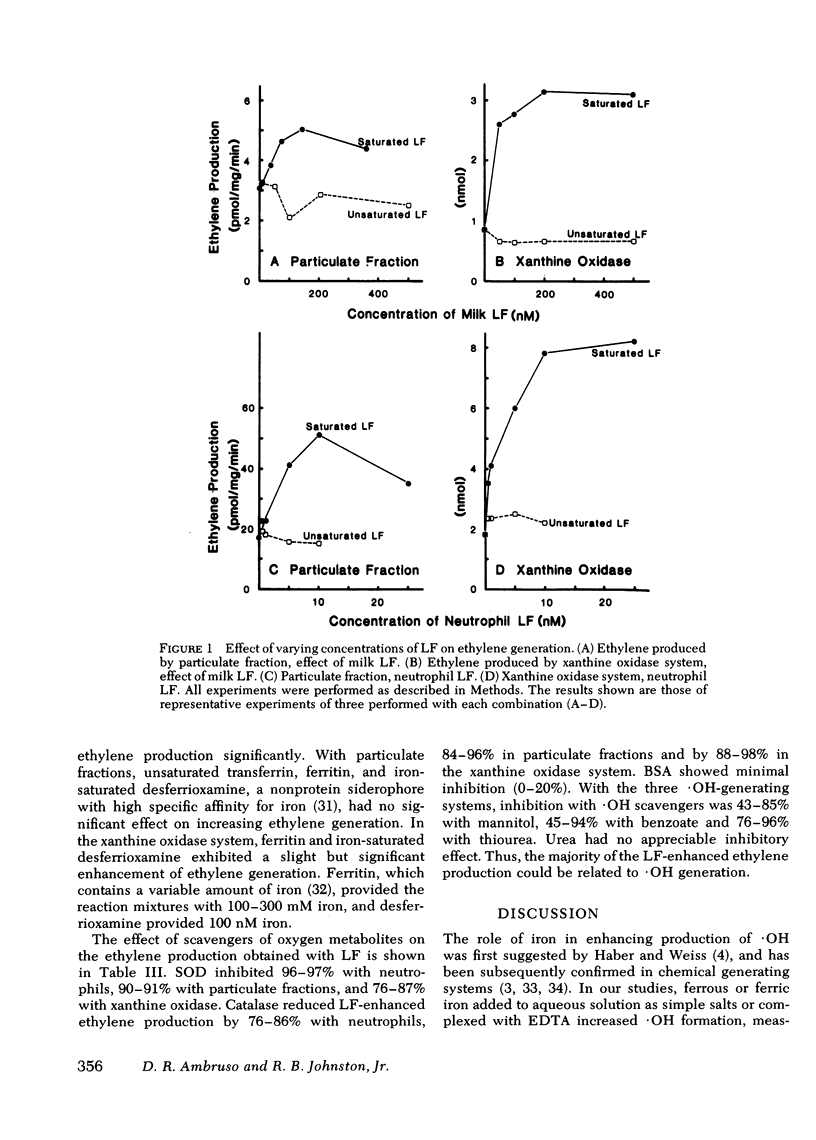
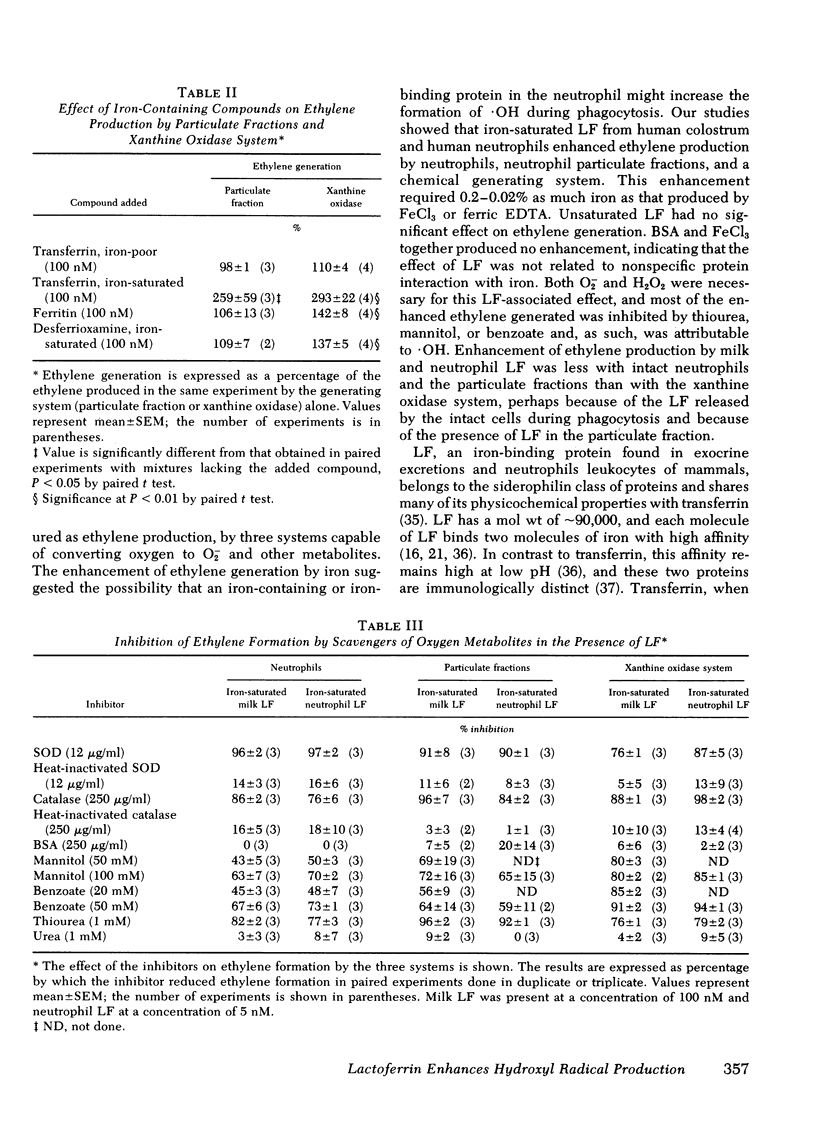
During phagocytosis, neutrophils take oxygen from the surrounding medium and convert it to superoxide anion (O2-) and hydrogen peroxide (H2O2). Hydroxyl radical (.OH), a particularly potent oxidant, is believed to be produced by interaction between O2- and H2O2 in the presence of iron, according to the Haber-Weiss reactions. Production of .OH by whole human neutrophils, by particulate fractions from human neutrophils disrupted after stimulation, and by a xanthine oxidase system was measured by conversion of alpha-keto-gamma-methiol butyric acid to ethylene. FeCl3 or ferric EDTA enhanced ethylene production in all three systems by 155--406% of base line at a concentration of 50--100 microM. Iron-saturated human milk lactoferrin, 100 nM, increased ethylene generation by 127--296%; and purified human neutrophil lactoferrin, 10 nM, enhanced ethylene production by 167--369%. Thus, iron bound to lactoferrin was approximately 5,000 times more effective in producing an enhancement in ethylene generation than iron derived from FeCl3 or ferric EDTA. O2- and H2O2 were required for ethylene production in the presence of lactoferrin, since superoxide dismutase inhibited ethylene formation in the three systems by 76--97% and catalase inhibited by 76--98%. Ethylene production in the presence of lactoferrin was inhibited by the .OH scavengers mannitol, benzoate, and thiourea by 43--85, 45--94, and 76--96%, respectively. Thus, most of the ethylene production could be attributed to oxidation of alpha-keto-gamma-methiol butyric acid by .OH. The ability of neutrophil lactoferrin to provide iron efficiently to the oxygen radical-generating systems is compatible with a role for lactoferrin as regulator of .OH production. As such, lactoferrin may be an important component in the microbicidal activity of neutrophils.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Aasa R., Malmström B. G., Vänngård T. Bicarbonate and the binding of iron to transferrin. J Biol Chem. 1967 May 25;242(10):2484–2490. [PubMed] [Google Scholar]
- Ambruso D. R., Altenburger K. M., Johnston R. B., Jr Defective oxidative metabolism in newborn neutrophils: discrepancy between superoxide anion and hydroxyl radical generation. Pediatrics. 1979 Nov;64(5 Pt 2 Suppl):722–725. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., De Duve C., Masson P. L., Heremans J. F. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970 Mar 1;131(3):559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bennett R. M., Kokocinski T. Lactoferrin content of peripheral blood cells. Br J Haematol. 1978 Aug;39(4):509–521. doi: 10.1111/j.1365-2141.1978.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Bors W., Lengfelder E., Saran M., Fuchs C., Michel C. Reactions of oxygen radical species with methional: a pulse radiolysis study. Biochem Biophys Res Commun. 1976 May 3;70(1):81–87. doi: 10.1016/0006-291x(76)91111-6. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J., Mason D. Y., Buriot D., Vilde J. L., Griscelli C. Lactoferrin deficiency as a consequence of a lack of specific granules in neutrophils from a patient with recurrent infections. Detection by immunoperoxidase staining for lactoferrin and cytochemical electron microscopy. Am J Pathol. 1980 May;99(2):413–428. [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. H. Studies on phagocytosis. I. Uptake of radio-iodinated (131-I) human serum albumin as a measure of the degree of phagocytosis in vitro. Exp Cell Res. 1969 Jan;54(1):42–48. doi: 10.1016/0014-4827(69)90290-0. [DOI] [PubMed] [Google Scholar]
- Cohen G. The generation of hydroxyl radicals in biologic systems: toxicological aspects. Photochem Photobiol. 1978 Oct-Nov;28(4-5):669–675. doi: 10.1111/j.1751-1097.1978.tb06993.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978 Dec 15;96(2):238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- Harrison P. M. Ferritin: an iron-storage molecule. Semin Hematol. 1977 Jan;14(1):55–70. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama A., Morosawa H., Nakahata T., Miyagawa Y., Akabane T. Abnormal neutrophil maturation in a neutrophil defect with morphologic abnormality and impaired function. J Pediatr. 1979 Jan;94(1):19–25. doi: 10.1016/s0022-3476(79)80343-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Intracellular and extracellular degranulation of human polymorphonuclear azurophil and specific granules induced by immune complexes. Infect Immun. 1974 Dec;10(6):1241–1249. doi: 10.1128/iai.10.6.1241-1249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestas A. N. The effect of pH upon human transferrin: selective labelling of the two iron-binding sites. Br J Haematol. 1976 Mar;32(3):341–350. doi: 10.1111/j.1365-2141.1976.tb00937.x. [DOI] [PubMed] [Google Scholar]
- MONTREUIL J., TONNELAT J., MULLET S. [Preparation and properties of lactosiderophilin (lactotransferrin) of human milk]. Biochim Biophys Acta. 1960 Dec 18;45:413–421. doi: 10.1016/0006-3002(60)91478-5. [DOI] [PubMed] [Google Scholar]
- Malmquist J., Thorell J. I., Wolheim F. A. Lactoferrin and lysozyme in arthritic exudates. Acta Med Scand. 1977;202(4):313–318. doi: 10.1111/j.0954-6820.1977.tb16834.x. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Johnston R. B., Jr Generation of chemiluminescence by a particulate fraction isolated from human neutrophils. Analysis of molecular events. J Clin Invest. 1979 Apr;63(4):648–655. doi: 10.1172/JCI109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn N., Eger R. R., Broxmeyer H. E., Moore M. A. Isolation of a granulocyte colony inhibitory factor derived from human polymorphonuclear neutrophils. Biochim Biophys Acta. 1978 Mar 28;533(1):238–247. doi: 10.1016/0005-2795(78)90567-6. [DOI] [PubMed] [Google Scholar]
- Parmley R. T., Ogawa M., Darby C. P., Jr, Spicer S. S. Congenital neutropenia: neutrophil proliferation with abnormal maturation. Blood. 1975 Nov;46(5):723–734. [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Strauss R. G., Bove K. E., Jones J. F., Mauer A. M., Fulginiti V. A. An anomaly of neutrophil morphology with impaired function. N Engl J Med. 1974 Feb 28;290(9):478–484. doi: 10.1056/NEJM197402282900903. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Gabig T. G., Babior B. M. Evidence for production of oxidizing radicals by the particulate O-2-forming system from human neutrophils. Blood. 1979 Apr;53(4):666–676. [PubMed] [Google Scholar]
- Wang-Iverson P., Pryzwansky K. B., Spitznagel J. K., Cooney M. H. Bactericidal capacity of phorbol myristate acetate-treated human polymorphonuclear leukocytes. Infect Immun. 1978 Dec;22(3):945–955. doi: 10.1128/iai.22.3.945-955.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrak J., Herman T., Werys R., Pryjma J., Gaweł J. Proteins in bronchial secretion of children with chronic pulmonary diseases. II. Relation to bronchoscopic and bronchographic examination. Scand J Respir Dis. 1979 Apr;60(2):69–75. [PubMed] [Google Scholar]



