Abstract
Underlying the importance of research on the biology of aging is the fact that many nations face the demographic reality of a rapidly aging populace and the looming healthcare challenges that it brings. This reality is a result of aging itself being the most significant risk factor for a range of the most prevalent diseases, including many cancers, cardiovascular disease and diabetes. Accordingly, interventions are sorely needed which would be able to delay or prevent diseases and disorders associated with the aging process and thereby increase the period of time that aging individuals are in good health (the “health-span”). Caloric restriction (CR) has emerged as a model of major interest as it is widely agreed that CR is the most potent environmental intervention that that delays the onset of aging and extends life span in diverse experimental organisms. A better understanding of the mechanisms by which CR delays aging will reveal new insights into the aging process and the underlying causes of disease vulnerability with age. These novel insights will allow the development of novel treatments and preventive measures for age-associated diseases and disorders.
Introduction
Caloric restriction (CR) is a long-term dietary intervention whereby caloric intake is reduced but malnutrition is avoided. CR applied at 10-30% reduced caloric intake extends lifespan in rodents in an inverse linear manner. The lower the intake of calories, the greater the extension in lifespan. There is a limit of course, and the beneficial effects are lost if animals approach malnutrition. A striking feature of CR that has been appreciated for many years from studies in rodents is its ability to oppose the development of a broad spectrum of age-associated pathological and physiological changes (Weindruch & Walford, 1988). Thus animals on CR stay “younger longer.” The field has rapidly grown with an increasing number of annual citations (Figure 1), powered in part by the highly mechanistic studies in non-mammalian species. Indeed, considerable insight has been gleaned through studies in yeast, nematodes and flies. The short lifespan, low cost, and relative ease of genetic manipulation of these simpler organisms has facilitated the type of analysis that, until recently, would have been inconceivable in mammalian systems.
Figure 1.
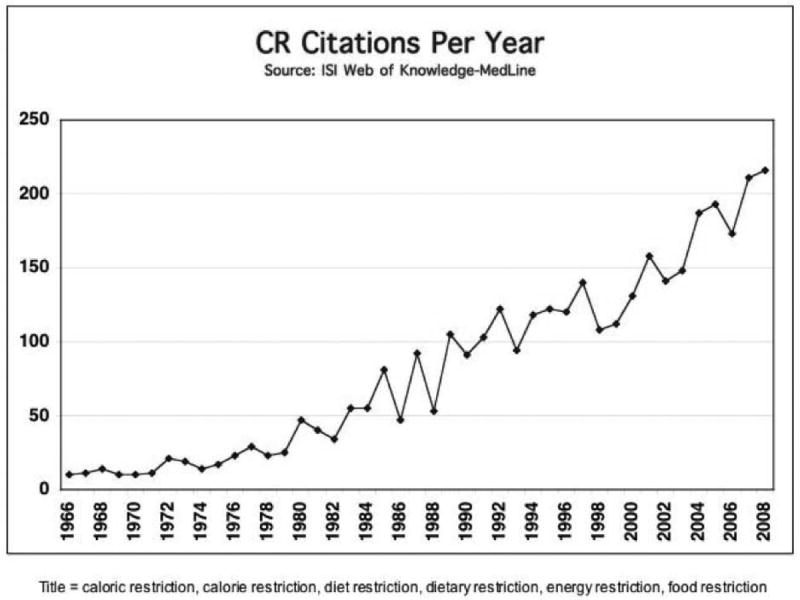
Caloric restriction is a growing area of study. The number of publications identified by a NCBI Pubmed search using the indicated keywords.
The early mammalian experiments were largely descriptive in nature. It was clear as early as the 1940’s that CR inhibited spontaneous tumor development and impeded progression of induced or implanted tumors in rats and mice (Weindruch & Walford, 1988). The mechanistic basis for the non-permissive tumor environment created by CR is only now beginning to be explored (De Lorenzo and others 2011; Jiang and others 2008; Yamaza and others 2010). In contrast, the involvement of single genes and signaling pathways in CR has been investigated in non-mammalian systems to some depth (Fontana and others 2010). Establishing whether mechanisms of CR identified in simple organisms are also relevant to mammalian CR is an active area of inquiry. Much of this work is being conducted in mice where conditional and tissue specific genetic manipulations can be employed to probe for factors involved in CR’s mechanisms. The beneficial impact of CR on health has been reported in non-human primates, including 3 independent studies in rhesus monkeys (see below) and one in squirrel monkeys (Roth and others 2000). In addition, a series of studies are currently underway using human subjects. So far the data are consistent with a high degree of similarity in the response of humans and non-human primates to CR, both of which mirror that observed in the earlier rodent studies. This conserved response supports the value of CR research for understanding human aging and age-associated disease vulnerability.
We herein discuss the three main questions that are being asked in CR studies:
By what molecular/cellular mechanisms does CR slow the aging process? Elucidation of the mechanisms of CR will provide crucial leads for understanding the aging process and will identify novel targets for disease prevention and treatment.
Can drugs or nutrients be discovered which mimic the actions of CR in organisms consuming normal levels of calories? The search for so-called “CR mimetics” is a rapidly growing area of inquiry that has huge potential to translate some of the benefits of CR to people consuming a normal caloric intake.
Will CR be effective in slowing aging in humans? We discuss data from long-term CR studies in rhesus monkeys as well as the increasing work being conducted in humans. We conclude by evaluating the implications of CR on healthy human aging.
Mechanisms
It has been 75 years since the impact of CR in extending lifespan and retarding the onset of age-associated disease in mammals was first described (McCay and others 1935). The mechanism of CR has remained elusive; however, because of the potential to radically improve our knowledge of aging, solving CR is a major focus in aging research. In the earlier stages of CR research, the anti-aging properties were thought to be a result of passive mechanism: a slower rate of living resulting in a slower rate of aging. Since then substantial evidence has emerged that CR actively induces a longevity-promoting program (Anderson and Weindruch 2010; Jazwinski 2000; Sinclair 2005). Analysis of data generated in mouse studies from the 1980’s shows that there is an inverse linear relationship between calorie intake and lifespan (Anderson and Weindruch 2010; Weindruch and others 1986; Weindruch and Walford 1988). Building on this observation we can suggest the following: first, nutrient sensitive elements are likely involved in relaying signals in response to lower calorie availability; second, pathways of fuel utilization are adapted to maximize efficiency; third, metabolites are diverted away from pathways of storage to permit full usage of all available fuel sources. All of these predictions point to one common theme, which is a change in the regulation of metabolism. We suggest that this change in metabolism protects cells and tissues from the rigors of age and activates pathways that confer increased disease resistance.
The advent of gene expression profiling technology has proven to be invaluable in providing insights into the complex processes of aging and anti-aging by CR. Large-scale analysis of age-associated transcriptional changes among 16 different mouse tissues demonstrates that while there is a high degree of tissue specificity in gene expression, and in the impact of age, a common signature across tissues is the decline in expression of genes involved in energy metabolism (Zahn and others 2007). Furthermore, a meta-analysis of age-related gene expression profiles from 27 datasets generated from human, rat and mouse tissues identified the same signature (de Magalhaes and others 2009). Gene expression profiling has also been used to determine the impact of CR on mouse aging (Lee and others 2002; Lee and others 1999; Lee and others 2000). At the level of individual tissues, CR prevents the majority of the age-associated changes in gene expression (Park and Prolla 2005).
These earlier studies analyzed gene expression in tissues from old Control and old CR mice and compared them to young Controls. This type of experiment provides information about aging and the impact of CR in delaying aging. In teasing out the data, two categories of transcriptional alterations induced by CR were identified: first, shifts that opposed age-related changes in gene expression and second, changes in the expression of genes that were impacted by CR independent of age. An alternate approach is to compare matched middle-aged adult animals fed Control or CR diets. In this way, changes in gene expression induced by aging are not contributing to the differences between groups of mice.
Using this latter approach we investigated the impact of CR on white adipose tissue (WAT). Animals on CR are smaller and lighter than their Control counterparts but the difference in size is not uniformly shared among the tissues. Proportionally more body fat is lost than any other tissue and for mice on a 35% CR diet, estimated total body fat is ~70% reduced. These differences in WAT may be important in the mechanisms of CR. A consensus is emerging that describes WAT in a key role in the central homeostatic mechanism that integrates metabolism and inflammation (Hotamisligil 2006). WAT-derived factors have been implicated in metabolic disorders linked to obesity (Lago and others 2007; Wisse 2004). Dietary excess and the resulting obesity can lead to metabolic syndrome, the manifestation of multiple conditions previously associated with aging. These include Type 2 diabetes, inflammation, hypertension and cardiovascular disease (Eckel and others 2005). Indeed aging is also associated with adverse alterations in body fat distribution as well as deregulated WAT function (Cartwright and others 2007; Clement and Langin 2007; Das and others 2004) (Figure 2).
Figure 2.
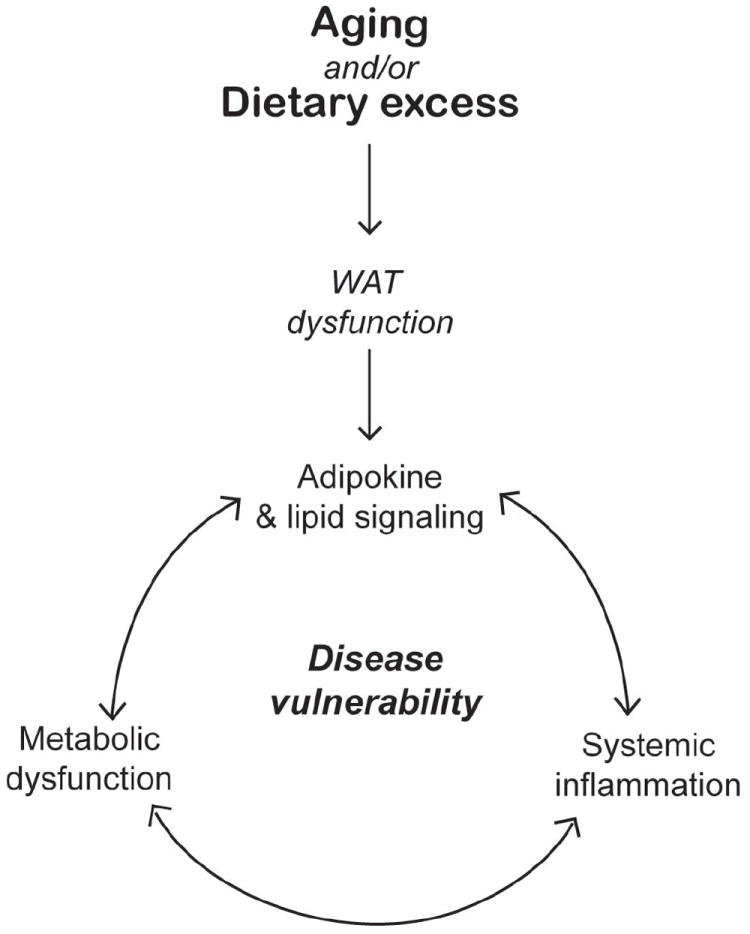
The influence of adipose tissue derived signaling factors on system wide metabolic balance and health.
In addition to shrinking the size of WAT depots, CR induces key shifts in genes involved in metabolism in WAT, including a striking increase in expression of genes involved in energy metabolism (Higami and others 2004). Furthermore, the expression of over 50 pro-inflammatory genes is reduced (Higami and others 2006). Reductions in systemic inflammatory tone caused by CR may underlie its ability to oppose a broad spectrum of age-associated diseases, including cancers and cardiovascular disease. The overt changes induced in WAT by CR may contribute to alterations in systemic metabolic homeostasis, influencing factors at the interface of metabolism and inflammation (Figure 2).
Targets for CR mimetic development
While improved health and increased longevity are enormously attractive to most people, a lifetime commitment to a reduced-calorie diet is rather unattractive. As a means to both have one’s cake and actually eat it, a range of nutraceuticals and drugs are being explored as a means to mimic the effects of CR. As outlined above, we propose that alterations in energy metabolism are critical in the mechanisms of CR and contribute to the increased disease resistance that is observed in animals on CR. Interventions that offer the most promise are those that target regulators of metabolism.
PGC-1a is a key regulator of mitochondria (Wu and others 1999) and a transcriptional co-activator. The targets of its co-activation are the nuclear receptor family of transcription factors that are involved in multiple aspects of metabolism (Canto and others 2009; Feige and Auwerx 2007; Rodgers and others 2008). SIRT1 is a positive regulator of PGC-1a (Nemoto and others 2005; Rodgers and others 2005) that has been implicated in the mechanisms of CR in several species including mice (Longo and Kennedy 2006). In mice fed a high fat diet, activation of SIRT1 enhances fat oxidation in peripheral tissues and enhances expression of genes involved in oxidative phosphorylation (Feige and others 2008). These metabolic alterations are likely responsible for the protective effect of increased SIRT1 in diet-induced obesity and are reminiscent of the metabolic shifts observed in tissues from CR animals. AMPK (AMP-activated protein kinase) is involved in the adaptive response to energy deficit. AMPK is also a PGC-1a activator that can induce mitochondrial biogenesis and increases activity of mitochondrial enzymes (Putman and others 2003; Zong and others 2002). The plant polyphenol resveratrol is an activator of AMPK that has been shown to produce CR-like alterations in gene transcription in multiple tissues in mice, although longevity does not appear to be extended in normally fed animals (Barger and others 2008a; Barger and others 2008b; Pearson and others 2008). Compounds that activate the AMPK/SIRT1/PGC-1a axis are strong candidates as CR mimetics.
Another factor that has emerged as a candidate in the mechanisms of CR is the nutrient sensing kinase TOR (Target of rapamycin). TOR signaling promotes cell growth in conditions of nutrient abundance (Wullschleger and others 2006). TOR has been implicated in the mechanisms of CR in yeast, where decreased signaling through TOR pathway extends lifespan (Kennedy and others 2007). Reduced signaling through the mammalian TOR (mTOR) has also been associated with increased lifespan in mice (Selman 2009). In addition, mTOR inhibition by rapamycin treatment extends lifespan in mice (Harrison and others 2009). The role of mTOR is likely to be highly tissue-specific and as yet little is known about this important regulatory molecule in primate aging and CR.
As the search for drugs and nutraceuticals continues, a variety of supplements are being tested for the ability to confer robust health effects. These include compounds that were originally derived from natural products, and compounds generated by pharmaceutical companies that have already been on the market for the treatment of other diseases and disorders. The National Institute on Aging at the National Institutes of Health has created the Interventions Testing Program where researchers are invited to propose compounds for testing as pro-longevity agents in mice. The list of compounds being tested to date includes resveratrol (activator of AMPK and SIRT1), rapamycin (inhibitor of mTOR), and metformin (AMPK activator). It will be very interesting to see the outcome of these exciting studies.
Nonhuman primates and humans
Much of what we have learned about the possible mechanisms of CR has been gleaned from the study of rodent and invertebrate species. There is always an issue of translatability to human aging for these shorter-lived species. Non-human primate species share a high degree of similarity to humans in their anatomy, physiology and behavior. Rhesus macaques (Macaca mulatta) have an average lifespan of ~27 years in captivity and a maximal lifespan ~40 years. Non-human primate aging studies, while very costly to conduct, promise considerable insight in the biology of aging and a high degree of translatability to human aging and age-associated disease.
In the late 1980’s, three independent Aging and CR studies were initiated utilizing rhesus macaques. The National Institute on Aging (NIA) and the Wisconsin National Primate Research Center (WNPRC) studies set out to test the suitability of rhesus monkeys as a model for human aging and to determine whether CR could delay aging and extend maximal lifespan in this species. The third study was a short-term intervention performed at the University of Maryland (UMD), and focused more specifically on obesity and glucoregulatory function.
Similar to the findings of numerous rodent studies, the most striking effect of CR in monkeys is the impact on body composition and glucoregulatory function. In all studies, CR lowers body weights, decreases fat mass and improves insulin sensitivity: UMD (Bodkin and others 2003), WNPRC (Colman and others 1999b; Gresl and others 2001; Kemnitz and others 1994) and the NIA (Lane and others 1995; Mattison and others 2007). Both the NIA and WNPRC studies have shown lower bone mass in the CR animals that can be accounted for by lower body mass (Black and others 2001; Colman and others 1999a). The WNPRC study has shown improved cardiovascular profiles in the restricted animals, including reduced levels of C-reactive protein (CRP) and decreased levels of triglyceride and phospholipids associated with low density lipoproteins (Edwards and others 1998). Furthermore, age-associated muscle mass loss (sarcopenia) is delayed and attenuated in CR animals (McKiernan and others 2011). Overall, the real test of the efficacy of CR in this species is its ability to delay the onset of age-related diseases and extend maximal lifespan. We recently published the findings of the WNPRC 20-year long study and showed that age-related diseases including diabetes, cardiovascular disease and cancer are delayed or prevented by CR (Colman and others 2009).
Evidence that the health-promoting effects of CR may also be conserved in humans comes from epidemiological studies, controlled short-term CR studies, and data from long-term CR practitioners. Although reduced caloric intake is prevalent in numerous global populations, it is often accompanied by malnutrition. A rare exception is found on the island of Okinawa. Okinawans reportedly eat fewer calories than individuals on mainland Japan as a whole and have a relatively large proportion of centenarians. (Chan and others 1997; Kagawa 1978; Suzuki and others 2001). The increase in longevity among these people has been taken as evidence that reduced caloric intake increases average lifespan.
The direct practice of CR is ongoing in two distinct groups. The NIA is currently funding a multicenter study (CALERIE) of the effects of short-term CR in women and men. The primary goal is to determine whether humans develop the same adaptive responses to CR that occur in rodents. The second group is made up of members of the Calorie Restriction Society, who are practicing long-term CR with optimal nutrition.
In the first phase of the NIH-funded CALERIE studies the impact of short-term (6 months and 1 year long) CR on overweight humans was assessed in parallel studies at 3 locations, Pennington Biomedical Research Center in Baton Rouge, Washington University in St. Louis and Human Nutrition Research Center on Aging at Tufts University in Massachusetts. In Louisiana, men and women on a 25% CR diet for 6 months demonstrated reductions in body weight and fasting levels of insulin (Heilbronn and others 2006). Weight loss was reflected in a 24% reduction in body fat including a 27% reduction in visceral fat (Redman and others 2007). Favorable changes in serum risk factors for cardiovascular disease were observed in CR individuals (Lefevre and others 2008). In Missouri, one year of 20% CR demonstrated improved glucose tolerance and insulin action, again in overweight but healthy people (Weiss and others 2006). In addition, substantial improvements in risk factors for cardiovascular disease were observed (Fontana and others 2007). CR resulted in a reduction in weight (~10%), and fat mass comprised 77% of that weight loss (Racette and others 2006). However, the extended period of CR revealed problems with feasibility for long term studies due to lack of adherence to the diet – participants attained ~11% CR rather than the 20% objective for the study. It will be of tremendous interest to determine whether CR is as effective in non-overweight individuals.
The study of long term CR is made possible through the cooperation of individuals that maintain a strict self-imposed restricted diet. Long-term practitioners of CR (on average 6 years) have reduced circulating levels of triglycerides, fasting glucose and fasting insulin compared to age and socioeconomic matched controls. Furthermore, blood pressure is lower in CR individuals and favorable lipoprotein profiles associated with reduced risk of atherosclerosis are observed (Fontana and others 2004; Meyer and others 2006). Clearly these early human studies are very promising and support continued exploration of the mechanism of aging retardation in other species and a means to understand human longevity.
Implications for human aging and health
The physiological response to CR in rodents has been well characterized. In addition to extended lifespan and lower disease incidence, hallmarks of CR include reduced adiposity, improved glucoregulatory function and preserved mitochondrial function. At this stage we know that many of these outcomes have also been confirmed in non-human primates on CR (except for the impact on lifespan extension, which is still unknown). In humans, we do not have definitive measures of CR’s impact on lifespan and are unlikely ever to have such data. Data on disease risk indicators in humans is promising, even if the impact on disease incidence is not yet known. A key distinction should be drawn at this time between the observation that CR appears to work in humans and a recommendation that individuals embark on its practice. Much remains to be understood about non-human primate and human CR. First, unlike the majority of laboratory rodents that are inbred, most non-human primate colonies are genetically diverse. As is the case for humans, in non-human primates individual variability is observed in feeding behavior, body composition and serum indicators of disease risk. This heterogeneity extends to the response to CR. Second, it is unclear whether or not there are time points in the lifespan when CR might not be effective or even imprudent; for example introducing a CR diet in youngsters that are still developing or in older persons that have already manifest some signs of aging. It will be of interest to discover the outcomes of the NIA rhesus monkey study where a CR was initiated in animals from a range of ages including juveniles (1-2 years), adolescents (3-5 years) and older adults (16-23).
First and foremost the take home message from CR research is that the rate of aging can be manipulated. Factors that contribute to age-associated disease can be influenced to disconnect chronological age from biological age. Based on our own work and that of others, we suggest that interventions that stimulate metabolism and/or activate WAT signaling hold great promise as mimics of CR. A number of strong candidates have emerged as potential CR effector molecules. These factors may not only hold the key to attenuating disease vulnerability with age, but also may have utility in diseases associated with dietary excess and obesity. A very recently published study supports the concept that this approach may be fruitful: dietary supplementation with resveratrol resulted in improvements in multiple serum health indicators in obese humans (Timmers and others 2011). In the meantime, establishing energy balance through sensible diet and staying active will go a long way toward improving and maintaining health.
Acknowledgments
Funding: NIH/NIA P01AG11915, R01AG037000 and NIH/NCRR/CTSA UL1RR025011
References cited
- Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010 doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutracuetical mixture mimics gene expression of long-term caloric restriction in mouse heart. Experimental Gerontology. 2008a doi: 10.1016/j.exger.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008b;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A, Allison DB, Shapses SA, Tilmont EM, Handy AM, Ingram DK, Roth GS, Lane MA. Calorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body size. J Gerontol A Biol Sci Med Sci. 2001;56(3):B98–107. doi: 10.1093/gerona/56.3.b98. [DOI] [PubMed] [Google Scholar]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003;58(3):212–9. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol. 2007;42(6):463–71. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, Suzuki M, Yamamoto S. Dietary, anthropometric, hematological and biochemical assessment of the nutritional status of centenarians and elderly people in Okinawa, Japan. J Am Coll Nutr. 1997;16(3):229–35. doi: 10.1080/07315724.1997.10718679. [DOI] [PubMed] [Google Scholar]
- Clement K, Langin D. Regulation of inflammation-related genes in human adipose tissue. J Intern Med. 2007;262(4):422–30. doi: 10.1111/j.1365-2796.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz JW. Skeletal effects of aging in male rhesus monkeys. Bone. 1999a;24(1):17–23. doi: 10.1016/s8756-3282(98)00147-1. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J Gerontol A Biol Sci Med Sci. 1999b;54(7):B283–90. doi: 10.1093/gerona/54.7.b283. [DOI] [PubMed] [Google Scholar]
- Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5(1):13–9. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzua P, Vatner SF, Rabson AB. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis. 2011;32(9):1381–7. doi: 10.1093/carcin/bgr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25(7):875–81. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Cefalu WT. Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J Gerontol A Biol Sci Med Sci. 1998;53(6):B443–8. doi: 10.1093/gerona/53a.6.b443. [DOI] [PubMed] [Google Scholar]
- Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17(6):292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8(5):347–58. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101(17):6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293(1):E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281(4):E757–65. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295(13):1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA, Weindruch R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136(2):343–52. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. Faseb J. 2004;18(2):415–7. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. Metabolic control and ageing. Trends Genet. 2000;16(11):506–11. doi: 10.1016/s0168-9525(00)02119-3. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68(13):5492–9. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7(2):205–17. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266(4 Pt 1):E540–7. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64(11):1323–8. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18(3-4):313–25. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ball SS, Ingram DK, Cutler RG, Engel J, Read V, Roth GS. Diet restriction in rhesus monkeys lowers fasting and glucose-stimulated glucoregulatory end points. Am J Physiol. 1995;268(5 Pt 1):E941–8. doi: 10.1152/ajpendo.1995.268.5.E941. [DOI] [PubMed] [Google Scholar]
- Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A. 2002;99(23):14988–93. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285(5432):1390–3. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25(3):294–7. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126(2):257–68. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Lane MA, Ingram DK. Dietary restriction in aging nonhuman primates. Interdiscip Top Gerontol. 2007;35:137–58. doi: 10.1159/000096560. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, Weindruch R. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol. 2011;46(1):23–9. doi: 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47(2):398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280(16):16456–60. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Park SK, Prolla TA. Lessons learned from gene expression profile studies of aging and caloric restriction. Ageing Res Rev. 2005;4(1):55–65. doi: 10.1016/j.arr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Kiricsi M, Pearcey J, MacLean IM, Bamford JA, Murdoch GK, Dixon WT, Pette D. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003;551(Pt 1):169–78. doi: 10.1113/jphysiol.2003.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61(9):943–50. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92(3):865–72. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582(1):46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Black A, Lane MA. Effects of reduced energy intake on the biology of aging: the primate model. Eur J Clin Nutr. 2000;54(Suppl 3):S15–20. doi: 10.1038/sj.ejcn.1601020. [DOI] [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126(9):987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Wilcox BJ, Wilcox CD. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr. 2001;10(2):165–71. doi: 10.1111/j.1440-6047.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–22. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–54. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Weindruch RH, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, Illinois, USA: Charles C Thomas; 1988. [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–42. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15(11):2792–800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, Chiba T, Mori R, Furuyama T, Mori N, et al. FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. 2010;9(3):372–82. doi: 10.1111/j.1474-9726.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- Zahn JM, Poosala S, Owen AB, Ingram DK, Lustig A, Carter A, Weeraratna AT, Taub DD, Gorospe M, Mazan-Mamczarz K, et al. AGEMAP: a gene expression database for aging in mice. PLoS Genet. 2007;3(11):e201. doi: 10.1371/journal.pgen.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99(25):15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]


