Abstract
Long-term patterns of depression, and associations with health and function were examined among 1115 individuals with rheumatoid arthritis, using 18 years of panel data, summarized in 9653 interviews. Depression was defined by scores on the Geriatric Depression Scale (6 or above). Participants were classified, using cluster analysis, into three distinct patterns of depression over repeated assessments: nondepressed (65.8%), intermittent (25.2%), and chronic (9.0%). GEE analyses assessed outcomes over time as a function of patterns of depression; controlling for demographic and clinical factors. Results indicated that patterns of depression had significant adverse effects on health and function over time.
Keywords: depression, rheumatoid arthritis
Introduction
Depression is an often debilitating illness that can substantially increase the disease burden and risk of morbidity, disability, and mortality among individuals with chronic illness (Ang et al., 2005; Brodaty et al., 2001; Katon and Ciechanowski, 2002), including rheumatoid arthritis (RA) (Ang et al., 2005). Depression erodes quality of life and function (Hays et al., 1995; Katz, 2004; Vilhjalmsson, 1998), disrupts interpersonal relationships (Katz, 2004; Katz and Morris, 2007), increases demands on caregivers (Farran et al., 1998), and elevates the risk of suicide among affected individuals (Angst et al., 2002; Angst et al., 1999; Katona and Bell, 1988).
Depression is also a leading cause of disability worldwide; accounting for billions of dollars in lost productivity each year (Callahan et al., 1996; Druss et al., 2000; Kessler et al., 1999; Panzarino, 1998; Pincus and Pettit, 2001; Simon et al., 2000, 2001; Stewart et al., 2003). Among individuals living with RA, depression increases functional limitations that are associated with RA, and is linked to poor health outcomes and quality of life (Simon et al., 2005), as well as the loss of valued life activities that is in itself associated with depression in RA (Katz and Yelin, 2001). Depression may also contribute to negative appraisals of symptoms and health that adversely affect health behaviors and undermine treatment effectiveness (Graves et al., 2009; Rosenberg et al., 1988; Turk et al., 1995).Unfortunately, depression is too often accepted as a natural consequence of chronic illness among health care providers and patients, and thus not treated as seriously as more obvious disease manifestations (Katon and Sullivan, 1990; Nicassio, 2010). In terms of disease mechanisms, it has been suggested that increased symptoms of RA might be associated with loss of function and increased disability, particularly in valued life activities that enrich life and imbue it with meaning and purpose for most people (Katz, 2004; Katz and Morris, 2007; Katz and Yelin, 1995, 2001; Van 't Land et al., 2010).
Depression and RA
In studies of depression and RA, prevalence estimates of depression among individuals with RA have ranged from 13–20 percent (Ang et al., 2005; Dickens et al., 2002; Katz and Yelin, 1993, 1995; Sheehy et al., 2006), compared with 6.6 percent in the general population (Kessler et al., 2003). However, few studies have assessed the prevalence of depression among individuals with RA over an extended period of time (Ang et al., 2005; Hawley and Wolfe, 1993; Wolfe and Hawley, 1998; Wolfe et al., 1991). Our study is unique in its focus on specific patterns of depressive symptoms and associations with health and function over time among a representative community sample of individuals with RA.
Objectives of the present study
In this study, our objectives were twofold: (1) to identify and to assess the prevalence of distinct patterns of depression among a representative community sample of individuals with RA followed for up to 18 years; and (2) to examine the unique impact of long-term patterns of depression on health and function. Outcomes assessed included disability in major life activities, and self-reported health. To address these study objectives, we have analyzed a panel study of 1115 persons with RA in whom 9653 person-years of observation have been accumulated.
We hypothesized that long-term depression would increase the odds of poor health and function over time, controlling for the effects of demographic, and time varying disease-related, medication, and physical impairment factors.
Methods
Sample
The participants included 1115 individuals with RA, who were part of a larger, ongoing panel study, and who had completed at least two annual interviews during 1988–2005. The University of California, San Francisco (UCSF) RA Panel study began in 1982, using a two-stage sampling frame, in which individuals with RA were recruited from a random sample of board-certified rheumatologists practicing in Northern California. Participants were recruited from lists maintained by participating rheumatologists of all persons with RA presenting to their offices over a one-month period and expressing an interest to participate in the study. The original RA Panel consisted of 822 participants who were enrolled between June 1982 and July 1983. There were subsequently four additional enrollment periods in 1989–1990, 1995, 1999, and 2003, during which 203, 131, 122, and 169 individuals were enrolled, respectively. The primary method of data collection for the ongoing RA Panel is an annual structured telephone interview that assesses factors such as RA symptoms, functioning, psychosocial factors, health insurance and service utilization, and medication use. Annual retention in the UCSF RA Panel has averaged 95 percent, excluding mortality.
Although the UCSF RA Panel began in 1982, a measure of depressive symptoms (the Geriatric Depression Scale (Yesavage, 1988) was not added until 1988. Participants in the study between 1988 and 2005 contributed 9653 interviews over the 18 study years, an average of 8.6 years of interview data (median = 7.0 years, SD = 5.4). Over the same period, 231 participants died (20.7%), 393 (35.2%) dropped out, primarily because of health reasons; and 22 (2.0%) were lost to follow-up. Analyses of attrition by patterns of depression and other study variables are described further in the results section. All of the study protocols have been reviewed and were approved by the institutional review board at UCSF.
Measures
Demographic characteristics
In the present analyses, demographic variables of interest were participant age, gender (female), marital/partner status, ethnic background (white versus other), and years of education.
Disease-related factors
Disease factors included in the analyses were disease duration, the presence of serious comorbidities in each year of the panel, counts of swollen joints, and global pain ratings. Comorbidities, counts of swollen joints, and global pain ratings, were treated as time-varying covariates in analyses. Comorbid illnesses included serious medical conditions such as high blood pressure, heart disease, stroke and neurological conditions, diabetes, lung disease (e.g. asthma, emphysema, etc.), cancer, and kidney disease, and were coded as 1 = any comorbid condition in each year, 0 = no comorbid conditions. Global ratings of pain were coded as 0–100, where 0 = ‘no pain’, and 100 = ‘very severe pain’ (Fries et al., 1980). Counts of swollen joints ranged from 0–10; and included knees, ankles, shoulders, elbows, and hands/fingers. In the clinical research literature, self-reported joint counts have generally been correlated with joint counts derived from physician assessments (Calvo et al., 1999; Mason et al., 1992).
Medication factors
Medication use was assessed in each wave of the panel study. In these analyses, we included the use of prednisone and disease modifying arthritis drugs (DMARDs) in each wave as covariates (1 = use, 0 = no use). Since the most commonly used DMARDs changed over the study period, we used an indicator variable for any DMARD use rather than the specific DMARDs taken.
Basic physical impairment due to RA
This was assessed using the Health Assessment Questionnaire (HAQ). The HAQ was specifically developed to measure basic physical function, such as mobility, toileting, bathing, and other forms of basic self-care, among persons with arthritis (Fries et al., 1980). Scores range from 0–3, with higher scores reflecting worse basic physical functioning.
Health and disability
Self-reported health was assessed using a single item, and was dichotomized to reflect the following: 1 = poor/fair health, 0 = good/excellent health. The test–retest reliability of this single item measure was .93 in the present study. A single item assessed functional disability in major life activities as the extent to which participants reported that ‘major activities’ were limited by medical conditions, including RA. The response categories were recoded and dichotomized to reflect the following: 1 = cannot perform usual major activities at all; and 0 = no limits/some limits in major life activities. The test–retest reliability for this single item was .90 in the present study.
Symptoms of depression
These were measured by the 15-item Geriatric Depression Scale-Short Form (GDS) (Yesavage, 1988; Yesavage et al., 1982). The GDS minimizes somatic aspects of depression, such as fatigue, and difficulties with sleeping, which are also often manifested as part of RA (Pincus & Callahan, 1993). The GDS has strong psychometric properties, both in the published literature and in the RA Panel, and has demonstrated high correspondence with psychiatric interviews (Dunn and Sacco, 1989). GDS scores range from 0–15. A score of 7 or above is considered to be indicative of major depression (Yesavage et al., 1982). In the present study, 12.2 percent of participants scored in this range on the GDS at baseline (n = 153), defined as either 1988 or the enrollment year, if later, in the panel. In the present study, the average Cronbach's reliability for the GDS over the 18-year period was .85 (range = .83–.87).
In order to capture distinct patterns of depression over time, depression scores were aggregated within individuals over repeated measurements to create the following depression variables, used in cluster analyses that are described below: (1) baseline depression score; (2) average depression score; (3) standard deviation of depression score; (4) maximum depression score; (5) minimum depression score; (6) percentage of assessments indicating depression (scoring 7 or higher on the GDS); (7) number of assessments indicating depression.
Analytic strategy
Our analytic strategy consisted of two phases. First, cluster analysis was used to identify distinct patterns of depressive symptoms among 1115 participants in the UCSF RA Panel using 18 years of panel data. Second, general estimating equations (GEE) analyses were conducted to assess the impact of these distinct patterns of depression over time on health/functional outcomes, controlling for demographic, and time varying disease-related and medication factors, and basic physical function (HAQ scores).
Cluster analyses
Characteristics of depression measured repeatedly were aggregated within individuals and used to identify distinct patterns of depression. These included the baseline and average scores of participants on the GDS (Yesavage, 1988), maximum and minimum scores, the within-person standard deviations of scores, and the percentage and number of assessments for which participants had a score of 7 or higher on the GDS, a score that is consistent with a diagnosis of depression in validation studies (Yesavage et al., 1982).
A K-means cluster analysis was used to identify distinct patterns of depression and to categorize participants on the basis of depression characteristics over time. Aggregate depression data were used for 1115 participants in the UCSF RA Panel study having at least two assessments, using SPSS version 17. Descriptive statistics were computed for each cluster. One-way ANOVAs, and post-hoc comparisons were used to assess cluster differences. In addition, analyses of variables that were exogenous to the cluster solution were conducted, in order to assess the concurrent validity of the three-cluster solution. A Bonferroni adjustment was applied to assess the statistical significance of these tests, and to minimize Type I error.
All depression variables were standardized prior to entering them into the cluster analysis, as recommended by Aldenderfer and Blashfield (1984), given that unequal variances create an unequal weighting of variables in determining the cluster solution. A three-cluster solution was selected on the basis of its theoretical and clinical relevance, interpretability, cluster size, and an assessment of cluster differences with respect to concurrently measured variables that were external to the cluster solution (Aldenderfer and Blashfield, 1984; Rapkin and Dumont, 2000; Rapkin and Luke, 1993). A split-half replication of the three-cluster solution yielded essentially the same pattern of results for these data, thus adding confirmatory evidence in support of the cluster solution.
One-way analyses of variance and chi-square tests were used to assess cluster differences on depression variables, as well as with respect to study variables that were external to the cluster solution.
GEE analyses
We used general estimating equations (GEE) to assess long-term effects of patterns of depression on health and function outcomes; controlling for the effects of demographic, and time-varying disease-related factors, medications, and physical impairment.
Dependent variables and covarying factors were measured in each year of the panel. Depression groups, based on all years of observation, were treated as categorical levels of a fixed variable (with nondepressed as reference group) in these GEE analyses, in order to assess their independent contribution to outcomes, after demographic, disease-related, and medication factors were accounted for.
Results
Cluster analyses
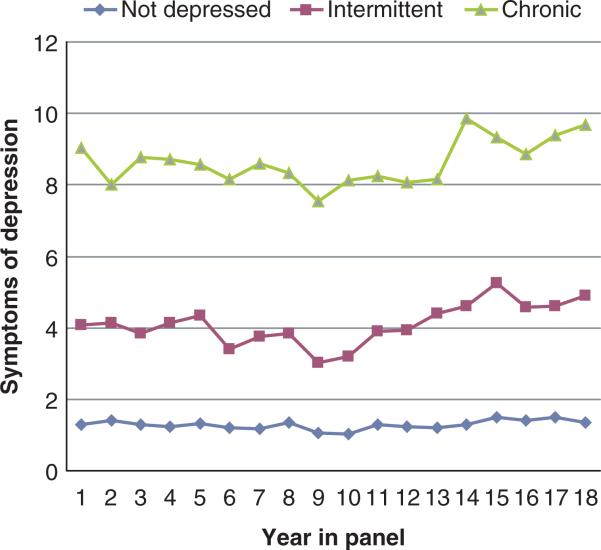
The results of cluster analyses are presented in Table 1. As can be seen in Table 1, three distinctive long-term patterns of depression were identified. Individuals in Cluster 1 (‘little/no depression’, n = 734) represented 65.8 percent of the sample. Cluster 2 participants ‘intermittent’ (n = 281), comprised 25.2 percent of the sample, and had intermittent patterns of depression (7+ symptoms on the GDS). Cluster 3 (‘chronic’, n = 100) constituted 9.0 percent of the sample, and scored 7 or higher on the GDS for most assessments. Figure 1 plots the mean levels of symptoms of depression by depression group over the duration of the panel study used in these analyses (1988–2005).
Table 1.
Patterns of depression and baseline characteristics among 1115 participants in the UCSF RA Panel from 1988–2005
| Depression factors | Low/none (n=734) | Intermittent (n=281) | Chronic (n=100) | F (2, 1112)/chi-square (2) |
|---|---|---|---|---|
| Mean baseline GDS (SD) | 1.35 (1.42) | 4.13 (2.86) | 8.96 (2.88) | 705.64*** |
| Within-person average GDS (SD) | 1.40 (1.05) | 4.36 (1.38) | 8.82 (1.84) | 1897.63*** |
| Within-person variability of GDS | 0.94 (.57) | 2.82 (.88) | 2.34 (1.12) | 755.53*** |
| Maximum GDS score | 2.97 (1.82) | 8.87 (1.99) | 11.74 (2.07) | 1647.90*** |
| Minimum GDS score | 0.46 (.84) | 1.40 (1.31) | 5.46 (2.89) | 676.73*** |
| % of assessments GDS (7+) | 0.17 (1.09) | 23.9 (16.0) | 78.9 (19.2) | 2991.28*** |
| # of assessments GDS (7+) | 0.02 (.15) | 1.77 (1.36) | 5.68 (3.40) | 1008.34*** |
| Demographic factors | ||||
| % Female (N) | 77.7 (570) | 83.3 (234) | 83.0 (83) | NS |
| % White (N) | 83.8 (615) | 79.7 (224) | 64.0 (64) | 22.77*** |
| % Married/partner | 68.6 (486) | 55.3 (152) | 51.0 (49) | NS |
| Age | 54.9 (13.8) | 56.2 (12.9) | 56.2 (12.6) | NS |
| Years of education | 13.72 (2.70) | 13.04 (3.14) | 11.84 (3.83) | 21.64*** |
| Disease-related factors | ||||
| Disease duration (years) | 9.7 (9.6) | 12.6 (11.2) | 12.6 (10.9) | 10.3*** |
| Comorbidities: % (n) | 36.9% (271) | 44.5% (125) | 58.0% (58) | 5.18* |
| Global pain rating: Mean (SD) | 28.59 (19.53) | 44.13 (20.55) | 56.83 (18.59) | 130.27*** |
| Swollen joint count: Mean (SD) | 2.58 (2.08) | 3.80 (2.30) | 4.92 (2.70) | 69.20*** |
| Medications | ||||
| DMARDS: % (n) | 81.3 (597) | 80.1 (225) | 76.0 (76) | NS |
| Prednisone: % (n) | 49.7 (365) | 60.5 (170) | 76.0 (76) | 29.4*** |
| Basic physical function (HAQ): Mean (SD) | 0.88 (.63) | 1.35 (.72) | 1.87 (.60) | 130.7*** |
| Health/function | ||||
| Disability in major life activities | 60.5 (444) | 80.1 (225) | 94.0 (94) | 69.3*** |
| Self-rated poor/fair health | 33.1 (243) | 59.1 (166) | 85.0 (85) | 129.26*** |
p < .05
**p < .01
p < .001
Figure 1.
Patterns of depression in a panel study of rheumatoid arthritis.
Participant characteristics
Overall, participants were predominantly female (79.6%), white (81.0%), and married/with a partner (63.7%). The mean age was 55.4 (median = 57.0, SD = 13.4), and average years of education was 13.4 years (median = 13.0, SD = 3.0). The average HAQ score was 1.09 (median = 1.0, SD = .72), and 5.7 percent (n = 63) of participants at baseline reported being unable to perform their usual major life activities, with another 62.8 percent (n = 700) reporting activity limits in other areas. Nine percent (n = 101) of participants reported being in poor/fair health.
There were significant differences among clusters in all categories of independent variables. Intermittent and chronic patterns of depression were far more prevalent among nonwhite participants (chi-square[2] = 22.8, p < .001), and participants with intermittent and chronic patterns also reported less education than was the case for the nondepressed group (F[2, 1112] = 21.6, p < .001). Intermittent and chronic patterns of depression were also significantly associated with longer disease duration (F[2, 1112] = 10.3, p < .001), more serious comorbidities (chi-square[2] = 5.2, p < .05) at baseline, higher ratings of global pain (F[2, 1112] = 130.3, p < .001), higher average swollen joint counts at baseline (F[2, 1112] = 130.7, p < .001), and higher average baseline HAQ scores, indicating greater limitations in basic physical function (F[2, 1112] = 21.6, p < .001). There were no group differences in the use of DMARDs at baseline, but the intermittent and chronic groups were more likely than the nondepressed group to report the use of prednisone (chi-square[2] = 29.4, p < .001).
There were significant and substantial group differences in the expected direction with respect to health and function variables at baseline: disability in major life activities (chi-square[2] = 69.3, p < .001), and self-rated poor/fair health (chi-square[2] = 129.3, p < .001).
Sample attrition
Analyses of baseline measures comparing participants who dropped out of the study (excluding participants who died), with those who remained revealed no statistically significant bivariate differences with respect to comorbidities, ethnicity, marital/partner status, gender, disease duration, or swollen joint counts. However, participants who dropped out tended to be significantly older, have less education, reported greater global pain, had worse basic physical function (HAQ scores), and greater symptoms of depression than participants who remained in the study.
Mortality
Patterns of depression, defined through cluster analysis of depression characteristics, were also significantly associated with mortality in bivariate analyses (chi-square[2] = 20.96, p < .01). Participants with intermittent and chronic patterns of depression were more likely than nondepressed participants to have died over the study period (26.0 percent and 34.0 percent, respectively, versus 17.2 percent). However, in multivariate analyses not presented here, patterns of depression were not significantly related to mortality, nor did they predict survival in Cox regression analyses, when adjusted for demographic, disease-related, medications, and basic function covariates.
GEE analyses predicting health and function over time
GEE analyses were used to assess the impact of patterns of depression on health and function controlling for demographic, time-varying disease-related, medication, and basic physical limitation factors. In these analyses, the reference group was the nondepressed group. The results of these analyses are summarized in Table 2. Overall, it can be seen that intermittent and chronic patterns of depression are associated with worse function and self-rated health. The effects are monotonic, with the outcomes increasingly poor for the intermittent and chronically depressed groups relative to the nondepressed group. Both of these outcomes are discussed, in turn, below.
Table 2.
GEE analyses of patterns of depression among 1115 participants in the UCSF RA Panel over an 18-year period: impact on health and function
| Disability in major life activities | Self-rated poor/fair health | |
|---|---|---|
| Demographic factors | ||
| • Gender (female) | 0.64 (0.52, 0.79)*** | 0.69 (0.54, 0.87)** |
| • Age | 1.00 (1.00, 1.01)+ | 1.00 (1.00, 1.01) |
| • Race (white vs other) | 1.24 (1.02, 1.52)* | 0.80 (0.64, 0.99)* |
| • Years of education+ | 0.95 (0.92, 0.98)** | 0.98 (0.95, 1.01) |
| • Married/partner | 0.95 (0.82, 1.09) | 1.18 (1.01, 1.38)* |
| Year in Panel (1–18) | 0.98 (0.97, 1.00)** | 1.02 (1.00, 1.03) |
| Disease factors | ||
| • Years with RA | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.01) |
| • Comorbidities (1/0) | 1.08 (0.96, 1.21) | 1.40 (1.28, 1.55)*** |
| • Global pain ratings (0–100) | 1.01 (1.01, 1.02)*** | 1.01 (1.01, 1.02)*** |
| • Swollen joint count (0–10) | 1.07 (1.04, 1.10)*** | 1.03 (1.01, 1.06)** |
| Medications | ||
| • DMARDS (1/0) | 0.96 (0.84, 1.11) | 1.10 (0.97, 1.24) |
| • Prednisone (1/0) | 1.16 (1.01, 1.33)* | 1.41 ((1.25, 1.58)** |
| Basic Physical Function (HAQ, 0–3) | 4.83 (4.21, 5.54)*** | 2.70 (2.40, 3.04)*** |
| Patterns of depressive symptoms (vs low/none) | ||
| • Intermittent | 1.63 (1.34, 1.98)*** | 2.31 (1.88, 2.84)*** |
| • Chronic | 2.96 (1.86, 4.70)*** | 4.23 (2.80, 6.40)*** |
p < .10
p < .05
p < .01
p < .001
Disability in major life activities
Patterns of depression were highly associated with increased odds of disability in life functions; both intermittent (OR = 1.63, CI = 1.34, 1.98, p < .001) and chronic patterns of (OR = 2.96, CI = 1.86, 4.70, p < .001) of depression increased risk.
Self-rated poor/fair health
As can be seen in Table 2, patterns of depression also significantly increased the likelihood of poor/fair health over time, with both intermittent (OR = 2.31, CI = 1.88, 2.84; p < .001) and chronic (OR = 4.23, CI = 2.80, 6.40; p < .001) patterns of depression substantially increasing the odds of poor outcomes.
Discussion
It seems evident that long-term patterns of depression are associated with worse function, and perceptions of poor/fair health, even after controlling for demographic, and time-varying disease-related factors, physical limitations, and medication factors. Thus, our study adds further evidence of the disease burden of symptoms of depression over time, among a representative sample of individuals with RA, as well as an estimate of the prevalence of chronic and intermittent patterns of depression over time. It was striking in our results that such a high proportion of individuals with RA are also affected by chronic and intermittent levels of depression. There is clearly an urgent need to identify individuals with high levels of depressive symptoms from year to year, even if these symptoms are ‘intermittent’ and do not meet threshold for a diagnosis of clinical depression. Addressing the symptoms of depression that individuals with RA are experiencing would contribute to an alleviation of disease burden and likely improve function in valued life activities and quality of life for affected individuals and their families.
It has been argued persuasively that individuals with chronic illness, such as RA should be routinely screened for depression and followed up with appropriate and timely interventions or referrals to mental health care providers or support resources (Panzarino, 1998; Perez-Stable et al., 1990; Pignone et al., 2002; Sheehy et al., 2006). There has been a welcome focus in the recent clinical literature on integrating evidence-based psychological treatments into RA care, including mindfulness-based stress reduction (Bohlmeijer et al., 2010), anti-depressants (Kapoor, 2010), and other psychological treatments (Van Straten et al., 2010).
In the current study, we observed higher unadjusted mortality rates for individuals with intermittent (26.0%) and chronic (34.0%) patterns of depression, compared with nonde-pressed individuals (17.2%). However, when we conducted multivariate analyses, controlling for demographic, disease-related, and functional variables, the relationship between patterns of depression and mortality, and patterns of depression and survival were not statistically significant. These findings are in contrast to those of Ang and colleagues (2005), who found that depression increased the risk of mortality among individuals with RA who were followed over an 18-year period. Our study differs with respect to sampling, methods, and covariates, which may account for the observed differences in mortality associated with depression.
In the present study there are several limitations which must be noted. First, this is an observational study that does not include clinical data on disease activity, nor clinical assessment of depression. Thus, limitations generically associated with our methods must be taken into account when considering our findings. These include biases that are inherent in self-report data, such as differences in recall, motivational biases, and the like. Second, although not statistically significant (p < .06), there were marginally significant differences in attrition, excluding mortality, across depression groups that might have biased our results. Finally, our measure of swollen joint counts did not include feet and wrists because these joint groups were not queried in all years of the study. Perhaps a more complete assessment of swollen joints would have shown more variability across depression groups.
Clearly, clinical interventions that address symptoms of depression as part of a comprehensive approach to treating individuals with RA are urgently needed. Indeed, there is accumulating evidence that integrating depression treatment into primary care and other specialty medical care is cost-effective, and linked to improvements in quality of life and function (Lin et al., 2003; Parker et al., 2003; Simon et al., 2007). It has been our goal in this study to contribute to the ongoing discussion and debate about the treatment of depression in RA, in order to improve quality of care and outcomes for individuals with RA.
Acknowledgements
This research was supported by a grant from NIAMS (R01 AR50015, Principal Investigator: Patricia P. Katz, PhD).
References
- Aldenderfer M, Blashfield R. Cluster Analysis. SAGE; Los Angeles, CA: 1984. [Google Scholar]
- Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid Depression Is an Independent Risk Factor for Mortality in Patients with Rheumatoid Arthritis. Journal of Rheumatology. 2005;32(6):1013–1019. [PubMed] [Google Scholar]
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of Patients with Mood Disorders: Follow-Up Over 34–38 Years. Journal of Affective Disorders. 2002;68(2–3):167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Angst J, Angst F, Stassen HH. Suicide Risk in Patients with Major Depressive Disorder. Journal of Clinical Psychiatry. 1999;60(Suppl. 2):57–62. discussion 75–56, 113–116. [PubMed] [Google Scholar]
- Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The Effects of Mindfulness-Based Stress Reduction Therapy on Mental Health of Adults with a Chronic Medical Disease: A Meta-Analysis. Journal of Psychosomatic Research. 2010;68(6):539–544. doi: 10.1016/j.jpsychores.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Luscombe G, Peisah C, Anstey K, Andrews G. A 25-Year Longitudinal, Comparison Study of the Outcome of Depression. Psychological Medicine. 2001;31(8):1347–1359. doi: 10.1017/s0033291701004743. [DOI] [PubMed] [Google Scholar]
- Callahan LF, Cordray DS, Wells G, Pincus T. Formal Education and Five-Year Mortality in Rheumatoid Arthritis: Mediation by Helplessness Scale Score. Arthritis Care and Research. 1996;9(6):463–472. doi: 10.1002/art.1790090608. [DOI] [PubMed] [Google Scholar]
- Calvo FA, Calvo A, Berrocal A, et al. Self-Administered Joint Counts in Rheumatoid Arthritis: Comparison with Standard Joint Counts. Journal of Rheumatology. 1999;26(3):536–539. [PubMed] [Google Scholar]
- Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in Rheumatoid Arthritis: A Systematic Review of the Literature with Meta-Analysis. Psychosomatic Medicine. 2002;64(1):52–60. doi: 10.1097/00006842-200201000-00008. [DOI] [PubMed] [Google Scholar]
- Druss BG, Rosenheck RA, Sledge WH. Health and Disability Costs of Depressive Illness in a Major U.S. Corporation. American Journal of Psychiatry. 2000;157(8):1274–1278. doi: 10.1176/appi.ajp.157.8.1274. [DOI] [PubMed] [Google Scholar]
- Dunn VK, Sacco WP. Psychometric Evaluation of the Geriatric Depression Scale and the Zung Self-Rating Depression Scale Using an Elderly Community Sample. Psychology and Aging. 1989;4(1):125–126. doi: 10.1037//0882-7974.4.1.125. [DOI] [PubMed] [Google Scholar]
- Farran CJ, Horton-Deutsch SL, Loukissa D, Johnson L. Psychiatric Home Care of Elderly Persons with Depression: Unmet Caregiver Needs. Home Health Care Services Quarterly. 1998;16(4):57–73. doi: 10.1300/J027v16n04_04. [DOI] [PubMed] [Google Scholar]
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of Patient Outcome in Arthritis. Arthritis and Rheumatism. 1980;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- Graves H, Scott DL, Lempp H, Weinman J. Illness Beliefs Predict Disability in Rheumatoid Arthritis. Journal of Psychosomatic Research. 2009;67(5):417–423. doi: 10.1016/j.jpsychores.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Hawley DJ, Wolfe F. Depression Is Not More Common in Rheumatoid Arthritis: A 10-Year Longitudinal Study of 6,153 Patients with Rheumatic Disease. Journal of Rheumatology. 1993;20(12):2025–2031. [PubMed] [Google Scholar]
- Hays RD, Wells KB, Sherbourne CD, Rogers W, Spritzer K. Functioning and Well-Being Outcomes of Patients with Depression Compared with Chronic General Medical Illnesses. Archives of General Psychiatry. 1995;52(1):11–19. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- Kapoor S. Antidepressants as an Often Overlooked Therapeutic Option for the Treatment and Management of Arthritis Pain: Comment on the Article by Wolfe and Michaud. Arthritis Care and Research. 2010;62(6):901–902. doi: 10.1002/acr.20120. [DOI] [PubMed] [Google Scholar]
- Katon W, Ciechanowski P. Impact of Major Depression on Chronic Medical Illness. Journal of Psychosomatic Research. 2002;53(4):859–863. doi: 10.1016/s0022-3999(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Katon W, Sullivan MD. Depression and Chronic Medical Illness. Journal of Clinical Psychiatry. 1990;51(Suppl.):3–11. discussion 12–14. [PubMed] [Google Scholar]
- Katona C, Bell G. Suicide Risk in Depression. British Journal of Hospital Medicine. 1988;39(2):170. [PubMed] [Google Scholar]
- Katz PP. Function, Disability, and Psychological Well-Being. Advances in Psychosomatic Medicine. 2004;25:41–62. doi: 10.1159/000079057. [DOI] [PubMed] [Google Scholar]
- Katz PP, Morris A. Time Use Patterns among Women with Rheumatoid Arthritis: Association with Functional Limitations and Psychological Status. Rheumatology. 2007;46(3):490–495. doi: 10.1093/rheumatology/kel299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PP, Yelin EH. Prevalence and Correlates of Depressive Symptoms among Persons with Rheumatoid Arthritis. Journal of Rheumatology. 1993;20(5):790–796. [PubMed] [Google Scholar]
- Katz PP, Yelin EH. The Development of Depressive Symptoms among Women with Rheumatoid Arthritis: The Role of Function. Arthritis and Rheumatism. 1995;38(1):49–56. doi: 10.1002/art.1780380108. [DOI] [PubMed] [Google Scholar]
- Katz PP, Yelin EH. Activity Loss and the Onset of Depressive Symptoms: Do Some Activities Matter More Than Others? Arthritis and Rheumatism. 2001;44(5):1194–1202. doi: 10.1002/1529-0131(200105)44:5<1194::AID-ANR203>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Barber C, Birnbaum HG, et al. Depression in the Workplace: Effects on Short-Term Disability. Health Affairs (Project Hope) 1999;18(5):163–171. doi: 10.1377/hlthaff.18.5.163. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, et al. The Epidemiology of Major Depressive Disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Lin EH, Katon W, Von Korff M, et al. Effect of Improving Depression Care on Pain and Functional Outcomes among Older Adults with Arthritis: A Randomized Controlled Trial. JAMA. 2003;290(18):2428–2429. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- Mason JH, Meenan RF, Anderson JJ. Do Self-Reported Arthritis Symptom (RADAR) and Health Status (AIMS2) Data Provide Duplicative or Complementary Information? Arthritis Care and Research. 1992;5(3):163–172. doi: 10.1002/art.1790050309. [DOI] [PubMed] [Google Scholar]
- Nicassio PM. Arthritis and Psychiatric Disorders: Disentangling the Relationship. Journal of Psychosomatic Research. 2010;68(2):183–185. doi: 10.1016/j.jpsychores.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzarino PJ., Jr The Costs of Depression: Direct and Indirect; Treatment versus Nontreatment. Journal of Clinical Psychiatry. 1998;59(Suppl. 20):11–14. [PubMed] [Google Scholar]
- Parker JC, Smarr KL, Slaughter JR, et al. Management of Depression in Rheumatoid Arthritis: A Combined Pharmacologic and Cognitive-Behavioral Approach. Arthritis and Rheumatism. 2003;49(6):766–777. doi: 10.1002/art.11459. [DOI] [PubMed] [Google Scholar]
- Perez-Stable EJ, Miranda J, Munoz RF, Ying YW. Depression in Medical Outpatients: Underrecognition and Misdiagnosis. Archives of Internal Medicine. 1990;150(5):1083–1088. doi: 10.1001/archinte.1990.00390170113024. [DOI] [PubMed] [Google Scholar]
- Pignone MP, Gaynes BN, Rushton JL, et al. Screening for Depression in Adults: A Summary of the Evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2002;136(10):765–776. doi: 10.7326/0003-4819-136-10-200205210-00013. [DOI] [PubMed] [Google Scholar]
- Pincus HA, Pettit AR. The Societal Costs of Chronic Major Depression. Journal of Clinical Psychiatry. 2001;62(Suppl. 6):5–9. [PubMed] [Google Scholar]
- Pincus T, Callahan LF. Depression Scales in Rheumatoid Arthritis: Criterion Contamination in Interpretation of Patient Responses. Patient Education and Counseling. 1993;20(2–3):133–143. doi: 10.1016/0738-3991(93)90127-i. [DOI] [PubMed] [Google Scholar]
- Rapkin BD, Dumont KA. Methods for Identifying and Assessing Groups in Health Behavioral Research. Addiction. 2000;95(Suppl. 3):S395–417. doi: 10.1080/09652140020004304. [DOI] [PubMed] [Google Scholar]
- Rapkin BD, Luke D. Cluster Analysis in Community Research: Epistomology and Practice. American Journal of Community Psychology. 1993;21(2):247–277. [Google Scholar]
- Rosenberg SJ, Peterson RA, Hayes JR, Hatcher J, Headen S. Depression in Medical In-Patients. British Journal of Medical Psychology. 1988;61(Pt 3):245–254. doi: 10.1111/j.2044-8341.1988.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Sheehy C, Murphy E, Barry M. Depression in Rheumatoid Arthritis: Underscoring the Problem. Rheumatology. 2006;45(11):1325–1327. doi: 10.1093/rheumatology/kel231. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Lin E. Clinical and Functional Outcomes of Depression Treatment in Patients with and without Chronic Medical Illness. Psychological Medicine. 2005;35(2):271–279. doi: 10.1017/s0033291704003071. [DOI] [PubMed] [Google Scholar]
- Simon GE, Barber C, Birnbaum HG, et al. Depression and Work Productivity: The Comparative Costs of Treatment versus Nontreatment. Journal of Occupational and Environmental Medicine. 2001;43(1):2–9. doi: 10.1097/00043764-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Simon GE, Katon WJ, Lin EH, et al. Cost-Effectiveness of Systematic Depression Treatment among People with Diabetes Mellitus. Archives of General Psychiatry. 2007;64(1):65–72. doi: 10.1001/archpsyc.64.1.65. [DOI] [PubMed] [Google Scholar]
- Simon GE, Revicki D, Heiligenstein J, et al. Recovery from Depression, Work Productivity, and Health Care Costs among Primary Care Patients. General Hospital Psychiatry. 2000;22(3):153–162. doi: 10.1016/s0163-8343(00)00072-4. [DOI] [PubMed] [Google Scholar]
- Stewart W, Ricci JA, Chee E. Cost of Lost Productive Work Time among US Workers with Depression. JAMA. 2003;289(23):3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Turk DC, Okifuji A, Scharff L. Chronic Pain and Depression: Role of Perceived Impact and Perceived Control in Different Age Cohorts. Pain. 1995;61(1):93–101. doi: 10.1016/0304-3959(94)00167-D. [DOI] [PubMed] [Google Scholar]
- Van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological Treatment of Depressive Symptoms in Patients with Medical Disorders: A Meta-Analysis. Journal of Psychosomatic Research. 2010;69(1):23–32. doi: 10.1016/j.jpsychores.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Van 't Land H, Verdurmen J, Ten Have M, Van Dorsselaer S, Beekman A, De Graaf R. The Association between Arthritis and Psychiatric Disorders: Results from a Longitudinal Population-Based Study. Journal of Psychosomatic Research. 2010;68(2):187–193. doi: 10.1016/j.jpsychores.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Vilhjalmsson R. Direct and Indirect Effects of Chronic Physical Conditions on Depression: A Preliminary Investigation. Social Science & Medicine. 1998;47(5):603–611. doi: 10.1016/s0277-9536(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Hawley DJ. The Longterm Outcomes of Rheumatoid Arthritis: Work Disability: A Prospective 18 Year Study of 823 Patients. Journal of Rheumatology. 1998;25(11):2108–2117. [PubMed] [Google Scholar]
- Wolfe F, Hawley DJ, Cathey MA. Clinical and Health Status Measures over Time: Prognosis and Outcome Assessment in Rheumatoid Arthritis. Journal of Rheumatology. 1991;18(9):1290–1297. [PubMed] [Google Scholar]
- Yesavage JA. Geriatric Depression Scale. Psychopharmacology Bulletin. 1988;24(4):709–711. [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]