Abstract
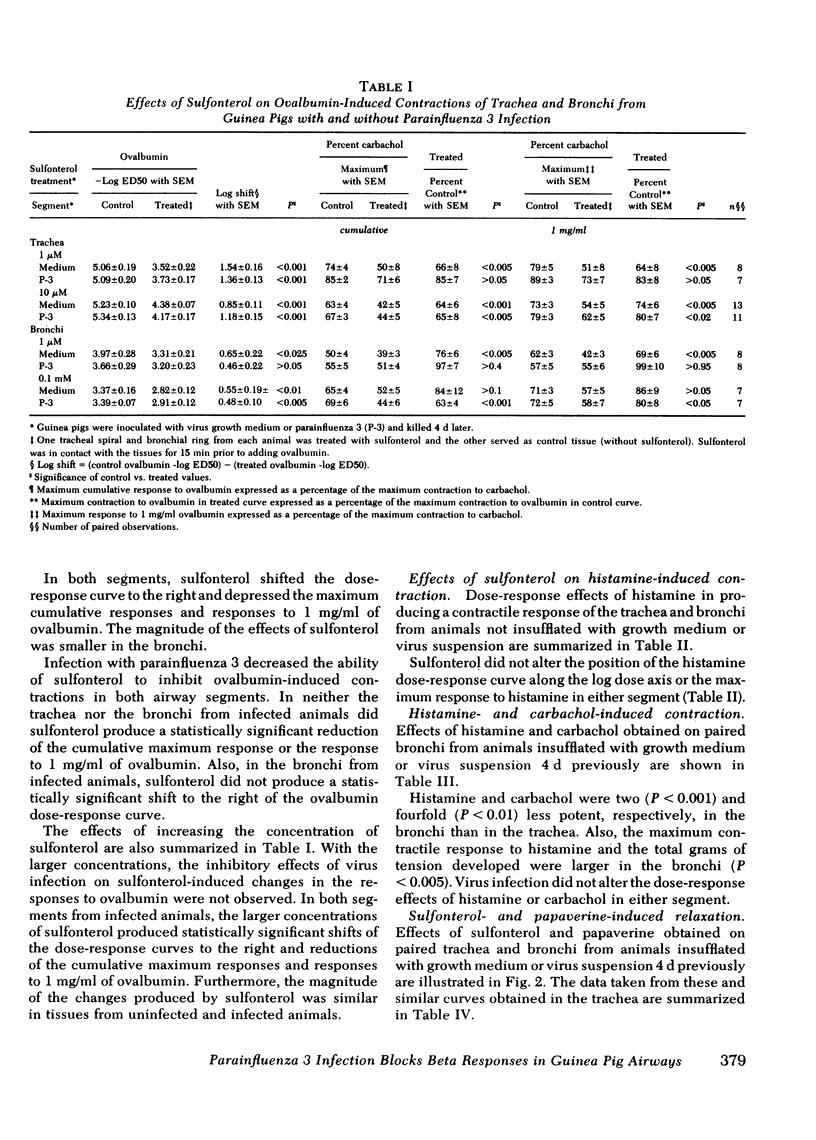
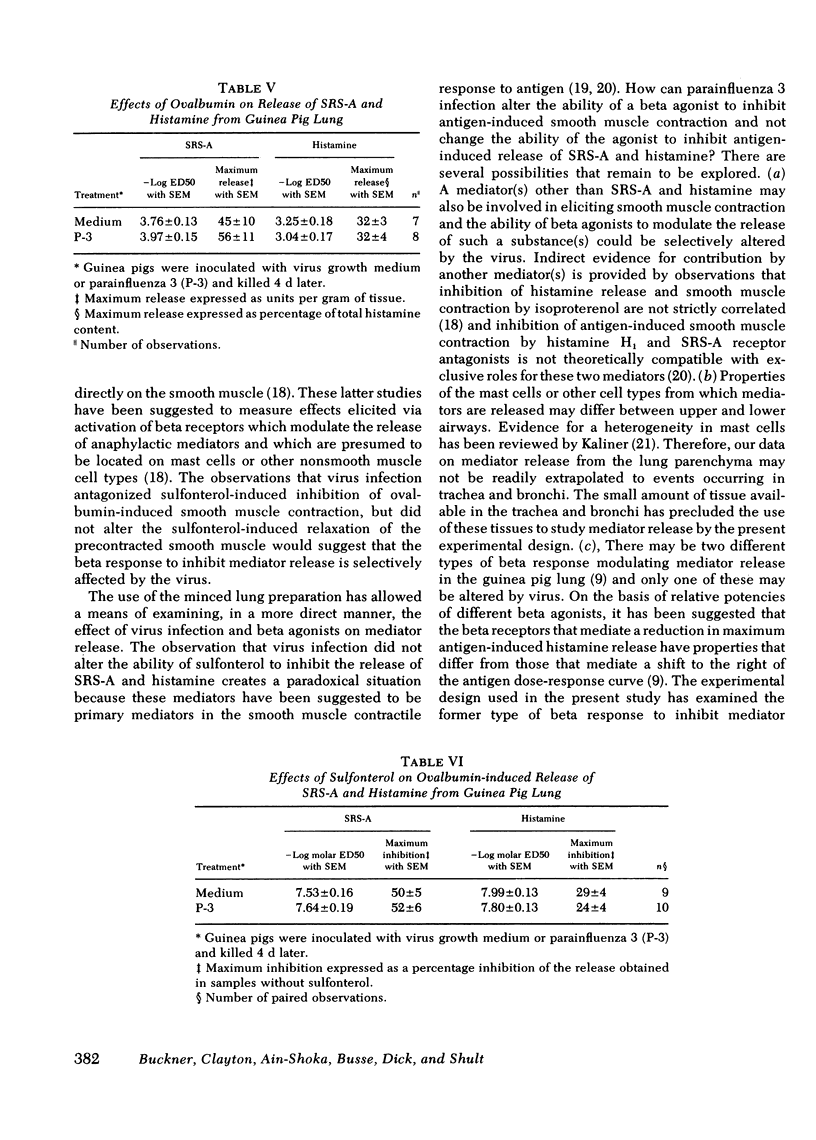
Guinea pigs, actively sensitized to ovalbumin, were inoculated by nasal insufflation with parainfluenza 3 or virus growth medium 4 d before performing in vitro pharmacological studies on tracheal and bronchial smooth muscle. In each airway segment, cumulative dose-response effects of ovalbumin were obtained in the absence and presence of a maximally effective concentration of a beta adrenergic receptor agonist, sulfonterol. Sulfonterol shifted the dose-response curve to the right and reduced the maximum smooth muscle contractile response to ovalbumin. Virus infection did not alter the dose-response effects of ovalbumin. However, the magnitude of the inhibitory effects of sulfonterol was smaller in segments taken from animals inoculated with virus. Blockade by virus infection of the inhibitory effect of sulfonterol was reversed when the concentrations of beta agonist were increased. Sulfonterol did not alter the dose-response effects of histamine at any of the concentrations that markedly antagonized the effects of ovalbumin. Virus infection did not alter the sensitivities to sulfonterol or papaverine in producing relaxation in either airway segment. The magnitude of relaxation produced by papaverine was significantly larger in bronchial rings taken from animals infected with virus for 4 d, but there was no alteration by virus of the dose-response effects of histamine or carbachol. In experiments measuring antigen-induced release of slow reacting substance of anaphylaxis and histamine from minced lung, virus infection did not alter the sensitivity or the maximum effects of ovalbumin. Also, the ability of sulfonterol to inhibit the release of slow reacting substance of anaphylaxis and histamine was not affected by virus infection.
These results demonstrate that infection of guinea pigs with respiratory virus results in a selective blockade of the beta adrenergic-mediated inhibition of antigen-induced contraction of airway smooth muscle. The guinea pig may serve as a useful model in physiological studies of virus-induced asthma.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. K., 3rd, Lichtenstein L. In vitro studies of antigen-induced bronchospasm: effect of antihistamine and SRS-A antagonist on response of sensitized guinea pig and human airways to antigen. J Immunol. 1979 Feb;122(2):555–562. [PubMed] [Google Scholar]
- Augstein J., Farmer J. B., Lee T. B., Sheard P., Tattersall M. L. Selective inhibitor of slow reacting substance of anaphylaxis. Nat New Biol. 1973 Oct 17;245(146):215–217. doi: 10.1038/newbio245215a0. [DOI] [PubMed] [Google Scholar]
- Buckner C. K., Hand J. M., Wong S. K. Inhibition by isoproterenol of ovalbumin-induced contraction of tracheal strips and release of histamine from lung isolated from the actively sensitized guinea-pig. Int J Immunopharmacol. 1979;1(3):173–182. doi: 10.1016/0192-0561(79)90039-0. [DOI] [PubMed] [Google Scholar]
- Buckner C. K., Saini R. K. On the use of functional antagonism to estimate dissociation constants for beta adrenergic receptor agonists in isolated guinea-pig trachea. J Pharmacol Exp Ther. 1975 Sep;194(3):565–574. [PubMed] [Google Scholar]
- Busse W. W., Cooper W., Warshauer D. M., Dick E. C., Wallow I. H., Albrecht R. Impairment of isoproterenol, H2 histamine, and prostaglandin E1 response of human granulocytes after incubation in vitro with live influenza vaccines. Am Rev Respir Dis. 1979 Apr;119(4):561–569. doi: 10.1164/arrd.1979.119.4.561. [DOI] [PubMed] [Google Scholar]
- Busse W. W. Decreased granulocyte response to isoproterenol in asthma during upper respiratory infections. Am Rev Respir Dis. 1977 May;115(5):783–791. doi: 10.1164/arrd.1977.115.5.783. [DOI] [PubMed] [Google Scholar]
- CHAKRAVARTY N. A method for the assay of 'slow reacting substance'. Acta Physiol Scand. 1959 Aug 31;46:298–313. doi: 10.1111/j.1748-1716.1959.tb01760.x. [DOI] [PubMed] [Google Scholar]
- CONSTANTINE J. W. THE SPIRALLY CUT TRACHEAL STRIP PREPARATION. J Pharm Pharmacol. 1965 Jun;17:384–385. doi: 10.1111/j.2042-7158.1965.tb07688.x. [DOI] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Jacobs L., Gold W. M., Nadel J. A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976 Feb;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Gold W. M., Meyers G. L., Dain D. S., Miller R. L., Bourne H. R. Changes in airway mast cells and histamine caused by antigen aerosol in allergic dogs. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):271–275. doi: 10.1152/jappl.1977.43.2.271. [DOI] [PubMed] [Google Scholar]
- Guerzon G. M., Paré P. D., Michoud M. C., Hogg J. C. The number and distribution of mast cells in monkey lungs. Am Rev Respir Dis. 1979 Jan;119(1):59–66. doi: 10.1164/arrd.1979.119.1.59. [DOI] [PubMed] [Google Scholar]
- Hand J. M., Buckner C. K. Effects of selected antagonists on ovalbumin-induced contraction of tracheal strips isolated from the actively sensitized guinea-pig. Int J Immunopharmacol. 1979;1(3):189–195. doi: 10.1016/0192-0561(79)90041-9. [DOI] [PubMed] [Google Scholar]
- Hooker C. S., Calkins P. J., Fleisch J. H. On the measurement of vascular and respiratory smooth muscle responses in vitro. Blood Vessels. 1977;14(1):1–11. doi: 10.1159/000158110. [DOI] [PubMed] [Google Scholar]
- Ida S., Hooks J. J., Siraganian R. P., Notkins A. L. Enhancement of IgE-mediated histamine release from human basophils by viruses: role of interferon. J Exp Med. 1977 Apr 1;145(4):892–906. doi: 10.1084/jem.145.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C., Schwartz M. S., Colella D. F., Wardell J. R., Jr Adrenergic agents. 3. Synthesis and adrenergic activity of some catecholamine analogs bearing a substituted sulfonyl or sulfonylalkyl group in the meta position. J Med Chem. 1975 Jul;18(7):674–683. doi: 10.1021/jm00241a006. [DOI] [PubMed] [Google Scholar]
- Kaliner M. A. Is a mast cell a mast cell a mast cell? J Allergy Clin Immunol. 1980 Jul;66(1):1–4. doi: 10.1016/0091-6749(80)90131-1. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Ellis E. F., Hoffman L. S., Lybass T. G., Eller J. J., Fulginiti V. A. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. J Pediatr. 1973 Apr;82(4):578–590. doi: 10.1016/S0022-3476(73)80582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor T. E., Dick E. C., DeMeo A. N., Ouellette J. J., Cohen M., Reed C. E. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974 Jan 21;227(3):292–298. [PubMed] [Google Scholar]
- Ouellette J. J., Reed C. E. Increased response of asthmatic subjects to methacholine after influenza vaccine. J Allergy. 1965 Nov-Dec;36(6):558–563. doi: 10.1016/0021-8707(65)90193-0. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Seale J. P. Non-immunological release of slow-reacting substance from guinea-pig lungs. Br J Pharmacol. 1979 Sep;67(1):67–72. [PMC free article] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Wasserman S. I., Goetzl E. J., Austen K. F. Inactivation of slow reacting substance of anaphylaxis by human eosinophil arylsulfatase. J Immunol. 1975 Feb;114(2 Pt 1):645–649. [PubMed] [Google Scholar]
- Wong S. K., Buckner C. K. Studies on the beta adrenergic receptors mediating inhibition of antigen-induced histamine release from the lung and heart isolated from the actively sensitized guinea pig. J Pharmacol Exp Ther. 1980 Jul;214(1):152–160. [PubMed] [Google Scholar]


