Abstract
Background. The segmentation of the common carotid artery (CCA) wall is imperative for the determination of the intima-media thickness (IMT) on B-mode ultrasound (US) images. The IMT is considered an important indicator in the evaluation of the risk for the development of atherosclerosis. In this paper, authors have discussed the relevance of measurements in clinical practices and the challenges that one has to face while approaching the segmentation of carotid artery on ultrasound images. The paper presents an overall review of commonly used methods for the CCA segmentation and IMT measurement along with the different performance metrics that have been proposed and used for performance validation. Summary and future directions are given in the conclusion.
1. Introduction
Cardiovascular disease (CVD) is one of the leading causes of deaths in the metropolitan cities. A recent survey by the World Health Organization revealed that up to 17.3 million people died from CVDs in 2008. Scientifically presaged the upcoming 2030, almost 23.3 million human deaths are resulting from CVDs [1]. CVD ailments are relative to atherosclerosis (arterial disease). Atherosclerosis is responsible for the thickening of the artery walls, and the IMT is used as a validated measure for the evaluation of atherosclerosis. Prominently, the increase in IMT jeopardizes the brain infarction or cardiac attack [2, 3].
Usually, the B-mode ultrasound scan of CCA in its common tract is used for the evaluation of the artery status and for the measurement of the IMT. Ultrasound methodology has manifest benefits of being real-time, noninvasive, low-cost, reliable, and absolutely safe for the patients. The essential drawbacks of this methodology are the B-mode image having low signal-to-noise ratio and ultrasounds are operator dependent [4]. Conventionally, the IMT is manually measured by the trained operator from the US scan images. This methodology is highly user dependent, time consuming, tedious, and infeasible in presence of large image databases [5, 6]. During the past 20 years, several computerized techniques have been developed for segmentation of CCA. These methods can be broadly classified into two categories: first category includes techniques that are completely automated, whereas second category includes those that require user interaction (semiautomated). This review will focus on the techniques that have been developed to perform CCA wall segmentation and IMT measurement in B-mode ultrasound images in both automated and semiautomated manners.
2. Clinical Significance of Vessel Wall Segmentation
2.1. Common Carotid Artery Intima-Media Thickness
The CCA longitudinal section is shown in Figure 1. It is characterized by a longitudinal tract (common carotid) that, after an enlargement (carotid sinus) containing a flow divider, bifurcates into two arteries, one internal and one external, on the basis of their position in relation to neck skin [7].
Figure 1.
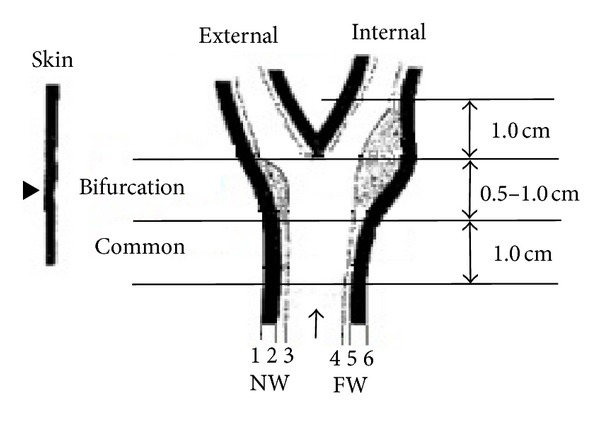
The carotid artery view with the interfaces: (1) periadventitia-adventitia (NW), (2) adventitia-media (NW), (3) intima-lumen (NW), (4) lumen-intima (FW), (5) media-adventitia (FW), and (6) adventitia-periadventitia (FW) [7].
The bifurcation and the internal carotid artery (ICA) are more threatening to atherosclerosis, due to stronger hemodynamic stresses in the bifurcation and branching zones, but it is difficult to visualize the “double-line” pattern in these locations. So an IMT measurement in the CCA is preferred in the development of segmentation algorithms and in clinical practice [8].
Classically, IMT is defined as a double-line pattern visualized by echotomography on both walls of the CCA in a longitudinal image. It consists of two parallel anatomical boundaries referred to as the lumen-intima and media-adventitia interfaces [9]. US waves are reflected differently by blood (vessel lumen) and wall layers because of their differences in density and elasticity. US waves are not reflected by vessel lumen and tunica media, thus allow detection of the lumen-intima (LI) and media-adventitia (MA) interfaces, as depicted in Figure 2 B-mode ultrasound CCA image [10].
Figure 2.
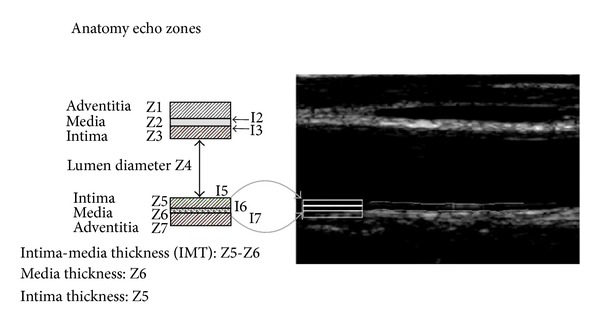
B-mode CCA image. The media layer thickness (MLT) is defined as the distance between the intima-media and the media-adventitia interface [11].
2.2. Importance of Carotid Arterial Intima-Media Thickness
The increased IMT reflects early stages of atherosclerosis and cardiovascular risk. Higher blood pressure and changes in shear stress are the potential causes of intimal thickening. Changes in shear stress and blood pressure may cause a local delay in lumen transportation of potentially atherogenic particles, which favors the accumulation of particles in the arterial wall and consequent plaque formation [12]. It is found that type 1 diabetes is a significant risk factor for increased carotid IMT in children [13]. It is confirmed that the increase in IMT is directly associated with an increase risk of myocardial infarction and stroke in older adults without a history of cardiovascular disease [14]. The assessment of IMT in prediction of the degrees of atherosclerosis and the risk of stroke and CVDs has been demonstrated by a lot of studies [15–17]. It is found that obesity especially abdominal obesity in childhood and adolescence is closely related to IMT [18]. The studies confirmed that the increase of the IMT value above 0.9-1.0 mm is indicative of a significant increase of CVD risk when taking into account the population of healthy elderly [4, 7]. In the following sections, we will discuss the difficulties confronted in the intima and media detection and review the recent advancements in CCA segmentation.
3. Difficulties of Intima and Adventitia Detection
The difficulties encountered in detecting intima and adventitia layers are as follows [19]:
presence of speckles in US image;
the structure of IM complex or the intimal layer changes due to diseases such as atherosclerotic plaques;
variation in echo characteristic on intima and adventitia on images with the variation in sonographic instrumentations.
4. Segmentation Techniques
Plethora of ultrasound-segmentation techniques have been reviewed in recent surveys by Noble and Boukerroui [29] and Molinari et al. [30]. Thereby authors are steadfast in reporting on recent techniques, used for the segmentation of the CCA on US images. These studies on the segmentation of the carotid artery boundaries include the application of dynamic programming, deformable snakes, hough transforms, and classification approaches to detect the carotid boundaries on longitudinally oriented images [27, 31–33]. For each methodology, the authors have described principles, performance, advantages, and limitations. Table 1 gives and summarizes the technique that is mentioned below.
Table 1.
Overview of recent CCA IMC segmentation techniques for ultrasound imaging.
| Name, year, and ref. no. |
Common carotid artery IMT segmentation technique | Advantages & limitations of the methods | Selection method of ROI (SA/FA) |
Performance metric | Processing time/frame T avg |
IMT error (mm) |
N |
|---|---|---|---|---|---|---|---|
| Ilea et al., 2013 [20] |
Model-based approach, video tracking procedure: spatially coherent algorithm | Advantages: Method can deal with inconsistencies in the appearance of the IMC over the cardiac cycle. Robustness with respect to data captured under different imaging conditions. | FA | MAD | 80 sec (8 sec in 1st frame & 72 sec in tracking 28 frames) |
(−0.007) ± 0.176 | 40* |
| Xu et al., 2012, [21] |
Hough transform and dual snake model | Advantages: It is less likely to be affected by noise, compensates the holes or missing boundaries, and can estimate the missing LI interface boundary. Limitations: The method would work fine for early thickening of IMC but fails for irregular boundaries in the presence of plaques and eliminates minor details. |
SA | MAD | 0.465 sec | 0.02 ± 0.03 | 50 |
| Molinari et al., 2012, [22] |
Multiresolution edge snapper | Advantages: Complete automation, robustness to noise, and real-time computation. Limitations: Robustness with respect to noise, but the LI/MA representation is less accurate. |
FA | MAD | Less than 15 sec | 0.078 ± 0.112 | 365 |
| Destrempes et al., 2011, [23] |
Nakagami distributions, Bayesian model | Advantages: Robust to a reasonable variability in the initialization, lowest tracing error for LI & MA, method is not sensitive to the degree of stenosis or calcification. Limitations: Depends on initial segmentation |
SA | MAD HD |
38 sec | — | 8988 |
| Petroudi et al., 2011, [24] | Active contours & active contours without edges | Advantages: Fully automated, fast, does not require any user interaction, and works well for noisy images. | FA | MAD | — | 0.09 ± 0.10 | 30 |
| Destrempes et al., 2009, [25] |
Nakagami distributions, stochastic optimization | Advantages: Reasonable average computation time, robust to the estimation procedures. Limitations: Method suitable for healthy arteries, extensive tuning & training, so computational cost is high, for different scanner requires retraining & retuning. |
SA | MAD HD |
24 sec | — | 7283 |
| Faita et al., 2008, [26] |
First-order absolute moment edge operator | Advantages: Suited for fast real-time implementation, operator can have immediate feedback on the quality of the images. Limitations: Depends on ROI selection. |
SA | MAD | — | 0.001 ± 0.035 | 150 |
| Liang et al., 2000, [27] |
Multiscale dynamic programming | Advantages: No initial human setting, capable of processing images of different quality, ambiguous cases user can intervene, and reduced interobserver variability. Limitations: Training required, for different scanner retraining needed, searched LI & MA interfaces may cross each other. |
FA | MAD | 0.7 min | 0.042 ± 0.02 | 50 |
| Gustavsson et al., 1994, [28] |
Dynamic programming | Advantages: Fully automated, low computational complexity; suitable for clinical purposes, human correction allowed. Limitations: Initial human setting & training required, fails for slanting IMC with weak boundary. |
SA | MAD | — | 0.03 ± 0.032 | 22 |
N: number of images/cases, SA: semiautomated, FA: fully automated, SD: standard deviation, HD: Hausdorff distance, MAD: mean absolute distance, *video sequences, T avg: average processing time/frame or image.
4.1. Dynamic Programming Techniques (DP)
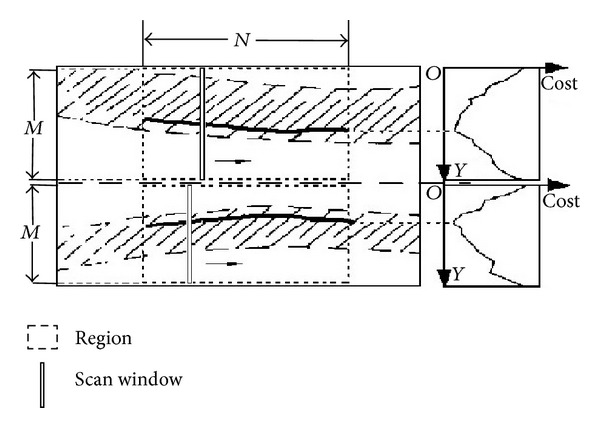
DP technique is concisely used to solve optimization problems, where desired segmentation minimizes the cost function defined by the particular application. Local measurements of echo intensity, edge strength, and boundary continuity are included as weighted terms in a cost function. All possible set of spatially consecutive points forming a polyline is being considered, and favor is given to that which minimizes the cost function [34].
The polylines are represented as a vector:
| (1) |
where i is the horizontal pixel position, P i−1 and P i are neighbor points, and N is the horizontal length of the search region. A vertical search window of size M × 1 pixels is used to scan the boundary from left to right at N horizontal positions as shown in Figure 3. At scan position i, the boundary point, P i, in (1) can be any pixel in this window. The optimized connection is searched for each point in this window and the cost accumulated. At the end of the scanning, the optimal polyline is the one that minimizes the cost function:
| (2) |
The local cost is a weighted sum of cost terms:
| (3) |
where W 1, W 2, and W 3 are weighting factors, C 1, C 2, and C 3 are the echo intensity, intensity gradient, and boundary continuity cost terms, respectively. Based on DP techniques in 1994 Gustavsson et al. [28] introduced a procedure for automatic ultrasonic measurements of the carotid artery, and lumen diameter (LD) and IMT were computed. Intermethod (auto versus manual) variability as well as inter- and intraobserver variability was studied by computing the conventional coefficient of variation (CV). A major advantage of this methodology was complete automation and low computational complexity, thus suitable for clinical purposes. This method requires interactive tools for manual tracing in order to correct the remaining detection errors. The major limitation of this technique is the need for training of the system. In 1997, Gustavsson et al. [34] have compared four algorithms: the dynamic programming, the maximum gradient, the model-based, and the matched filter algorithm and confirmed that the DP algorithm provides superior performance in terms of accuracy and robustness. In 2008 Liu et al. [35] proposed a segmentation method in which the energy definition of active contour model was used and DP was employed to search the shortest path. To reduce the effects of speckle noise, anisotropic diffusion method was adopted. It is advantageous as it requires less manual input. Holdfeldt et al. [36] proposed a method based on DP for boundary detection in ultrasound image sequences. According to the author, this method gives favorable results on both synthetic and real ultrasound data. Cheng and Jiang [37] proposed a novel dual dynamic programming (DDP) technique that detected intimal and adventitial layers of the CCA of the B-mode US images. In this, the robustness against the speckles was increased by embedding the anatomical knowledge into its structure. Therefore, the researcher reported that the DDP technique achieved a detection performance comparable to manual segmentation.
Figure 3.
Detecting interfaces I2 and I7 in an artery image.
4.2. Hough Transform (HT)
HT technique used to detect straight lines. A straight line at a distance s and orientation θ can be represented by
| (4) |
The HT of this line is just a point in the (s, θ) plane; that is all the points on this line map into a single point. This fact is utilized to detect straight lines in a given set of boundary points. If given boundary points are (x i, y i), i = 1,…, N for some selected quantized values of parameter s and θ, map each (x i, y i) into the (s, θ) space and count C(s, θ), the number of edge points that map into the location (s, θ); that is, set
| (5) |
Then the local maxima of C(s, θ) give the different straight line segments through the edge points. Generalized HT can be used to detect curves other than the straight lines [38]. Segmentation algorithm based on the HT was demonstrated by Golemati et al. [39] in 2007 to segment both longitudinal and transverse images. The HT is effective in detecting lines (longitudinal images) or circles (transverse images), but it may fail in detecting curved vessels. In 2008, Stoitsis et al. [40] proposed the HT-initialized active contour methodology. In 2010, Petroudi et al. [41] proposed a fully automated method that was proposed for the delineation of the intima-media complex (IMC). In this technique after speckle removal and HT used for boundary detection followed by image normalization, the corresponding results were used to provide the initial statistical information needed for a Markov random field (MRF). In 2011, Matsakou et al. [42] proposed a method in which an HT-based methodology was used for the definition of the initial snake followed by a gradient vector flow (GVF) snake deformation for the final contour detection. The author reported that the sensitivity, specificity, and accuracy were 0.97, 0.99 and 0.98, respectively, for both diastolic and systolic cases. Recently Xu et al. [21] proposed a segmentation method using HT and dual snake model; two contours are initialized from line segments generated by HT. Author admits that the technique is not suitable for irregular boundaries and decimate minor details.
4.3. Nakagami Mixture Modelling
In 2009, Destrempes et al. [25] introduced a segmentation technique based on Nakagami mixture modeling and stochastic optimization. The echogenicity of the region of interest (ROI) comprising the intima-media layers, the lumen, and the adventitia in an ultrasonic B-mode image is modeled by a mixture of three Nakagami distributions. In a first step the expectation maximization (EM) algorithm was used to compute the maximum A posterior (MAP) estimator of the proposed model, then computes the optimal segmentation based on the estimated distributions as well as a statistical prior for disease-free IMT using a variant of the exploration/selection (ES) algorithm. This method requires minimal manual initialization. Destrempes et al. [43] proposed a method for segmentation of plaques in sequences of ultrasound B-mode images of carotid arteries based on motion estimation and Nakagami distributions. In it, a local geometrical smoothness constraint and an original spatiotemporal cohesion constraint were incorporated, envisaging the segmented plaque based on motion field estimation. In 2011, Destrempes et al. [23] proposed a method for the segmentation of plaques in the sequence of ultrasound B-mode images of carotid arteries based on motion estimation and a Bayesian model. Authors have reported that the algorithm was not sensitive to the degree of stenosis or calcification.
4.4. Active Contour
The basic concept of active contour model is to fit a contour to local image information, for example, gradient. There exist several implementations of this basic idea such as snakes [44], discrete dynamic contour model [45], and level sets [46]. Based on the involved feature image, they can be categorized as edge based [47], region based [48, 49], and higher level knowledge based [50, 51]. Several studies have adopted the traditional snake model as proposed by Williams and Shah [52]. Snakes are also called active parametric contours, which have been widely used in medical image segmentation. The major limitations of this method are sensitive to noise, depends on the initial contour that is provided by the user, need for optimization of the parameters. In 2008, Moursi and El-Sakka [53] proposed an active contour-based segmentation technique, in which user only requires to place seed points in the ROI with the aim of reducing user interaction. Author admitted that the computational time depends on the size of the carotid artery and the location of the seed point. In 2010, Bastida-Jumilla et al. [54] used geodesic active contours for IMC detection. In 2011, Petroudi et al. [24] proposed the fully automated segmentation algorithm based on active contours, and active contours without edges were proposed in which anatomical information was incorporated to achieve accurate segmentation. The segmented regions were used to automatically achieve image normalization, which is followed by speckle removal. The resulting smoothed LI boundary combined with anatomical information provides an excellent initialization for parametric active contours that provide the final IMC segmentation. No information about an inter- and intraobserver variability and its effect on segmentations was given by author.
4.5. Edge Detection and Gradient-Based Techniques
The edge detection methods could detect the variation of gray levels, but it is sensitive to noise and may suffer from the focusing artifact. In 2001, Liguori et al. [7] proposed the segmentation technique based on an edge detection, in which image gradient was used. In this method, for each column of the image the gradient of the intensity profile has been computed. It was assumed that pixels belonging to lumen were black and that the carotid wall layers originate with gradient transitions. It is a semiautomatic method; ROI is selected by the user. The pattern recognition, edge detection (PRED) algorithm, and the measurement algorithm were used for carotid IMT measurement. Its main task is to find out all the pixels belonging to the two required interfaces (LI and MA) for each wall. The measured intensity gradient was different from the theoretical one, due to noise. In order to reduce the effect of noise, a statistical thresholding was adopted before computing the image gradient. Robustness of the edge detection algorithm had been evaluated with respect to the ROI. The gradient-based segmentation mainly suffers from the problem of superimposed noise, which precludes a proper individuation of the LI and MA transitions. In 2008, Faita et al. [26] proposed a method in which the gradient performance was improved by the use of a first-order absolute moment edge operator (FOAM) and a pattern recognition approach. The overall performance of this methodology was very high: IMT measurement error was equal to 10.0 ± 38.0 μm. Moreover, FOAM operator and intelligent procedure determines maxima, ensuring a good robustness to noise. As the technique is real-time, it suits well to clinical application. It is a semiautomatic technique. Recently, Mahmoud et al. [55] introduced a method, which employs a multistep gradient-based algorithm. This method principally uses intensity, intensity gradient, and interface continuity of pixels to determine the ultrasound interface. Author reported that this technique eliminates subjectivity associated with conventional manual tracing and semiautomated gradient methods that employ seed point selection.
4.6. Combined Approaches
Delsanto et al. [6] proposed a combined approach for classification and a snake-based segmentation to perform IMT measurement. Completely user-independent layer extraction based on signal analysis (CULEXsa) is a completely user independent algorithm for IMT measurement. Firstly ROI is identified automatically followed by gradient-based initial segmentation, and then active contour technique is used for segmentation refinement. For improving segmentation performance, Molinari et al. [56] have combined the three IMT segmentation methods: (i) signal processing approach, combined with snakes and fuzzy clustering, (ii) integrated approach based on seed and line detection, followed by probability-based connectivity and classification, and (iii) morphological approach and fused the resulting boundaries using a greedy method described by the “ball and basket” to minimize the system error. In 2010, Molinari et al. [57] proposed a completely automated layer extraction technique (named CALEXia). The IMT measurement error was equal to 0.87 ± 0.56 pixels (0.054 ± 0.035 mm). Author admitted that CALEXia showed limited performance in segmenting the LI interface. Meiburger et al. [58] introduced the Carotid Automated Double-Line Extraction System based on the Edge Flow (CADLES-EF). It is characterized and validated by comparing the output of the algorithm with CALEXia and CULEXsa. Validation was performed on a multi-institutional database of 300 longitudinal B-mode carotid images with normal and pathologic arteries. CADLES-EF showed an IMT bias of 0.043 ± 0.097 mm in comparison with CALEXia and CULEXsa that showed 0.134 ± 0.088 mm and 0.74 ± 0.092 mm, respectively. The system's Figure of Merit (FoM) showed an improvement when compared with CALEXia and CULEXsa, leading to values of 84.7%, 91.5%, while CADLES-EF performed the best with 94.8%. In 2011, Molinari et al. [59] proposed a method called CARES 3.0 (a patented technology). CARES 3.0 is completely automated and adopts an integrated approach for segmentation of carotid artery in the image frame. The FoM of CARES 3.0 was 97.4%. In 2012, Molinari et al. [22] proposed completely automated multiresolution edge snapper (named CAMES). In it carotid artery is recognized automatically using a scale-space and statistical classification in a multiresolution framework. Recently, Ilea et al. [20] proposed a fully automated segmentation and tracking of the intima-media thickness in ultrasound video sequences of the CCA. For the video tracking procedure, a spatially coherent algorithm is introduced, which prevents the tracking process from converging to wrong arterial interfaces. Author reported that method can deal with inconsistencies in the appearance of the IMC over the cardiac cycle.
5. Validation/Quantitative Performance Assessment
Validation experiments are necessary in order to quantify the performance of a segmentation method. Validation is usually performed using truth models such as phantom studies, animal model studies, simulation, comparing the automated segmentation method with manually obtained segmentations. Following are the most used performance metrics to validate IMT measurements and computer traced boundaries [30]:
Mean absolute distance (MAD),
Hausdorff distance (HD),
Polyline distance metric (PDM),
Percent statistic test,
Reproducibility of manual procedures. It is assessed by calculating intraoperator and interoperator variability using either of CV, MAD, HD, regression analysis, and Bland-Altman statistics,
Manual and computer-measured IMT (intermethod). Comparison between the two sets was done using correlation or Bland-Altman plot.
6. Conclusion
This paper reports an extensive review of ultrasound carotid artery IMT segmentation techniques. Active contours, dynamic programming, and integrated approaches have been presented to segment the carotid wall and trace the boundaries of the LI and MA interfaces. None of the existing techniques were overwhelmingly good in all aspects. Characterization and validation studies will be required in order to carefully assess the effect of such variability on segmentation performance. Finally, we recognize that in the future, more work is likely to be done in segmentation based on adaptive segmentation for determination of IMT in ultrasound images of CCA with high IMT measurement accuracy, robustness, automation and reducing processing time.
As in the case of fully automatic techniques, detection is not reliable, since it may detect the jugular veins edges. In addition performance of semiautomatic segmentation techniques is better than fully automatic segmentation techniques. Therefore, in future we will develop techniques in which human operator will select an ROI manually, and methodology will be based on adaptive segmentation with the aim of high accuracy, great robustness, and with reducing processing time. The proposed accuracy of detection of IMT algorithm falls within the inter- and intraobserver variability for the manual determination.
References
- 1.World Health Organization. Cardiovascular Disease. http://www.who.int/cardiovascular_diseases/en/
- 2.Pu L-N, Zhao Z, Zhang Y-T. Investigation on cardiovascular risk prediction using genetic information. IEEE Information Technology in Biomedicine. 2012;16:795–808. doi: 10.1109/TITB.2012.2205009. [DOI] [PubMed] [Google Scholar]
- 3.Touboul P, Elbaz A, Koller C, et al. Common carotid artery intima-media thickness and brain infarction: the etude du profil genetique de l’infarctus cerebral (GENIC) case-control study. Circulation. 2000;102(3):313–318. doi: 10.1161/01.cir.102.3.313. [DOI] [PubMed] [Google Scholar]
- 4.Delsanto S, Molinari F, Giustetto P, Liboni W, Badalamenti S, Suri JS. Characterization of a completely user-independent algorithm for carotid artery segmentation in 2-D ultrasound images. IEEE Transactions on Instrumentation and Measurement. 2007;56(4):1265–1274. [Google Scholar]
- 5.Santhiyakumari N, Madheswaran M. Extraction of intima- media layer of arteria-carotis and evaluation of its thickness using active contour approach. Proceedings of the International Conference on Intelligent and Advanced Systems (ICIAS '07); November 2007; pp. 582–586. [Google Scholar]
- 6.Delsanto S, Molinari F, Giustetto P, Liboni W, Badalamenti S. CULEX-completely user-independent layers EXtraction: ultrasonic carotid artery images segmentation. Proceedings of the 27th Annual International Conference of the Engineering in Medicine and Biology Society (IEEE-EMBS '05); September 2005; pp. 6468–6471. [DOI] [PubMed] [Google Scholar]
- 7.Liguori C, Paolillo A, Pietrosanto A. An automatic measurement system for the evaluation of carotid intima-media thickness. IEEE Transactions on Instrumentation and Measurement. 2001;50(6):1684–1691. [Google Scholar]
- 8.Halenka M. Noninvasive measurement of early atherosclerosis by high-resolution B-mode ultrasonography. Acta Universitatis Palackianae Olomucensis Facultatis Medicae. 1999;142:7–11. [PubMed] [Google Scholar]
- 9.Touboul P-J, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006) Cerebrovascular Diseases. 2007;23(1):75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 10.Faita F, Gemignani V, Bianehini E, Giannarelli C, Demi M. Real-time measurement system for the evaluation of the Intima Media Thickness with a new edge detector. Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS '06); September 2006; pp. 715–718. [DOI] [PubMed] [Google Scholar]
- 11.Loizou CP, Pattichis CS, Nicolaides AN, Pantziaris M. Manual and automated media and intima thickness measurements of the common carotid artery. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2009;56(5):983–994. doi: 10.1109/TUFFC.2009.1130. [DOI] [PubMed] [Google Scholar]
- 12.Van Der Meer IM, Bots ML, Hofman A, Del Sol AI, Van Der Kuip DAM, Witteman JCM. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: The Rotterdam Study. Circulation. 2004;109(9):1089–1094. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 13.Järvisalo MJ, Putto-Laurila A, Jartti L, et al. Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes. 2002;51(2):493–498. doi: 10.2337/diabetes.51.2.493. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. The New England Journal of Medicine. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 15.Huang C-C, Chen T, Chao T-H, Juan Y-F. The investigation of the relationship between carotid intima-media thickness and vascular compliance in patients with coronary artery disease. Biomedical Engineering. 2004;16(1):37–42. [Google Scholar]
- 16.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 17.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima—media thickness in predicting clinical coronary events. Annals of Internal Medicine. 1998;128(4):262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 18.Fang J, Zhang JP, Luo CX, Yu XM, Lv LQ. Carotid intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. International Journal of Medical Sciences. 2010;7(5):278–283. doi: 10.7150/ijms.7.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng D-C, Jiang X, Schmidt-Trucksäss A, Cheng K. Automatic intima-media thickness measurement of carotid artery wall in B-mode sonographic images. Proceedings of the 3rd IEEE International Symposium on Biomedical Imaging: from Nano to Macro; April 2006; pp. 912–915. [Google Scholar]
- 20.Ilea DE, Duffy C, Kavanagh L, Stanton A, Whelan PF. Fully automated segmentation and tracking of the intima media thickness in ultrasound video sequences of the common carotid artery. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 2013;60:158–177. doi: 10.1109/TUFFC.2013.2547. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Zhou Y, Cheng X, Song E, Li G. Ultrasound intima-media segmentation using Hough transform and dual snake model. Computerized Medical Imaging and Graphics. 2012;36(3):248–258. doi: 10.1016/j.compmedimag.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Molinari F, Pattichis CS, Zeng G, et al. Completely automated multiresolution edge snapper—a new technique for an accurate carotid ultrasound IMT measurement: clinical validation and benchmarking on a multi-institutional database. IEEE Transactions on Image Processing. 2012;21(3):1211–1222. doi: 10.1109/TIP.2011.2169270. [DOI] [PubMed] [Google Scholar]
- 23.Destrempes F, Meunier J, Giroux M, Soulez G, Cloutier G. Segmentation of plaques in sequences of ultrasonic B-mode images of carotid arteries based on motion estimation and a bayesian model. IEEE Transactions on Biomedical Engineering. 2011;58(8):2202–2211. doi: 10.1109/TBME.2011.2127476. [DOI] [PubMed] [Google Scholar]
- 24.Petroudi S, Loizou C, Pantziaris M, Pattichis M, Pattichis C. A fully automated method using active contours for the evaluation of the intima-media thickness in carotid US images. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS '11); September 2011; pp. 8053–8057. [DOI] [PubMed] [Google Scholar]
- 25.Destrempes F, Meunier J, Giroux M, Soulez G, Cloutier G. Segmentation in ultrasonic B-mode images of healthy carotid arteries using mixtures of Nakagami distributions and stochastic optimization. IEEE Transactions on Medical Imaging. 2009;28(2):215–229. doi: 10.1109/TMI.2008.929098. [DOI] [PubMed] [Google Scholar]
- 26.Faita F, Gemignani V, Bianchini E, Giannarelli C, Ghiadoni L, Demi M. Real-time measurement system for evaluation of the carotid intima-media thickness with a robust edge operator. Journal of Ultrasound in Medicine. 2008;27(9):1353–1361. doi: 10.7863/jum.2008.27.9.1353. [DOI] [PubMed] [Google Scholar]
- 27.Liang Q, Wendelhag I, Wikstrand J, Gustavsson T. A multiscale dynamic programming procedure for boundary detection in ultrasonic artery images. IEEE Transactions on Medical Imaging. 2000;19(2):127–142. doi: 10.1109/42.836372. [DOI] [PubMed] [Google Scholar]
- 28.Gustavsson T, Liang Q, Wendelhag I, Wikstrand J. A dynamic programming procedure for automated ultrasonic measurement of the carotid artery. Proceedings of the IEEE Computers in Cardiology Conference; 1994; pp. 297–300. [Google Scholar]
- 29.Noble JA, Boukerroui D. Ultrasound image segmentation: a survey. IEEE Transactions on Medical Imaging. 2006;25(8):987–1010. doi: 10.1109/tmi.2006.877092. [DOI] [PubMed] [Google Scholar]
- 30.Molinari F, Zeng G, Suri JS. A state of the art review on intima-media thickness (IMT) measurement and wall segmentation techniques for carotid ultrasound. Computer Methods and Programs in Biomedicine. 2010;100(3):201–221. doi: 10.1016/j.cmpb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Loizou CP, Pattichis CS, Pantziaris M, Tyllis T, Nicolaides A. Snakes based segmentation of the common carotid artery intima media. Medical and Biological Engineering and Computing. 2007;45(1):35–49. doi: 10.1007/s11517-006-0140-3. [DOI] [PubMed] [Google Scholar]
- 32.Golemati S, Stoitsis J, Balkizas T, Nikita KS. Comparison of B-mode, M-mode and Hough transform methods for measurement of arterial diastolic and systolic diameters. Proceedings of the 27th Annual International Conference of the Engineering in Medicine and Biology Society (IEEE-EMBS '05); September 2005; pp. 1758–1761. [DOI] [PubMed] [Google Scholar]
- 33.Molinari F, Zeng G, Suri JS. An integrated approach to computer-based automated tracing and its validation for 200 common carotid arterial wall ultrasound images: a new technique. Journal of Ultrasound in Medicine. 2010;29(3):399–418. doi: 10.7863/jum.2010.29.3.399. [DOI] [PubMed] [Google Scholar]
- 34.Gustavsson T, Abu-Gharbieh R, Hamarneh G, Liang Q. Implementation and comparison of four different boundary detection algorithms for quantitative ultrasonic measurements of the human carotid artery. Proceedings of the 24th Annual Meeting on Computers in Cardiology; September 1997; pp. 69–72. [Google Scholar]
- 35.Liu G, Wang B, Liu DC. Detection of intima-media layer of common carotid artery with dynamic programming based active contour model. Proceedings of the Chinese Conference on Pattern Recognition (CCPR '08); October 2008; pp. 1–6. [Google Scholar]
- 36.Holdfeldt P, Viberg M, Gustavsson T. A new method based on dynamic programming for boundary detection in ultrasound image sequences. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society Conference. 2008;2008:3072–3074. doi: 10.1109/IEMBS.2008.4649852. [DOI] [PubMed] [Google Scholar]
- 37.Cheng D-C, Jiang X. Detections of arterial wall in sonographic artery images using dual dynamic programming. IEEE Transactions on Information Technology in Biomedicine. 2008;12(6):792–799. doi: 10.1109/TITB.2008.926413. [DOI] [PubMed] [Google Scholar]
- 38.Jain AK. Fundamentals of Digital Image Processing. PHI; [Google Scholar]
- 39.Golemati S, Stoitsis J, Sifakis EG, Balkizas T, Nikita KS. Using the hough transform to segment ultrasound images of longitudinal and transverse sections of the carotid artery. Ultrasound in Medicine and Biology. 2007;33(12):1918–1932. doi: 10.1016/j.ultrasmedbio.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Stoitsis J, Golemati S, Kendros S, Nikita KS. Automated detection of the carotid artery wall in B-mode ultrasound images using active contours initialized by the Hough Transform. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2008;2008:3146–3149. doi: 10.1109/IEMBS.2008.4649871. [DOI] [PubMed] [Google Scholar]
- 41.Petroudi S, Loizou CP, Pattichis CS. Atherosclerotic carotid wall segmentation in ultrasound images using Markov random fields. Proceedings of the 10th International Conference on Information Technology and Applications in Biomedicine (ITAB '10); November 2010; pp. 1–5. [Google Scholar]
- 42.Matsakou AI, Golemati S, Stoitsis JS, Nikita KS. Automated detection of the carotid artery wall in longitudinal B-mode images using active contours initialized by the Hough transform. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS '11); September 2011; pp. 571–574. [DOI] [PubMed] [Google Scholar]
- 43.Destrempes F, Soulez G, Giroux M, Meunier J, Cloutier G. Segmentation of plaques in sequences of ultrasonic B-mode images of carotid arteries based on motion estimation and Nakagami distributions. Proceedings of the IEEE International Ultrasonics Symposium (IUS '09); September 2009; pp. 2480–2483. [Google Scholar]
- 44.Kass M, Witkin A, Terzopoulos D. Snakes: active contour models. International Journal of Computer Vision. 1988;1(4):321–331. [Google Scholar]
- 45.Lobregt S, Viergever MA. Discrete dynamic contour model. IEEE Transactions on Medical Imaging. 1995;14(1):12–24. doi: 10.1109/42.370398. [DOI] [PubMed] [Google Scholar]
- 46.Sethian JA. Level Set Methods and Fast Marching Methods: Evolving Interfaces in Computational Geometry, Fluid Mechanics, Computer Vision and Materials Science. Cambridge University; 1999. [Google Scholar]
- 47.Caselles V, Kimmel R, Sapiro G. Geodesic active contours. International Journal of Computer Vision. 1997;22(1):61–79. [Google Scholar]
- 48.Li C, Kao C, Gore JC, Ding Z. Minimization of region-scalable fitting energy for image segmentation. IEEE Transactions on Image Processing. 2008;17(10):1940–1949. doi: 10.1109/TIP.2008.2002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu SC. Region competition: unifying snakes, region growing, and bayes/mdl for multiband image segmentation. IEEE Transactions on Pattern Analysis and Machine Intelligence. 1996;18(9):884–900. [Google Scholar]
- 50.Cremers D, Tischhäuser F, Weickert J, Schnörr C. Diffusion snakes: Introducing statistical shape knowledge into the Mumford-Shah functional. International Journal of Computer Vision. 2002;50(3):295–313. [Google Scholar]
- 51.Michael EL, Grimson WEL, Olivier F. Statistical shape influence in geodesic active contours. Computer Vision and Image Understanding. 2000;1:316–323. [Google Scholar]
- 52.Williams DJ, Shah M. A Fast algorithm for active contours and curvature estimation. CVGIP. 1992;55(1):14–26. [Google Scholar]
- 53.Moursi SG, El-Sakka MR. Active contours initialization for ultrasound carotid artery images. Proceedings of the 6th IEEE/ACS International Conference on Computer Systems and Applications (AICCSA '08); April 2008; pp. 629–636. [Google Scholar]
- 54.Bastida-Jumilla MC, Morales-Sánchez J, Verdú-Monedero R, Larrey-Ruiz J, Sancho-Gómez JL. Detection of the intima and media walls of the carotid artery with geodesic active contours. Proceedings of the 17th IEEE International Conference on Image Processing (ICIP '10); September 2010; pp. 2213–2216. [Google Scholar]
- 55.Mahmoud A, Morsy A, De Groot E. A new gradient-based algorithm for edge detection in ultrasonic carotid artery images. Proceedings of the 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC '10); September 2010; pp. 5165–5168. [DOI] [PubMed] [Google Scholar]
- 56.Molinari F, Zeng G, Suri JS. Intima-media thickness: setting a standard for a completely automated method of ultrasound measurement. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2010;57(5):1112–1124. doi: 10.1109/TUFFC.2010.1522. [DOI] [PubMed] [Google Scholar]
- 57.Molinari F, Zeng G, Suri JS. Inter-Greedy technique for fusion of different carotid segmentation boundaries leading to high-performance IMT measurement. Proceedings of the 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC '10); September 2010; pp. 4769–4772. [DOI] [PubMed] [Google Scholar]
- 58.Meiburger KM, Molinari F, Zeng G, Saba L, Suri JS. Carotid automated ultrasound double line extraction system (CADLES) via Edge-Flow. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS '11); September 2011; pp. 575–578. [DOI] [PubMed] [Google Scholar]
- 59.Molinari F, Meiburger KM, Acharya UR, et al. CARES 3.0: a two stage system combining feature-based recognition and edge-based segmentation for CIMT measurement on a multi-institutional ultrasound database of 300 images. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS '11); September 2011; pp. 5149–5152. [DOI] [PubMed] [Google Scholar]


