Short abstract
Most computational models of abdominal aortic aneurysms address either the hemodynamics within the lesion or the mechanics of the wall. More recently, however, some models have appropriately begun to account for the evolving mechanics of the wall in response to the changing hemodynamic loads. Collectively, this large body of work has provided tremendous insight into this life-threatening condition and has provided important guidance for current research. Nevertheless, there has yet to be a comprehensive model that addresses the mechanobiology, biochemistry, and biomechanics of thrombus-laden abdominal aortic aneurysms. That is, there is a pressing need to include effects of the hemodynamics on both the development of the nearly ubiquitous intraluminal thrombus and the evolving mechanics of the wall, which depends in part on biochemical effects of the adjacent thrombus. Indeed, there is increasing evidence that intraluminal thrombus in abdominal aortic aneurysms is biologically active and should not be treated as homogeneous inert material. In this review paper, we bring together diverse findings from the literature to encourage next generation models that account for the biochemomechanics of growth and remodeling in patient-specific, thrombus-laden abdominal aortic aneurysms.
Keywords: aorta, hemodynamics, stress analysis, biomechanics, inflammation, proteases, growth and remodeling
1. Introduction
Abdominal aortic aneurysms (AAAs) are focal dilatations of the infrarenal aorta; they tend to enlarge over years but rupture abruptly when wall stress exceeds wall strength. These lesions are prevalent in the aging population and, consequently, are increasingly responsible for morbidity and mortality. Although the pathogenesis is still not well understood, there is a wealth of biological, clinical, histopathological, and mechanical data on AAAs, and there has been significant progress in the biomechanical modeling of these lesions [1,2]. Nevertheless, our ability to predict whether a specific lesion will arrest, continue to enlarge either slowly or rapidly, or ultimately rupture remains wanting. Clinical interventions thus continue to be based primarily on the maximum dimension or expansion rate of the lesion as well as symptoms (e.g., pain) despite the observation that many small lesions rupture whereas larger lesions may remain asymptomatic [3]. There is clearly a need for increased understanding.
One possible reason for this variability in clinical outcomes that has garnered increased attention is the intraluminal thrombus (ILT). The majority of AAAs harbor an ILT, which may vary in thickness from a few millimeters to multiple centimeters. These thrombi have complex natural histories and structures, and their biological and mechanical roles in the progression and potential rupture of AAAs remains controversial. The goals of this paper, therefore, are twofold. First, we review salient aspects of the development and progression of ILT, its evolving mechanical properties, and its potential role in the biochemomechanics that govern the natural history of AAAs. Second, we discuss past successes and future needs regarding mathematical modeling of the role of ILT in AAA progression, both from a classical mechanical and a contemporary growth and remodeling (G&R) perspective. Toward this end, we submit that an increased ability to understand and quantify the evolving heterogeneous biochemomechanical properties of ILT and its effects on the natural history of the aneurysmal wall will not only help resolve controversies that have arisen from past consideration of the ILT as a homogeneous and/or inert structure, it will also guide future clinically motivated experimental and computational efforts to understand, predict, and therapeutically address the roles of ILT in this challenging and important vascular pathology.
2. Hemodynamics and ILT Development
The disturbed hemodynamics that characterizes blood flow in AAAs likely plays a major role in initiating and sustaining the cascade of processes that promote ILT. Several experimental and computational studies of blood flow in idealized and patient-specific models of AAAs suggest that particular characteristics might be recurrent [4–8]. For example, vortical structures may develop during systole and then propagate and dissipate during diastole [8–10]. The outer regions of these vortices, in turn, may allow regions of high fluid shear stress that can exist away from the arterial wall. At the aneurysmal wall, however, shear stress has been consistently reported to be lower compared with values typically computed on the endothelium of the healthy aorta [5,7,8]. Thus, the hemodynamic environment in nascent AAAs may simultaneously contain regions favorable to platelet activation (due to high shear stresses in the lumen where platelets activate) and adhesion (due to low wall shear stresses where platelets may aggregate on a vulnerable endothelium).
Note, therefore, that early thrombus development is characterized by aggregation of activated platelets and entrapment of erythrocytes and a small number of leukocytes within an evolving fibrin mesh; this fibrin accumulates as activated thrombin cleaves fibrinogen into fibrin, which then polymerizes to form the underlying mesh. Fibrin is stabilized via crosslinking by activated factor XIII (i.e., fXIIIa). Primary thick fibrin fibers may form around the initially plentiful erythrocytes, but as the fibrin-dominated matrix grows under the activity of additional platelets recruited from the bloodstream, focal areas of higher fibrin density may arise as old erythrocytes lyse and the porous structure of the matrix becomes sufficiently small to exclude the influx of new cells from the lumen [11]. With continued loss of erythrocytes, increased secondary fibers form between the thick primary fibers, which could contribute to a stiffening of the fibrin matrix (cf. Ref. [12]). Interestingly, plasma samples from AAA patients form denser, lower porosity clots that resist fibrinolysis more than controls, with the degree of resistance correlating with aneurysm size [13]. These findings appear to be independent of fibrinogen or changes in thrombin generation; yet, other agents such as lipoprotein (a) or activated factor XII (fXIIa) may affect clot structure independent of thrombin [13]. Notably, lipoprotein (a) is elevated in AAA plasma versus control [14], and fXII activity correlates with aneurysm size [15]. These correlates may also contribute to the increased stiffness observed during the aging of ILT [12].
The process of coagulation, the mechanical properties of fibrin, and fibrinolysis are described in detail in several reviews [16–19] and recent papers [11,20,21]. A number of computational models have also been proposed for thrombus development, many of which are reviewed in Xu et al. [22]. In general, two approaches have been used to model coagulation and the development of thrombus within flowing blood. Continuum models at the macrolevel include reaction-diffusion (differential) equations that attempt to describe changes in the concentration and activation of essential hemostatic factors. Illustrative examples of this approach include Lobanov and Starozhilova [23], who were among the first to model effects of flow on fibrin deposition to a thrombus attached to a vessel wall and the subsequent effect of the growing clot on the flow, as well as Anand et al. [24], who focused on both the biochemical and mechanical factors governing thrombus formation and lysis. Another, increasingly popular, approach includes hybrid formulations that combine discrete and continuum models. For example, since thrombin is generated on a nanoscale while blood flows at a macroscale, a hybrid formulation attempts to integrate these processes spatiotemporally and to take advantage of individual strengths of the different models. Illustrative examples include Xu et al. [25,26], who combined a discrete cellular Potts model for platelets, red blood cells, and white blood cells with the continuum Navier–Stokes equations, and Biasetti et al. [27], who investigated the relationship of flow vortices, coagulation, and thrombus deposition while including both plasma and membrane phase biochemical reactions. See, too, the works by Leiderman and Fogelson [28], Scianna and Preziosi [29], Flamm and Diamond [30], and others.
Many phenomenological models have also been employed to identify potentially useful metrics for predicting the location and timing of thrombus development within the aneurysmal sac. For example, a particle-hemodynamics analysis by Basciano et al. [31] suggested a generally greater maximum time-averaged shear stress on simulated particles in the proximal and middle AAA as well as a potential correlation between measures of near-wall particle transit times and future thrombus deposition. These findings indirectly support computational results by Biasetti et al. [9,32], which suggest that platelets can activate within proximal regions of high fluid shear stress (e.g., flow vortices) and convect toward the wall distally to deposit in zones of low wall shear stress. Although larger sample sizes are needed to confirm and extend these results, such studies are promising, indeed prerequisite, to modeling the development of thrombus-laden AAAs over physiologically relevant timeframes. Timing and location of thrombus deposition alone represent, however, only one aspect of understanding the role of ILT in AAA progression. Thus, we turn our attention to the heterogeneous structure and inherent mechanical properties of the thrombus itself.
3. ILT Structure and Mechanical Properties
Based on gross examination, two major types of ILT have been described in AAAs: “discrete transition” and “continuous transition” [33]. In the discrete type, the thrombus appears to be layered, with sharp demarcations and weak attachments between adjacent layers. Properties, therefore, are generally distinct among the multiple (often three primary) layers. In contrast, in the continuous transition thrombus, there are gradual transmural changes in structure with strong radial connectivity. We suggest that these differences could reflect the nature in which the thrombus was formed; that is, by temporally separated discrete deposition events resulting in various thicknesses versus a slow gradual build-up. Interestingly, the aforementioned study by Basciano et al. [31] appears to have captured a potential discrete deposition event (see Fig. 1). Furthermore, noting that multiple studies have observed both continuous and stepwise (or “staccato”) expansion of AAAs [34,35], we hypothesize that the type of thrombus deposition (i.e., continuous versus discrete) may reflect, if not contribute to, these modes of expansion. Future studies will be required to investigate this possibility, however.
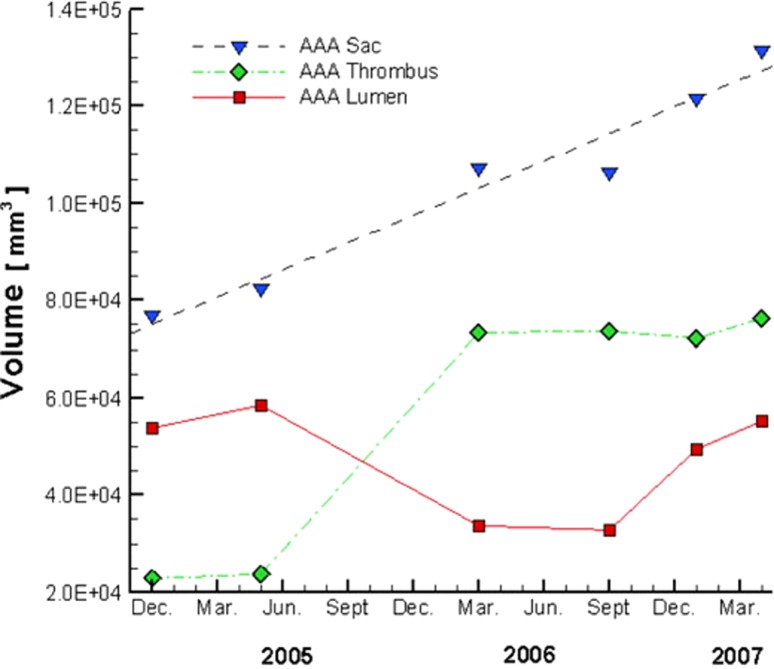
Fig. 1.
Patient's AAA volume history. Note the changes in volume of the lumen, ILT (thrombus), and aneurysmal sac over time, with a possible discrete deposition of thrombus between Jun. 2005 and Mar. 2006. From Basciano et al. [31], with kind permission from Springer Science and Business Media.
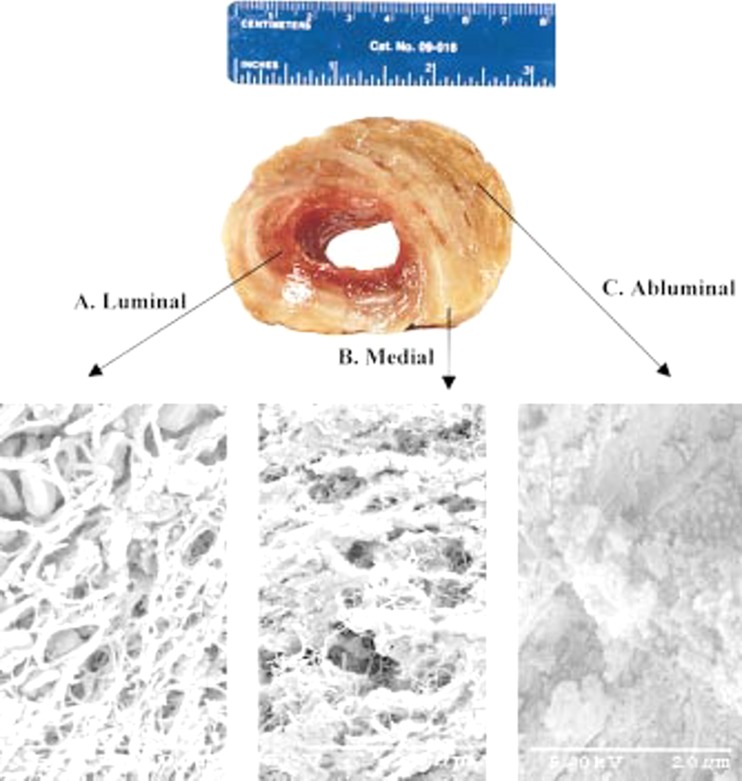
The three regions of layered ILT (Fig. 2) are often referred to as luminal (nearest the blood), medial (or middle), and abluminal (nearest the aneurysmal wall). The luminal layer appears red due to the high content of erythrocytes, while the medial layer appears white to yellow, due in part to the lysing of erythrocytes. In contrast, in thick ILT, the deepest (abluminal) layer may appear brown [33,36]. In some cases (up to 40% of cases in one study [37]), a liquid phase is also found at the interface between the abluminal layer and the wall, suggestive of local liquefaction. It should be noted, however, that not all ILT contain all three layers. Thin thrombi may consist entirely of “luminal-type” thrombus, while thicker thrombi may have a combination of distinct layers. There is, therefore, a need to develop a more consistent and inclusive terminology to describe the type of thrombus observed in future studies. Given clear differences in biochemical and biomechanical properties of the three layers, as determined by gross morphology, histology, and mechanical tests, we suggest further that the terms “luminal,” “medial,” and “abluminal” be used to refer to intrinsic differences in clot type, not simply positions relative to the wall. That is, a luminal thrombus should include all thrombi with certain specific characteristics (e.g., high numbers of activated platelets and infiltrating neutrophils) even if part of it is immediately adjacent to the aortic wall. Thus, over time, a luminal thrombus may be buried by subsequent clot deposition and evolve into medial and eventually abluminal thrombi following erythrocyte lysis, leukocyte apoptosis, and various degrees of fibrinolysis.
Fig. 2.
Gross and ultrastructural appearance of a layered intraluminal thrombus from a human AAA. Note the entrapped cells in the luminal thrombus. From Wang et al. [36], with permission.
In terms of fibrin structure, the luminal layer is characterized by thick primary fibers of fibrin with fine interconnecting secondary fibers forming a well-organized matrix. These secondary interconnections are lost in the medial layer with evidence of progressing matrix degradation [33,36], consistent with the observation that thin fibrin fibers cleave more quickly than thick ones [38]. This finding is also consistent with the observation that small interconnected channels termed “canaliculi” found throughout the ILT appear to increase in mean area from the luminal toward the abluminal layer [39]. Within the abluminal layer, the matrix is almost completely degraded, with minimal fibrin organization [36].
Just as our understanding of structural characteristics of ILT has increased significantly over the past few decades, so too has our understanding of the mechanical properties. An early study by Di Martino et al. [40] reported uniaxial, tensile data for circumferentially oriented surgical samples of ILT and used a simple linearly elastic Hookean model. While no distinction was made as to the layer of ILT tested, only “well-organized” thrombi were selected for testing, which likely reflects the luminal layer; reported Young's moduli were 0.05 MPa to 0.2 MPa. To quantify potential viscous properties of ILT, both linear [33] and nonlinear [41] viscoelastic models have been proposed. These results suggest that elastic behavior dominates in multiple layers, though viscous effects may be important in attenuating pressure waves. Although linearized constitutive relations continue to be used, it has become increasingly clear that such models cannot capture all responses [42,43]. This finding should not be unexpected given that Vorp et al. [44] demonstrated many years ago that ILT may undergo large strains in vivo.
Wang et al. [36] conducted uniaxial tensile tests on luminal and medial layers of ILT in both circumferential and axial directions and fit the data with a nonlinear, hyperelastic constitutive relation. They reported distinct mechanical properties for two layers, with increased stiffness and strength exhibited by the luminal layer; results from circumferential and axial tests further suggested material isotropy. Vande Geest et al. [45] similarly concluded that the luminal layer was isotropic and nonlinearly hyperelastic using biaxial tests; they were only able to test the luminal layer, however, noting that medial and abluminal layers were too weak. Interestingly, they noted significant differences in the predicted mechanical behavior when comparing results from the biaxial and prior uniaxial results, highlighting the importance of appropriate protocols when formulating constitutive relations. Indeed, although tests are usually designed to explore tensile behavior, Ashton et al. [46] used unconfined compression tests to calculate a drained secant modulus at 5% strain for all three layers. Interestingly, they reported significantly higher compressive stiffness for abluminal than for medial and luminal layers, a gradient opposite that observed in most tensile tests.
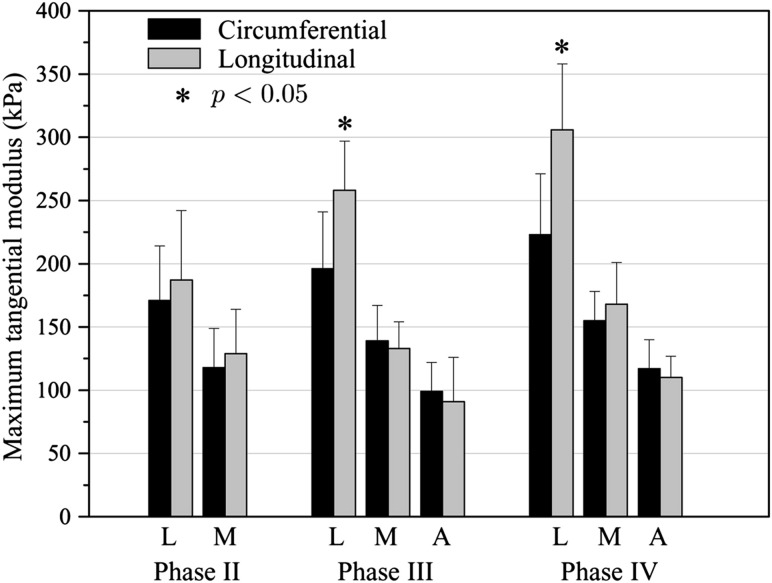
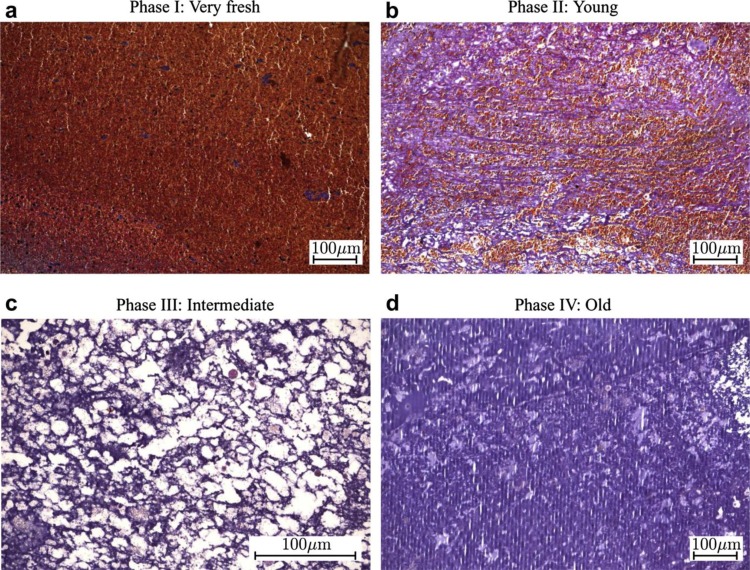
Recently, biaxial tests on all three layers by Tong et al. [12] suggested the need to classify ILT even further by both position and age, due to possible evolving phases of development of the mechanical and histological properties of each of the three layers. Specifically, they observed a decreasing maximum tangential modulus and tensile stress at failure from the luminal to the abluminal layer but a potentially increasing tensile modulus for each layer as it evolves through four phases over time (Fig. 3), most notably in the axial direction for the luminal layer [12]. These phases were histologically distinct (Fig. 4): Phase I (very fresh) was characterized by an abundance of erythrocytes, Phase II (young) by loose fibrin networks entrapping the erythrocytes, Phase III (intermediate) by disrupted erythrocytes and a condensing fibrin network, and Phase IV (old) by disrupted fibrin networks and condensed residue proteins. They also reported a subclass of generally older luminal ILT that demonstrated anisotropic behavior with increased stiffness in the axial direction. Interestingly, associated mechanical testing of the underlying aortic wall similarly revealed increased anisotropy when located directly underneath an older, anisotropic ILT. Thus, biochemomechanical models of ILT may not only require delineation of the layers of thrombus, but also their unique evolution in properties and their influence on the mechanics of the underlying wall. This consideration leads us naturally to the associated spatiotemporal biochemical activity of ILT.
Fig. 3.
Maximum tangential moduli of ILT from human AAAs separated by luminal (L), medial (M), and adventitial (A) layers and phase (a proposed indicator of age; see Fig. 4). From Tong et al. [12], with permission.
Fig. 4.
Proposed histological phases of ILT maturation. From Tong et al. [12], with permission.
4. The Biologically Active ILT
Thrombus may occur throughout the body, including other focal areas of ILT (e.g., deep vein or coronary thrombosis), yet thrombi within AAAs are characterized by unique biochemical and biomechanical properties secondary to their persistent contact with flowing blood and their apparent inability to heal through mesenchymal cell invasion and collagen deposition [47]. As a result, ILT within AAA may contribute to the often chronic inflammation of the underlying aortic wall, with persistent renewal of cellular activity at the luminal interface via the aggregation, entrapment, and recruitment of activated platelets and inflammatory cells. Consequently, ILT can serve as a reservoir for myriad proteases that can be released and activated during fibrinolysis. Any attempt to understand and model the effects of ILT on AAA progression thus requires insight into not only its mechanical properties and its often eccentric spatiotemporal distribution within an aneurysm [48], but also its heterogeneous synthesis, storage, and release of relevant biomolecules: mitogenic, synthetic, proteolytic, and so forth.
4.1. Prominent Role of the Luminal Layer in ILT Activity and Renewal.
The luminal layer is the primary site in the ILT for new thrombus deposition via activated platelets and cellular activity. Interestingly, cellular content almost exclusively remains isolated to the luminal layer, with cells (including neutrophils, macrophages, and lymphocytes) generally found only to a depth of ∼1 cm, despite the aforementioned network of interconnected canaliculi that permeate the ILT [39]. Circulating leukocytes, consisting predominately of neutrophils, are actively recruited by and retained in the luminal layer, aided by the exposure of P-selectin by activated platelets and the affinity of neutrophils for binding to the copolymer of fibrin-fibronectin in the clot [37]. Neutrophils in the luminal layer can produce interleukin-8 (IL-8) and leukotriene B4, which reinforce further neutrophil invasion [49,50]. Indeed, in vitro tests confirm a potent neutrophil chemotactic activity of the luminal layer that can be inhibited by antibodies to RANTES or IL-8 or by the IL-8 receptor antagonist, reparixin [49]. Ultimately, the neutrophil content of the luminal layer can be 12-fold greater than that of blood [51].
Neutrophils are highly active cells that express and release a number of proteolytic enzymes, including myeloperoxidase (MPO), leukocyte elastase (LE), matrix metalloproteinases (MMPs) 8 and 9, and urokinase-type plasminogen activator (uPA) [52]. Lucent halos have even been observed around neutrophils in regions of fibrin degradation [53], likely due to local activation of plasmin via uPA or direct activity of secreted LE or cathepsin G [54]. Notably, MMP-8 is a potent collagenase for type I collagen whereas MMP-9 has specificity for partially degraded collagen [52]. Both of these MMPs, along with MMP-2, are elevated in AAAs, while the MMP inhibitors TIMP-1 and -2 are decreased [52,55]. Nevertheless, both TIMP-1, released from platelet α-granules [55], and the elastase inhibitor α1-antitypsin (α1-AT) exist in the luminal layer, potentially explaining (along with latency of the proform of the enzymes) some reports of high MMP-9 and elastase levels in the luminal layer but low activity [56]. Indeed, a negative gradient of latent MMP-9 but positive gradient of active MMP-9 has been reported from luminal to abluminal layers of ILT [37]. Importantly, binding of an inhibitor to its target can depend strongly on whether the protease is cell/matrix bound or freely soluble; thus, there is a need for careful control and documentation of methods of measurement. Indeed, LE, MMP-9, plasmin, and tissue plasminogen activator (tPA) can bind to fibrin [47], whereas clot retraction and fibrinolysis can release proMMP-9, elastase/α1-AT, MMP/NGAL (neutrophil gelatinase-associated lipocalin), and RANTES [37,49].
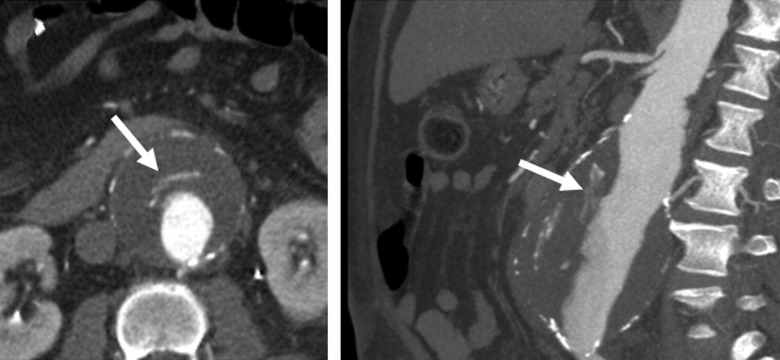
Most proMMPs can be activated by other proteases (e.g., the serine protease plasmin) or by reactive oxygen species and nitric oxide [55]. An exception is the activation of proMMP-2, which generally utilizes a membrane-type MMP on the cell surface, such as MT1-MMP. While MMP-9 is frequently produced by inflammatory cells, the main source of MMP-2 in the aortic wall appears to be smooth muscle cells (SMCs). This difference may explain the predominant role of MMP-2 in small AAAs, when SMCs are likely more abundant, compared to larger AAAs where MMP-9 activity is increased and SMCs are decreased [55,57]. Of note, MMP-2 has a greater ability to degrade elastin than does MMP-9 [55], though MMP-2, -7, -9, and -12 all have activity against insoluble elastin [58]. Future studies are needed to quantify spatiotemporal fluctuations of MMP levels and their respective regulation by gene expression, zymogen activation, inhibition, and inactivation [59]. In theory, temporal imbalances between proteases and inhibitors could lead to a destabilizing increase in matrix degradation (i.e., a “spike” in protease activity) that could overwhelm the currently available inhibitors. For example, delivery of fresh blood deep within an ILT due to hemorrhage into the thrombus (radiographically known as the “crescent sign”; see Fig. 5) could release and activate numerous bound proteases from the fibrin matrix that could overwhelm inhibitors and lead to rapid degradation of the wall. Notably, in one study, the crescent sign was seen in 21% (11/52) of ruptured aneurysms but 0% (0/56) of asymptomatic nonruptured aneurysms [60].
Fig. 5.
Note the “crescent sign” on a contrast-enhanced CT study of a 59 year-old male with an AAA. (Left—axial image; Right—maximum-intensity projection reconstruction on an oblique sagittal projection). From Labruto et al. [124], with permission.
While most thrombi tend to resolve over time, those in AAAs show few signs of healing by endothelial cell coverage or mesenchymal cell invasion and replacement of the fibrin network with collagen (cf. Ref. [61]). In vitro experiments reveal the inability of SMCs to seed the luminal layer, likely due to the high proteolytic activity. Indeed, luminal extracts induce anoikis of SMCs and stromal cells of bone marrow origin, although this effect can be blocked by protease inhibitors, including α1-AT. Notably, LE can degrade fibronectin, a key adhesive protein for adherent cell survival, and LE has been identified in neutrophils and the matrix around dying cells in the luminal layer [47]. Neutrophils are also capable of detaching endothelial cells from their underlying extracellular matrix [47]. Ultimately, many neutrophils undergo apoptosis (common in the luminal layer), which may lead to further expression of chemotactic and procoagulant factors. Additionally, the luminal layer is characterized by high levels of hemagglutination as erythrocytes deteriorate and release free hemoglobin, a powerful oxidizing agent capable of forming free radicals [51].
Of the cell-derived proteases produced within the luminal layer, LE and MMP-9 are two of the most frequently studied enzymes in AAA development. Notably, a significant correlation has been observed between LE and MMP-9 when compared within the luminal, as well as abluminal, layer [56]. While MMP-9 can be inhibited by TIMP-1, neutrophils can upregulate MMP-9 activity by releasing NGAL, which binds MMP-9 and prevents its inactivation [48]. Interestingly, NGAL has been identified in ILT, interphase fluid, and even aortic media not covered by thrombus, consistent with the ability of NGAL to diffuse from thrombus to wall and/or to be produced by leukocytes invading from the vasa vasorum, including monocyte derived macrophages [55]. LE can also increase MMP-9 activity by directly activating proMMP-9, indirectly activating it through the activation of proMMP-3 [54], or by degrading TIMP-1, thereby protecting MMP-9 from inhibition [52]. Of note, TIMP-1 not only can inhibit active MMP-9, it can also inhibit the activation of proMMPs and neoangiogenesis [55].
In addition to cell-derived proteolytic activity, the luminal layer is characterized by high levels of fibrinolytic activity, as evidenced by elevated release of plasmin-antiplasmin complexes (PAPs) and D-dimers (a measure of cross-linked fibrin degradation), greater immunostaining for plasminogen and plasminogen activators, and greater 99mTc-Aprotinin activity (a potential imaging agent that binds serine proteases like plasmin and elastase) compared with other layers [62]. Although fibrin may deposit throughout the thrombus, it decreases significantly toward the abluminal layer, consistent with activated platelets being predominately confined to the luminal layer [39]. Indeed, Touat et al. [53] demonstrated that procoagulant activity and markers of platelet activation were three to five fold higher in the luminal compared with deeper layers. Yet, because activated platelets degranulate within the clot [39], there is a potential for diffusion/convection of biomolecules through the canaliculi. Overall, these findings suggest possible continual cycles of fibrin deposition and degradation within the luminal layer, indicative of an active remodeling and renewal of the fibrin matrix [13,54]. Whether fibrin deposition within the luminal layer could eventually become dense enough to exclude further cellular infiltration (thus rendering the layer more inert) remains to be proven, although findings by Tong et al. [12] for older luminal layers having few erythrocytes supports this possibility.
4.2. Luminal ILT—Aortic Interactions.
Because the ILT is a heterogeneous structure, with its primary cellular activity in the luminal layer, those elements of the aortic wall nearest the luminal layer would be expected to be most at risk for proteolytic attack. Indeed, aneurysmal walls covered by a thin (≤1.0 cm) thrombus have significantly higher levels of LE activity, concentrations of active MMP-9 and total MMP-8, and ratios of active MMP-9/TIMP-1 than those covered by a thick (≥2.5 cm) thrombus [52]. MMP-9 and LE concentrations are also higher in luminal layers compared with deeper layers, and a significant negative correlation exists between the concentration and activity of LE in the abluminal layer and ILT volume, suggesting that LE may not penetrate well a thick ILT [56]. Of note, the slightly alkaline environment in the aneurysmal wall next to a thin ILT may also accelerate local degradation by elastase [52].
In summary, the luminal layer of an ILT is a site of renewable biochemical activity via the accumulation of platelets, recruitment of proteolytically active leukocytes (notably neutrophils), formation of fibrin, erythrocyte hemagglutination, and retention of tPA and plasminogen for postponed progressive fibrinolysis [51]. With continued deposition of thrombus, either gradually or discretely, the luminal layer may eventually become buried. This process could trigger a unique evolution of properties of the ILT and underlying aortic wall that must be considered in future modeling efforts.
4.3. Medial and Abluminal Layers.
The medial layer is characterized by a transition from the active cellular luminal layer and the disorganized, degrading abluminal layer. It is generally devoid of intact erythrocytes and only rarely has infiltrating leukocytes despite a dense fibrin network. The abluminal layer is distinguished by its location adjacent to the aortic wall in thick ILTs, a brown discoloration, lack of cells, degraded fibrin network, and weak gelatinous material with high compressive stiffness [46,53]. Its interface with the aneurysmal wall may exist as a liquid interphase with high levels of soluble proteins [37]. The lack of cellular content in the deeper layers of the ILT renders the clot unable to synthesize new proteases in these regions; nevertheless, biomolecules bound to the fibrin matrix during its formation in the luminal layer may persist and be released and/or activated. As a result, measurements of proteolytic activity in these layers may potentially vary in time and space as well as from patient to patient, depending on past and current fibrinolytic activity. For example, Folkesson et al. [56] found abluminal proteases to be predominately inactive whereas Fontaine et al. [37] reported active MMP-9 at the liquid interface between the ILT and wall.
The ability of the degrading fibrin matrix to release bound proMMPs in the presence of plasmin (potentially formed by a ready supply of plasminogen activators, such as tPA, in the immediately adjacent wall [62]) may activate MMPs at the interface and cause subsequent degradation of the underlying wall. Indeed, the aneurysmal wall has shown 100-fold higher tPA activity than the ILT [63], and MMP-9 activity has been localized to the inner wall of AAA [64] as has elastase activity [52] and plasmin [65], consistent with an ILT-mediated degradation of the wall. Yet, remnant SMCs, infiltrating macrophages, and endothelial cells from the vasa vasorum may also produce MMP-9 [66], which may relate to the ability of these cells to migrate toward and neovascularize the injured aortic wall. For example, noting that plasmin is a key activator of proMMP-9, plasminogen-/- mice had reduced macrophage migration through the extracellular matrix that was rescued with administration of active MMP-9 [67]. Nevertheless, increased MMP-9 activity in the wall significantly exceeded the increase in MMP-9 expression [66], consistent with diffusion/convection from the ILT and/or increased release and activation of stored MMPs.
It remains to be shown whether deeper layers of ILT may eventually become depleted of stored pro-enzymes as they degrade. If so, their level of proteolytic activity may vary depending on the spatiotemporal availability of activators and inhibitors, leading to a potentially significant source of patient variability in proteolytic activity. Indeed, there is a large range of reported MMP-9 levels in the ILT, interface, and wall (e.g., see Fig. 3 in Fontaine et al. [37]), thus highlighting the need to develop improved patient-specific methods of determining proteolytic content and activity in thrombi. We suggest, therefore, that while averaged data on biochemical potential and activity are insightful for population-based studies, patient-specific determinations of rupture risk may benefit from individualized spatiotemporal assessment of key enzymes and regulators or at least localized measures of net proteolytic activity.
4.4. The Fibrinolytic System and AAAs.
Just as LE, MMPs, and other proteases are important in AAA development and progression, the fibrinolytic system is fundamental to regulating the ILT. Plasminogen, synthesized by the liver and released into the blood, is naturally bound within the fibrin matrix during its formation and is present in large quantities in thrombus [54]. Cleavage of plasminogen, classically by uPA or tPA, to the potent serine protease plasmin results in many proteolytic consequences, including direct degradation of fibrin and extracellular matrix [54], direct or indirect activation of various proMMPs (including MMP-1, -3, -7, -9, -10, -12, and -13) [55,62,67], regulation of growth factors and chemokines [68,69], and proteolysis of cellular anchoring fibers, which may induce anoikis [51,70]. Plasmin can also exert a fibrin-catalyzed positive feedback to activate additional plasminogen [68]. The major inhibitory proteins in the fibrinolytic system are plasminogen activator inhibitor 1 (PAI-1), which inhibits uPA and tPA, and α2-antiplasmin (α2-AP), which binds to and inhibits active plasmin to form PAP complexes. Interestingly, while plasmin can activate MMPs, MMPs can also affect the fibrinolytic system. For example, activation of proMMP-9 by MMP-3 can cleave plasminogen and inactivate PAI-1, α2-AP, and α1-AT [54]. Similarly, elastase can indirectly activate plasminogen through alternative pathways [56].
While some report that the luminal layer releases most of the D-dimers [62], indicating degradation of fibrin, the medial and especially abluminal layer appear to undergo greater net fibrinolysis, likely due to the lack of significant new fibrin deposition within these deeper layers. Suggestive of abluminal plasminogen activation by activators in the inner wall, Fontaine et al. [37] reported that free uPA and tPA activities were only noted in the wall, while plasminogen was identified only in the ILT; however, Houard et al. [62] later reported that while tPA in the wall localizes to the inner media (and vasa vasorum in the adventitia), abundant tPA and uPA immunostaining are also present in the luminal ILT, potentially bound to fibrin and associated with neutrophils, respectively. Co-incubation of ILT and wall extracts in the presence of fibrin also produces plasmin in vitro [37]. The degree to which patient-specific and layer-specific variability in the samples of thrombi tested influence these results may require further consideration.
Notably, PAI-1 and α2-AP may not be as effective in inhibiting plasmin activity when their targets are bound to fibrin or the cell surface [54,70]. Since plasminogen, tPA, and plasmin can each bind to fibrin (as can α2-AP) [54,71] and localize to fibrin-rich areas in the luminal layer [62], they could potentially exert local activity before becoming soluble and thereby vulnerable to increased inhibition. Thus, PAPs are often present in areas of active fibrinolysis, such as the thrombus-wall interface [37], even though they are inhibitory complexes. In fact, a positive correlation exists between PAPs and D-dimers in all ILT layers [62]. In addition to decreasing inactivation, binding of plasminogen, such as to an inducible cell surface receptor, may also increase its activation and serve to direct its proteolytic activity to specific targets [68]. Clearly, therefore, it is important to report and interpret measures of fibrinolytic enzymes (and MMPs) carefully in terms of their expression (mRNA), storage/binding (stained), or release (solubilized) as well as whether the protein is latent, activated, or inhibited. For example, upregulation of a particular gene may lead to increased transcription and translation of a protease or cytokine but no immediate increase in its extracellular activity due to intracellular storage or release of only the latent form.
Synthesis of tPA and PAI-1 appear isolated to the wall, while uPA is expressed by inflammatory cells within the wall and luminal layer of the ILT [37,62]. Interestingly, SMCs can use constitutive tPA on their cell surface to locally activate plasmin and cause pericellular proteolysis of attachment molecules like fibronectin [70], a process potentially involved in activation, migration, and/or anoikis in the aortic media; tPA also localizes to the adventitia, possibly related to its release by endothelial cells of the vasa vasorum [62]. As for PAI-1, it has been highly stained in the luminal layer of the ILT but most readily released from the medial and abluminal layers consistent with its strong affinity for the γγ-fibrin chain, which is intact in the luminal layer but degraded in deeper layers [62]. Given the significant ability of the fibrinolytic system both to remodel the ILT and regulate proteolysis of the extracellular matrix of the underlying wall, the spatiotemporal distribution of plasmin, its activators, and its inhibitors represent key data that could improve future patient-specific models of AAA progression and rupture risk.
4.5. A Unique Role for Matrikines?
Despite appropriate emphasis on proteolytic enzymes that directly affect the remodeling of fibrin and extracellular matrix, degradation products of matrilysis (including elastin [72], laminin [73], and fibrin [74]) are not simply removed. Rather, these products may serve as active signaling and catalytic molecules, termed matrikines [54], which help regulate inflammation, repair, angiogenesis, and other processes. Of these, the elastin degradation products (EDPs) are likely of particular significance in AAAs given their chemotaxis for monocytes, neutrophils, fibroblasts, and endothelial cells [75], stimulation of the release of elastase in the wall by neutrophils [52], augmentation of MMP-1 production by aortic SMCs, promotion of angiogenesis [75], and influence on the differentiation of Th1 cells that can upregulate interferon-γ (IFN-γ) and interleukin-2 (IL-2) [48].
Interestingly, Nackman et al. [75] demonstrated that perfusion of a segment of the infrarenal aorta in a rat with an elastin peptide fragment (VGVAPG) could reproduce the extensive neovascularization of the wall that occurs in a classic rodent model of AAAs (by direct perfusion of the aorta with elastase), despite the aortic diameter increasing only 26% following perfusion with the peptide fragment instead of 142% with elastase. This importance of elastin breakdown products stimulating neovascularization (as opposed to just hypoxia from a thickening ILT) may explain, in part, why significant differences in neovascularization were noted between aneurysmal walls and control aorta but not between thick versus thin thrombus covered aneurysmal walls [65] or thrombus covered versus noncovered aneurysmal walls [76]. Faisal et al. [73] similarly showed that a laminin peptide found in AAAs, due to release from the degrading ECM, can upregulate uPA and MMP-9 in macrophages. Notably, laminin is a susceptible target of plasmin degradation [77]. Finally, fibrinogen derived peptides may increase leukocyte chemotaxis and permeability of the aneurysmal wall [78].
In healthy aorta, the media has been considered an immunologically “privileged” site to which immune cells have minimal access [79,80]; yet, when the well-organized elastic lamellar units are disrupted, otherwise inaccessible antigens and degradation products may become exposed and participate in the observed shift from innate to adaptive immunity in AAAs. In addition to EDPs, these local mediators may include post-translationally modified proteins, reactive oxygen species (ROS), and bi-products of SMC apoptosis [78]. Proteolysis of extracellular matrix can also release and activate transforming growth factor β (TGF-β), a key biomolecule in the regulation of collagen synthesis and secretion [78]. Notably, plasmin at cell surfaces is an activator of TGF-β [81], and whether via TGF-β or other factors, increase in pro-collagen gene expression in AAAs is mediated by soluble tissue factors [82]. Indeed, even in regions beyond the infrarenal aorta, AAAs have been associated with increased aortic collagen, elevated expression of MMP-2 by SMCs, diffuse arteriomegaly, arterial elongation, and peripheral aneurysms, which suggest a widespread process [82–85]. Whether this process is causal or responsive to the aneurysm is yet unknown.
4.6. Convection/Diffusion.
Adding to the complexity of identifying the major proteolytic enzymes and signaling molecules that influence AAA progression, these macromolecules likely convect/diffuse through the canaliculi of the ILT toward the underlying wall. Adolph et al. [39] showed, for example, that ILT is fluid permeable, although diffusion becomes limited across very thick thrombi. Levels of LE and MMP-9, which are produced predominately in the active luminal layer, decrease through the depth of the ILT [56]. Similarly, proteases, degradation products, antigens, and other soluble biomolecules may be transported radially through the aneurysmal wall toward the adventitia, which can play a major role in arterial responses to disease, including fibroblast activation, neovascularization, immunoinflammation, and fibrosis [78]. It is also likely that the permeability increases as the endothelial lining is lost [76] and the organized structure of the healthy aortic wall is degraded [51]. Along these lines, recent attempts at modeling the thrombus and aneurysmal wall as porohyperelastic structures [86,87] may provide insight into the complex interstitial fluid flows in thrombus-laden AAAs. Thus, a complete model of the biochemical influences of various mediators may require accurate quantification of the transmural hydraulic conductance, diffusion down concentration gradients, binding affinities, and wall permeability [78].
4.7. Role of Animal Models.
In concluding our discussion of biochemical processes in ILT, note that diverse animal models have been used to study AAAs because of the difficulty of obtaining human tissue, particularly under controlled longitudinal conditions. The two most common animal models of AAAs that can also lead to formation of thrombus are the angiotensin II (Ang-II) infusion model [88] and the elastase intraluminal perfusion model [89]. The former involves subcutaneous implantation of an osmotic mini-pump whereas the latter involves direct perfusion of the infrarenal aorta during open surgery. Note that the Ang-II model is typically used in Apo-E deficient mice and produces dissecting aneurysms in the suprarenal aorta, as opposed to dilatory aneurysms in the infrarenal aorta as classically seen in humans. Moreover, intramural thrombi within the dissected wall in the Ang-II model can heal through cellular invasion and eventual replacement of the fibrin matrix with collagen [61]. Thus, the natural history of AAAs in the Ang-II infusion model may have distinct differences from the human pathology, particularly in terms of thrombus formation and evolution.
In contrast, although the elastase perfusion model tends not to yield ILT in mice, the same experiment in rats often does, and the aneurysms are appropriately in the infrarenal aorta. Using this model, Coutard et al. [71] demonstrated higher levels of MMP-9, elastase, uPA, plasmin, and microparticles in the ILT compared with the wall, while MMP-2 activation was similar. Interestingly, ILT correlated positively with wall levels of pro- and active MMP-2, elastase, and plasmin, whereas total wall MMP-9, MMP-2 activation, plasmin activity, and microparticle release correlated with aneurysm diameter. The potential importance of these and other factors in future modeling and therapeutic efforts is highlighted by the attenuation of aneurysmal development in various experimental models of AAA by depletion of neutrophils, T-cells, macrophages, or mast cells [76,90], inhibition of platelet activation [91], inhibition of neutrophil recruitment [92,93], knockout of plasminogen [67], deficiency of uPA [94], knockout of MMP-2, -9, or -12 [95,96], or increases in PAI-1 [97], TIMP-1 [98], or catalase [99].
5. Role of ILT in AAA Wall Mechanics
5.1. Clinical and Experimental Observations.
While our primary focus thus far has been on biomechanical properties of and biochemical processes within the ILT itself, numerous studies have attempted to determine effects of the ILT on the underlying wall. Compared to a thrombus-free wall, the wall beneath an ILT may have increased inflammation and neovascularization as well as fewer elastic fibers and smooth muscle cells, many of which are apoptotic [100]. An increase in phenotypically synthetic SMCs, many with elongated processes suggesting ongoing migration, has also been noted [76,100]. In comparison, an aneurysmal wall without ILT can have a dense collagenous matrix with phenotypically contractile SMCs and increased staining for α-actin [100], although expression of MMP-1, -7, -9, and -12 can be higher than in the covered wall [101]. While both thrombus-covered and thrombus-free wall exhibit increased CD68+ macrophage counts and caspase-3 staining for apoptosis, the thrombus-covered wall can also have increased CD3+ T-cells, CD8+ cytotoxic T-cells, and CD20+ B-cells, along with increased TUNEL staining for DNA fragmentation in the intima and media, often in association with inflammatory cells [100]. Clearly, there are significant differences in the structure of the matrix, cellular content, and inflammatory status of the wall underneath an ILT that require further investigation to improve future modeling.
Interestingly, the thrombus has been found to be thinner, on average, in ruptured versus nonruptured aneurysms [52], though thick thrombus covered walls may have fewer remaining elastic fibers, more inflammation, and lower tensile strength compared with walls covered with thin ILT [65]. A possible reason for this apparent discrepancy is that any wall covered by thick thrombus likely was originally covered by thin thrombus (i.e., a luminal layer in direct contact with the wall); thus, changes may reflect cumulative damage from the past because intact lamellae are not regenerated in adulthood. One may hypothesize, therefore, that the most active degeneration of the wall occurs in regions of thin thrombus and that thickening of the ILT with subsequent deposition events may be somewhat protective, at least insofar as the wall is farther from the active luminal layer. In fact, if a thin thrombus presents excess proteases to the wall, which in turn heightens wall degeneration and lesion expansion, and if expansion leads to hemodynamic changes conducive to new clot deposition, then one would naturally expect thrombus to thicken in regions of significant wall degeneration (provided that a rupture does not occur first). Indeed, loss of elastin may primarily cause a decrease in axial support, leading to an elongation and tortuosity of the aorta that may induce endothelial injury due to disturbed blood flow and abnormal wall stress [48]. Thus, despite an increased aneurysmal diameter correlating with both increased rupture risk and ILT volume in some reports [48,55], one must interpret cautiously possible causation as the expanding geometry and associated hemodynamic changes may naturally precipitate further ILT deposition. Nevertheless, the growth rate of ILT in moderately sized AAAs may be a better predictor of rupture risk than total amount of ILT or the common metric of maximum diameter of the lesion [102], which potentially explains why a simple static ratio of lesion size to ILT volume has been unrevealing when comparing ruptured and nonruptured AAAs [48,103].
Alternatively, or perhaps complementarily, the fibrinolysis that predominates in the abluminal layer may release and activate previously bound or soluble proteases (e.g., plasmin or MMPs) that could then diffuse into the adjacent wall and directly degrade the ECM or activate other proteases, thus contributing to the increased degradation behind thicker thrombi. Additionally, thick thrombi may also exacerbate hypoxic conditions in the wall that may contribute to neovascularization from the vasa vasorum or even alter collagen production [65,104], though a convection/diffusion model of oxygen transport by Sun et al. [105] suggested that thickening of the ILT beyond approximately 5 mm may not have much more effect on mural oxygen tension. Of note, formation of these neovessels not only requires proteases for endothelial cell invasion, it establishes a direct path for circulating inflammatory cells to invade the degenerating media. Indeed, inflammatory cell retention, elastin degradation, and lymphoid neogenesis all correlate with adventitial neovascularization [78].
5.2. Implications of Spatial Distribution—The Shoulder and Neck Regions.
Multiple studies have focused on the shoulder regions of the AAA (i.e., proximal and distal regions between the dilated and nondilated aorta) as they may both reflect “early” stages of aneurysmal development (which are difficult to study due to the asymptomatic nature of the early disease process) and represent regions that may dictate the axial expansion of the lesion into the proximal and distal necks (i.e., the adjacent nondilated areas) of the infrarenal aorta. Moreover, the proximal neck is particularly important clinically as the location for seating the endovascular stents that are increasingly used for AAA repair.
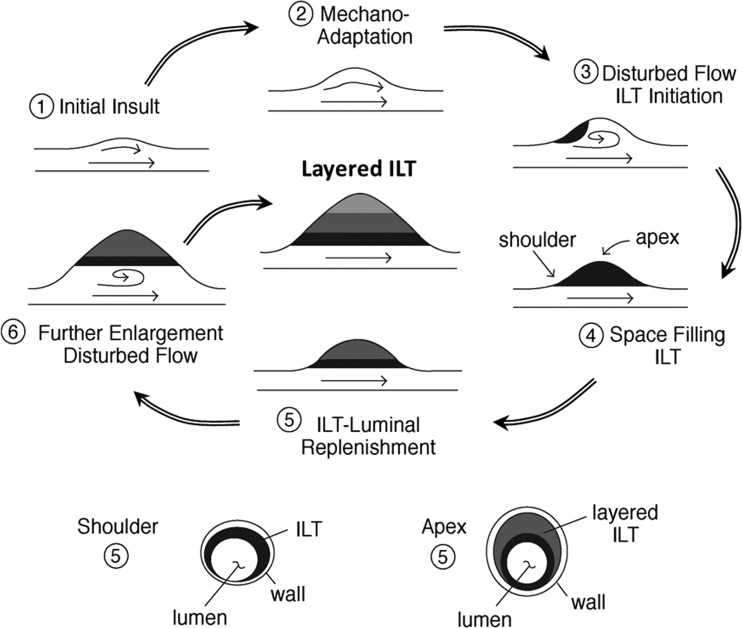
Assuming the thrombus approximately “space-fills” the dilated portions of the body of an AAA (see Fig. 6), the active luminal layer of the ILT would always remain in contact with the evolving shoulder of an aneurysm. As suggested by Yoshimura et al. [106], this situation would predict increased thrombus-derived proteases in the shoulders of the lesion. Indeed, Curci et al. [58] observed that areas of “active” elastic lamellar degradation predominated in the proximal shoulder region, while the body of the lesion consisted of mostly “amorphous” fibrocollagenous regions with few remaining elastic fibers and only scattered “inflammatory” and active regions. The shoulder also appears to be a site of increased MMP-9 activity, decreased medial thickness, fewer intact lamellar units, and increased phosphorylated c-Jun N-terminal kinase (JNK), inflammation, and neovascularization [66]. Notably, JNK may be a pivotal intracellular signaling molecule in SMCs and macrophages that can be upregulated by a plethora of biomolecules frequently associated with AAAs, including MMP-2 and 9, Ang-II, histamine, interleukins, IFN-γ, and tumor necrosis factor α (TNF-α) [66,76]. Interestingly, expression of both TNF-α and its activating enzyme TACE are not only higher in AAAs compared to control aortas, they are also higher in the neck compared with the apex of the lesion, where they colocalize with macrophages of the media and adventitia. TNF-α can also induce other inflammatory cytokines and several MMPs, promote apoptosis, and increase uPA, tPA, and PAI-1 levels (which are pro-angiogenic) [107]. The delivery of a TNF-α specific binding protein has even been shown to prevent AAA formation in a rat elastase perfusion model [107].
Fig. 6.
Top: Simplified schema of the possible formation of a layered “space filling” ILT by multiple cycles of AAA sac enlargement and ILT deposition secondary to disturbed flow. Note that the specific initiation site in 3 is merely schematic. Bottom: Cross-sectional view at two different axial locations at one instant, when the luminal ILT layer (black) may remain in contact with the wall at the shoulder region while being distant from the anterior wall at the apex of the lesion. (Diagram by Carolyn Valentín).
Early damage to the aortic wall is not confined to the shoulder, however. Increased degeneration in the nonaneurysmal neck region has been associated with maximum AAA diameter [66], and the neck can be a site of early inflammatory cell infiltration with elevated levels of MMP-12 strongly bound to elastic fiber fragments, which may augment its elastolytic activity [58]. In comparing samples of aneurysmal necks from different patients, those with mild degeneration were characterized by damage to inner medial elastin with some mid-medial and patchy outer-medial loss of SMCs, while more heavily damaged samples showed widespread degeneration with significant loss of smooth muscle in the outer media [66]. This observation may suggest an initial process of damage to the inner wall, particularly to the elastic fibers, followed by a strong adventitial response.
Computationally, many finite element studies suggest that the highest values of wall stress occur at sites other than the maximal diameter. Li et al. [108] specifically suggested that elevated shoulder stress associates with rapidly expanding AAAs. Our own growth and remodeling simulations [109] also suggest that the shoulder represents a region where elastin may fail mechanically as an aneurysm enlarges, thus leading to steep gradients in stress. Our computational models of AAAs expanding axially against fixed ends (e.g., at the renal arteries and iliac bifurcations) also predict decreasing axial stress in the neck and shoulder regions, which suggests a potentially increased risk of tortuosity (e.g., buckling) [110]. Consistent with the idea that elongation and tortuosity may result from elastin degradation [48], our computational results may potentially link changes in the neck and shoulder regions with the induction of disturbed flow and subsequent thrombus deposition. Interestingly, hemodynamic simulations within our lab and others also demonstrate regions of backflow in the proximal neck of the aneurysm, which may convect important biomolecules, including proteases and inflammatory mediators, from nearby thrombus to the proximal neck. Indeed, NGAL-MMP-9 complexes, which are abundant in the luminal layer, have been found in the neck [66], though they might also originate from early infiltrating leukocytes in the wall as well. In addition, thrombin and fibrin from the nearby thrombus may activate endothelial cells, which are still present in the neck and possibly shoulder regions, to increase production of tPA and uPA [24] and thus potentially affect ILT remodeling.
We suggest, therefore, that particular attention be given to the neck and shoulder regions of the AAA, including their biomechanical and biochemical properties, their role in the axial expansion of the aneurysm, and their relation to nearby thrombus. Future data regarding changes in these regions may provide important clues to understand early aneurysm formation and tortuosity that may ultimately guide new therapeutics, such as stent seating and design, and improve future G&R models, which must account for the evolving nonaneurysmal aortic regions in the modeling domain.
5.3. ILT Shielding, Attachment, and Compressibility.
It has long been appreciated that mathematical models of the aneurysmal wall can provide important insight into the biomechanics, and thus structural stability, of these potentially life-threatening lesions. While the earliest models did not include ILT, their incorporation into models of AAAs has developed over the past two decades from axisymmetric [111,112] to 2D eccentric [40] and finally to fully 3D patient-specific geometries [113–116]. The majority of these computational studies, as well as an in vitro experimental study using a synthetic AAA model with thrombus [117], support the initial work by Inzoli et al. [111] that an ILT may provide a mechanically protective effect (i.e., a “cushioning” or “shielding”) by reducing the stress on the aneurysmal wall; yet, others question the ability of the porous thrombus to reduce wall stress consistently, as assessed computationally [118] or by direct intraoperative pressure measurements in vivo [119]. Clearly, controversy remains.
Two important factors in determining the presence or extent of this potential stress shielding are the attachment of the thrombus to the underlying wall and the degrees of porosity and compressibility of the ILT. Adhesion of platelets to collagen has been investigated extensively, but connection of fibrin to the aneurysmal wall has not. As noted earlier, several papers report a “liquid phase” at the interface between the ILT and wall [37,48], suggesting that the thrombus may not be attached to the wall, at least in some instances. The type of thrombus layer in contact with the wall may also affect the degree of attachment; that is, an actively remodeling thin luminal layer in direct contact with an aneurysmal wall could be attached differently than a degenerated abluminal layer as observed in many thick ILT. Most finite element analyses that include ILT have assumed its perfect attachment to the wall. While a potential reduction of intraluminal pressurization of the wall by the ILT could reduce wall stress, Meyer et al. [118] showed that the thrombus can still have a stress-reducing role even if it does not directly reduce pressurization on the wall as long as the ILT is fully attached. With only partial attachment, however, a porous thrombus capable of transmitting pressure also has the potential to generate stress concentrations and increase wall stress. Clearly, there is a pressing need for greater experimental insight to resolve this question since any estimation of stress distributions, and thus rupture risk, will depend on the mechanical tethering of the ILT and wall.
The compressibility of the ILT will also directly affect its mechanical behavior and its ability to shield the aneurysmal wall. Using noninvasive ultrasound, Vorp et al. [44] suggested that ILT is incompressible, and the vast majority of computational works to date have invoked incompressibility when modeling ILT. Yet, some question this assumption. In vitro experiments on fibrin gels demonstrated a dramatic decrease in clot volume due to water expulsion (and thus manifold increase in protein content) with negative compressibility upon stretching [120]. This volume change may be associated with the exposure of hydrophobic groups upon protein unfolding and bundling of stretched fibrin fibers. Of note, the compressibility of the fibrin gels was limited to strains of ∼0.5, as further stretching did not alter volume. Provocatively, an in vivo study by Truijers et al. [121] that quantified volumes of lumen, intraluminal thrombus, and whole AAA through the cardiac cycle in 17 patients using dynamic electrocardiographically-gated CT angiography showed wide patient-specific variability (0.4% to 43.6% compressibility by volume between diastole and peak systole). No correlation was found between compressibility of thrombus and aneurysm size, thrombus volume, or pulse pressure. Since this study analyzed the volume of thrombus within the entire aneurysm, no distinction can be made between the compressibility of the heterogeneous layers of ILT that may possess different inherent mechanical properties and times of deposition; nevertheless, this study strongly suggests that not all ILT are incompressible. Furthermore, since thrombus is constantly evolving, the compressibility of a given ILT may change over time. The potentially evolving compressibility of ILT may not only affect its stress-shielding of the wall (and require a reformulation of the constitutive relations used for its mechanical modeling), it may also significantly affect the transport of key biomolecules produced by the ILT. Clearly, studies are needed to determine the compressibility, attachment, and stress buffering capability of ILT in a patient-specific manner, as some ILT may provide significant shielding effects while others may not.
5.4. Strength and Failure of the ILT and Wall.
Aneurysms rupture when local wall stress exceeds wall strength [3]. Hence, including the potential ability of ILT to alter spatiotemporally both the mechanical stress and the inherent strength of the underlying aneurysmal wall may improve our ability to predict patient-specific rupture risk. Indeed, Vande Geest et al. [122] already proposed ILT thickness as a key parameter in formulating a statistical estimate of wall strength, and Tong et al. [12] showed that aneurysmal wall underneath older thrombus was generally weaker in the high loading domain during biaxial testing compared with that under young thrombus.
Gasser et al. [123] tested the hypothesis that mechanical failure of ILT itself could lead to subsequent AAA rupture. They subjected specimens of luminal, medial, and abluminal ILT to uniaxial fatigue and strength tests and concluded that ILT fails before the wall. Indeed, CT evidence suggests that focal hemorrhage into the ILT, described radiographically as a “crescent sign” (Fig. 5, Ref. [124]), associates with an increased risk of rupture or impending rupture [54]. Such a failure could not only expose the wall to increased stress (if the assumption of stress-shielding by the ILT is true), the fresh hemorrhage into the ILT could also weaken the underlying wall structurally via the delivery and release of active proteases (as discussed earlier), both of which may increase rupture risk.
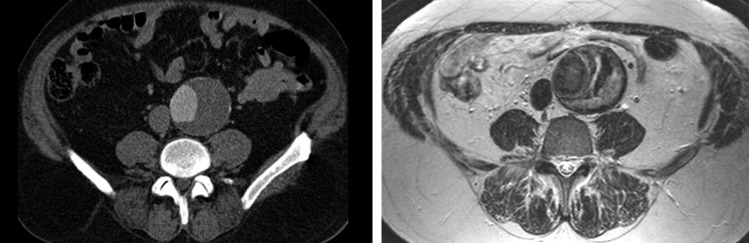
In addition to revealing the crescent sign, medical imaging may provide further insights into the mechanics and interactions of the ILT and aortic wall that could significantly advance the assessment of rupture risk by revealing patient-specific properties of the thrombus and wall noninvasively. For example, Nchimi et al. [125] reported a correlation of phagocytic leukocytes and changes in magnetic resonance signal via the use of superparamagnetic iron oxide (SPIO) at the luminal interface of the thrombus, thus suggesting a potential means of determining activity of the luminal layer. Later, Richards et al. [126] showed that distinct mural uptake of ultrasmall SPIO (or USPIO) in the aneurysmal wall associated with a three-fold higher expansion rate than no or nonspecific USPIO uptake despite similar maximum diameters. This finding is consistent with the idea that active areas of inflammation augment the degradation and remodeling of the extracellular matrix and hence influence expansion. Moreover, MRI may help differentiate distinct layers of ILT within some aneurysms in vivo (e.g., see Fig. 7 [124]). Whether these layers correspond to the biochemically and/or biomechanically distinct layers discussed herein requires future investigation. Nevertheless, the potential for medical imaging to inform the next generation of patient-specific G&R models of AAAs holds significant promise.
Fig. 7.
Comparison of a contrast-enhanced CT image (left) and a T2-weighted MR image (right) of an AAA in a 49 year-old male. Note the clear layers of the ILT evident in the MRI that are not delineated by CT. From Labruto et al. [124], with permission.
6. Modeling Aneurysmal Growth and Remodeling
As the word itself implies, biomechanics requires an understanding and description of both the biological and the mechanical aspects of a system. In this review, we have attempted to highlight both of these aspects in regards to intraluminal thrombus in abdominal aortic aneurysms. What remains, however, is a merging of the two into a computational framework capable of quantifying and predicting how the biology and mechanics interrelate. For example, hyperelasticity may be used to describe a thrombus at a given instant, but such a description could just as easily be assigned to a nonliving polymer gel. What distinguishes the metabolically active thrombus from the inanimate gel is the ability of the incorporated platelets and cells to actively change the mass and interconnectedness of the fibrin matrix over time, that is, to grow and remodel. A G&R approach to modeling biological systems thus has the potential to describe evolving mechanical properties (e.g., mass, stiffness, failure criteria) over physiologically relevant timeframes as a function of local biological, chemical, and mechanical factors such as cellular content, protease levels, and hemodynamic loads. Ultimately, this is the challenge facing the clinician—not only determining immediate risk of rupture but also risk over the subsequent months to years so that the timing of follow-up with reimaging or surgical intervention may be selected appropriately for a given patient.
The first G&R model to describe AAA evolution was presented by Watton et al. [127] in 2004. They modeled the wall as a two layered cylindrical membrane described by a constrained mixture [128], with structural properties determined by the nonlinearly elastic collagen and elastin. They considered the development of both axisymmetric and asymmetric aneurysms caused by prescribed focal degradations of elastin, with potential limitation of posterior expansion due to the spine. This G&R framework was later extended to include fluid-solid-growth (FSG) modeling by Watton et al. [129] in 2009.
Other recent models [109,110,130,131] also employed the concept of evolving constrained mixtures to investigate AAA mechanics but with different constitutive functions and implementations. For example, instead of using a “recruitment stretch” to characterize when collagen becomes engaged mechanically, they used the concept of a “deposition stretch” to model incorporation of prestressed fibers within the in vivo geometry. Since new fibers are always added at a prescribed homeostatic deposition stretch while old fibers are removed, the wall as a whole can reestablish a homeostatic state provided that the forces favoring expansion (e.g., intravascular pressure and degradation of extracellular matrix) do not exceed the capacity of the wall to produce new material. Wilson et al. [110] further suggested that the range of potential clinical endpoints (arrest, continued expansion, or rupture) depended strongly on G&R parameters governing collagen turnover (e.g., production rates, degradation rates, deposition stretch, and stiffness). A more detailed review of past finite element studies of AAA wall stress and growth and remodeling can be found in Humphrey and Holzapfel [2], and a review of the need for fluid-solid-growth models of AAAs can be found in Humphrey and Taylor [1].
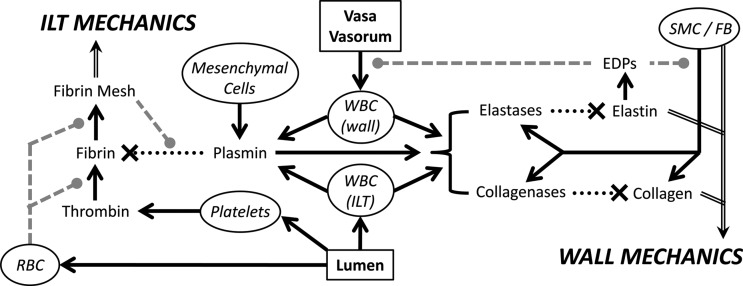
Nevertheless, current models of aneurysmal G&R do not include directly the biochemical effects of intraluminal thrombus. For example, the aforementioned release of plasmin in a particular region of the ILT may activate available proMMPs and lead to a local change in the G&R parameter controlling the degradation rate of collagen. At the same time, the now active MMPs could activate latent TGF-β that had been sequestered in the extracellular matrix and, thereby, increase the G&R parameter governing growth factor mediated collagen production. We suggest, therefore, that there is a pressing need to develop biochemomechanical constitutive models to correlate the spatiotemporal activity of key proteases and mediators (e.g., plasmin, MMPs, elastase, TGF-β, TIMPs) produced and activated by the ILT and wall with the governing G&R parameters that control the turnover of the primary load-bearing constituents within the wall, most notably, collagen, elastin, and smooth muscle. Indeed, similar models of the turnover of load-bearing constituents within the ILT will also be needed [132]. Given the inherent complexities in the biology and mechanical modeling, advances will likely need to proceed stepwise, building from lumped parameter models towards eventual multiscale, multiphysics models. Towards this end, we present an initial schema (Fig. 8) outlining the interactions of key contributors likely affecting the growth and remodeling of thrombus-laden AAAs. Indeed, the ability to identify and measure such lumped parameters noninvasively and patient-specifically (e.g., leukocyte content of the ILT by USPIO uptake or collagenase activity in the wall by MMP-targeted MRI agents) is increasingly becoming available to provide the necessary data to inform the next generation of biochemomechanical G&R models of AAAs.
Fig. 8.
Schema of some of the primary effectors governing the evolution of the mechanics of both the ILT and aneurysmal wall. Solid black arrow – “increases the amount/activity,” dotted black line—“degrades,” dashed gray line—“modulates the effect.” (ILT: intraluminal thrombus, SMC: smooth muscle cell, FB: fibroblast, WBC: white blood cell, RBC: red blood cell).
7. Conclusions
We have attempted to draw attention to complementary biological, chemical, and mechanical characteristics of ILT and their potential roles in the natural history of AAAs. Given this background, we suggest that appropriate biochemomechanical models of the G&R of thrombus-laden AAAs can be constructed using existing theoretical frameworks, including the constrained mixture theory [128]. These models require three classes of constituent-specific constitutive relations: stored energy functions that describe the mechanical behaviors plus production and removal functions that describe chemomechanical mediated turnover of structural constituents. To encourage experimentation that will provide the requisite data for developing and validating such models, we suggest four aspects of the evolution of thrombus-laden AAAs that require continued investigation:
-
(i)
Structurally motivated stress-strain relations—quantify the nonlinear heterogeneous mechanical properties of both the ILT and aneurysmal wall, preferably including constituent specific correlations with histological characteristics as a lesion evolves.
-
(ii)
Thrombus deposition and evolution—identify criteria that govern the initial and subsequent hemodynamically influenced deposition of thrombus and its evolving properties, including its compressibility, attachment to the wall, and continual renewal of the luminal layer but degradation of underlying layers.
-
(iii)
Biomolecular kinetics—identify key biomolecules, or classes of biomolecules, that affect the G&R of the ILT and AAA, including cell sources and relevant rates of production, activation, inhibition, convection/diffusion, and degradation.
-
(iv)
Failure/rupture—quantify regionally the strength of the ILT and aneurysmal wall and formulate models of failure that include the propagation of dissections or fissures.
Experimental advances and constitutive formulations along these lines would significantly enhance our ability to develop fluid-solid-growth (FSG) models that will bring us closer to understanding and predicting the complex, biochemomechanical effects of ILT on AAA progression, with the ultimate goal of improving patient-specific risk stratification and clinical outcome. Appreciating early on the underlying complexity of this serious vascular disease is essential, however, to establish the scope of the problem and to coordinate effectively future experimental and computational investigations.
Acknowledgment
This work was supported, in part, by NIH Grants R01 HL086418 and R21 HL107768 and an Installation Grant from the Croatian Science Foundation.
Contributor Information
J. S. Wilson, Department of Biomedical Engineering, Yale University, New Haven, CT 06520
L. Virag, Faculty of Mechanical Engineering and Naval Architecture, University of Zagreb, 10000 Zagreb, Croatia
P. Di Achille, Department of Biomedical Engineering, Yale University, New Haven, CT 06520
I. Karšaj, Faculty of Mechanical Engineering and Naval Architecture, University of Zagreb, 10000 Zagreb, Croatia
J. D. Humphrey, Fellow ASME, Department of Biomedical Engineering, Yale University, New Haven, CT 06520; Vascular Biology and Therapeutics Program, Yale School of Medicine, New Haven, CT 06520, e-mail: jay.humphrey@yale.edu.
References
- [1]. Humphrey, J. D. , and Taylor, C. A. , 2008, “Intracranial and Abdominal Aortic Aneurysms: Similarities, Differences, and Need for a New Class of Computational Models,” Annu. Rev. Biomed. Eng., 10, pp. 221–246. 10.1146/annurev.bioeng.10.061807.160439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Humphrey, J. D. , and Holzapfel, G. A. , 2012, “Mechanics, Mechanobiology, and Modeling of Human Abdominal Aorta and Aneurysms,” J. Biomech., 45(5), pp. 805–814. 10.1016/j.jbiomech.2011.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Vorp, D. A. , 2007, “Biomechanics of Abdominal Aortic Aneurysm,” J. Biomech., 40(9), pp. 1887–1902. 10.1016/j.jbiomech.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Salsac, A. V. , Sparks, S. R. , and Lasheras, J. C. , 2004, “Hemodynamic Changes Occurring During the Progressive Enlargement of Abdominal Aortic Aneurysms,” Ann. Vasc. Surg., 18(1), pp. 14–21. 10.1007/s10016-003-0101-3 [DOI] [PubMed] [Google Scholar]
- [5]. Salsac, A.-V. , Sparks, S. R. , Chomaz, J. M. , and Lasheras, J. C. , 2006, “Evolution of the Wall Shear Stresses During the Progressive Enlargement of Symmetric Abdominal Aortic Aneurysms,” J. Fluid Mech., 550, pp. 19–51. 10.1017/S002211200600036X [DOI] [Google Scholar]
- [6]. Bluestein, D. , Niu, L. , Schoephoerster, R. T. , and Dewanjee, M. K. , 1996, “Steady Flow in an Aneurysm Model: Correlation Between Fluid Dynamics and Blood Platelet Deposition,” J. Biomech. Eng., 118(3), pp. 280–286. 10.1115/1.2796008 [DOI] [PubMed] [Google Scholar]
- [7]. Les, A. S. , Shadden, S. C. , Figueroa, C. A. , Park, J. M. , Tedesco, M. M. , Herfkens, R. J. , Dalman, R. L. , and Taylor, C. A. , 2010, “Quantification of Hemodynamics in Abdominal Aortic Aneurysms During Rest and Exercise Using Magnetic Resonance Imaging and Computational Fluid Dynamics,” Ann. Biomed. Eng., 38(4), pp. 1288–1313. 10.1007/s10439-010-9949-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. O'Rourke, M. J. , McCullough, J. P. , and Kelly, S. , 2012, “An Investigation of the Relationship Between Hemodynamics and Thrombus Deposition Within Patient-Specific Models of Abdominal Aortic Aneurysm,” Proc. Inst. Mech. Eng., Part H: J. Eng. Med., 226(7), pp. 548–564. 10.1177/0954411912444080 [DOI] [PubMed] [Google Scholar]
- [9]. Biasetti, J. , Hussain, F. , and Gasser, T. C. , 2011, “Blood Flow and Coherent Vortices in the Normal and Aneurysmatic Aortas: A Fluid Dynamical Approach to Intra-Luminal Thrombus Formation,” J. R. Soc., Interface, 8(63), pp. 1449–1461. 10.1098/rsif.2011.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Arzani, A. , and Shadden, S. C. , 2012, “Characterization of the Transport Topology in Patient-Specific Abdominal Aortic Aneurysm Models,” Phys. Fluids, 24(8), p. 081901. 10.1063/1.4744984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Gersh, K. C. , Nagaswami, C. , and Weisel, J. W. , 2009, “Fibrin Network Structure and Clot Mechanical Properties are Altered by Incorporation of Erythrocytes,” Thromb. Haemostasis, 102(6), pp. 1169–1175. 10.1160/TH09-03-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Tong, J. , Cohnert, T. , Regitnig, P. , and Holzapfel, G. A. , 2011, “Effects of Age on the Elastic Properties of the Intraluminal Thrombus and the Thrombus-Covered Wall in Abdominal Aortic Aneurysms: Biaxial Extension Behaviour and Material Modelling,” Eur. J. Vasc. Endovasc. Surg., 42(2), pp. 207–219. 10.1016/j.ejvs.2011.02.017 [DOI] [PubMed] [Google Scholar]
- [13]. Scott, D. J. , Prasad, P. , Philippou, H. , Rashid, S. T. , Sohrabi, S. , Whalley, D. , Kordowicz, A. , Tang, Q. , West, R. M. , Johnson, A. , Woods, J. , Ajjan, R. A. , and Ariëns, R. A. , 2011, “Clot Architecture is Altered in Abdominal Aortic Aneurysms and Correlates With Aneurysm Size,” Arterioscler., Thromb., Vasc. Biol., 31(12), pp. 3004–3010. 10.1161/ATVBAHA.111.236786 [DOI] [PubMed] [Google Scholar]
- [14]. Takagi, H. , Manabe, H. , Kawai, N. , Goto, S. N. , and Umemoto, T. , 2009, “Circulating Lipoprotein(a) Concentrations and Abdominal Aortic Aneurysm Presence,” Interact Cardiovasc. Thorac. Surg., 9(3), pp. 467–470. 10.1510/icvts.2009.208843 [DOI] [PubMed] [Google Scholar]
- [15]. Pulinx, B. , Hellenthal, F. A. , Hamulyák, K. , van Dieijen-Visser, M. P. , Schurink, G. W. , and Wodzig, W. K. , 2011, “Differential Protein Expression in Serum of Abdominal Aortic Aneurysm Patients—A Proteomic Approach,” Eur. J. Vasc. Endovasc. Surg., 42(5), pp. 563–570. 10.1016/j.ejvs.2011.07.019 [DOI] [PubMed] [Google Scholar]
- [16]. Mann, K. G. , 2003, “Thrombin Formation,” Chest, 124(3 Suppl), pp. 4S–10S. 10.1378/chest.124.3_suppl.4S [DOI] [PubMed] [Google Scholar]
- [17]. Weisel, J. W. , 2004, “The Mechanical Properties of Fibrin for Basic Scientists and Clinicians,” Biophys. Chem., 112(2–3), pp. 267–276. 10.1016/j.bpc.2004.07.029 [DOI] [PubMed] [Google Scholar]
- [18]. Weisel, J. W. , 2007, “Structure of Fibrin: Impact on Clot Stability,” J. Thromb. Haemost., 5(Suppl 1), pp. 116–124. 10.1111/j.1538-7836.2007.02504.x [DOI] [PubMed] [Google Scholar]
- [19]. Furie, B. , and Furie, B. C. , 2007, “ In Vivo Thrombus Formation,” J. Thromb. Haemost., 5(Suppl 1), pp. 12–17. 10.1111/j.1538-7836.2007.02482.x [DOI] [PubMed] [Google Scholar]
- [20]. Gersh, K. C. , Edmondson, K. E. , and Weisel, J. W. , 2010, “Flow Rate and Fibrin Fiber Alignment,” J. Thromb. Haemost., 8(12), pp. 2826–2828. 10.1111/j.1538-7836.2010.04118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Varjú, I. , Sótonyi, P. , Machovich, R. , Szabó, L. , Tenekedjiev, K. , Silva, M. M. , Longstaff, C. , and Kolev, K. , 2011, “Hindered Dissolution of Fibrin Formed Under Mechanical Stress,” J. Thromb. Haemost., 9(5), pp. 979–986. 10.1111/j.1538-7836.2011.04203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Xu, Z. , Kamocka, M. , Alber, M. , and Rosen, E. D. , 2011, “Computational Approaches to Studying Thrombus Development,” Arterioscler., Thromb., Vasc. Biol., 31(3), pp. 500–505. 10.1161/ATVBAHA.110.213397 [DOI] [PubMed] [Google Scholar]
- [23]. Lobanov, A. I. , and Starozhilova, T. K. , 2005, “The Effect of Convective Flows on Blood Coagulation Processes,” Pathophysiol. Haemost. Thromb., 34(2–3), pp. 121–134. 10.1159/000089932 [DOI] [PubMed] [Google Scholar]
- [24]. Anand, M. , Rajagopal, K. , and Rajagopal, K. R. , 2005, “A Model for the Formation and Lysis of Blood Clots,” Pathophysiol. Haemost. Thromb., 34(2–3), pp. 109–120. 10.1159/000089931 [DOI] [PubMed] [Google Scholar]
- [25]. Xu, Z. , Chen, N. , Kamocka, M. M. , Rosen, E. D. , and Alber, M. , 2008, “A Multiscale Model of Thrombus Development,” J. R. Soc., Interface, 5(24), pp. 705–722. 10.1098/rsif.2007.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Xu, Z. , Lioi, J. , Mu, J. , Kamocka, M. M. , Liu, X. , Chen, D. Z. , Rosen, E. D. , and Alber, M. , 2010, “A Multiscale Model of Venous Thrombus Formation With Surface-Mediated Control of Blood Coagulation Cascade,” Biophys. J., 98(9), pp. 1723–1732. 10.1016/j.bpj.2009.12.4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Biasetti, J. , Spazzini, P. G. , Swedenborg, J. , and Gasser, T. C. , 2012, “An Integrated Fluid-Chemical Model Toward Modeling the Formation of Intra-Luminal Thrombus in Abdominal Aortic Aneurysms,” Front. Physiol., 3, pp. 266–270. 10.3389/fphys.2012.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Leiderman, K. , and Fogelson, A. L. , 2011, “Grow With the Flow: A Spatial-Temporal Model of Platelet Deposition and Blood Coagulation Under Flow,” Math. Med. Biol., 28(1), pp. 47–84. 10.1093/imammb/dqq005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Scianna, M. , and Preziosi, L. , 2012, “Multiscale Developments of the Cellular Potts Model,” Multiscale Model. Simul., 10(2), pp. 342–382. 10.1137/100812951 [DOI] [Google Scholar]
- [30]. Flamm, M. H. , and Diamond, S. L. , 2012, “Multiscale Systems Biology and Physics of Thrombosis Under Flow,” Ann. Biomed. Eng., 40(11), pp. 2355–2364. 10.1007/s10439-012-0557-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Basciano, C. , Kleinstreuer, C. , Hyun, S. , and Finol, E. A. , 2011, “A Relation Between Near-Wall Particle-Hemodynamics and Onset of Thrombus Formation in Abdominal Aortic Aneurysms,” Ann. Biomed. Eng., 39(7), pp. 2010–2026. 10.1007/s10439-011-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Biasetti, J. , Gasser, T. C. , Auer, M. , Hedin, U. , and Labruto, F. , 2010, “Hemodynamics of the Normal Aorta Compared to Fusiform and Saccular Abdominal Aortic Aneurysms With Emphasis on a Potential Thrombus Formation Mechanism,” Ann. Biomed. Eng., 38(2), pp. 380–390. 10.1007/s10439-009-9843-6 [DOI] [PubMed] [Google Scholar]
- [33]. van Dam, E. A. , Dams, S. D. , Peters, G. W. , Rutten, M. C. , Schurink, G. W. , Buth, J. , and van de Vosse, F. N. , 2006, “Determination of Linear Viscoelastic Behavior of Abdominal Aortic Aneurysm Thrombus,” Biorheology, 43(6), pp. 695–707. [PubMed] [Google Scholar]
- [34]. Brady, A. R. , Thompson, S. G. , Fowkes, F. G. , Greenhalgh, R. M. , and Powell, J. T. , 2004, “Abdominal Aortic Aneurysm Expansion: Risk Factors and Time Intervals for Surveillance,” Circulation, 110(1), pp. 16–21. 10.1161/01.CIR.0000133279.07468.9F [DOI] [PubMed] [Google Scholar]
- [35]. Kurvers, H. , Veith, F. J. , Lipsitz, E. C. , Ohki, T. , Gargiulo, N. J. , Cayne, N. S. , Suggs, W. D. , Timaran, C. H. , Kwon, G. Y. , Rhee, S. J. , and Santiago, C. , 2004, “Discontinuous, Staccato Growth of Abdominal Aortic Aneurysms,” J. Am. Coll. Surg., 199(5), pp. 709–715. 10.1016/j.jamcollsurg.2004.07.031 [DOI] [PubMed] [Google Scholar]
- [36]. Wang, D. H. , Makaroun, M. , Webster, M. W. , and Vorp, D. A. , 2001, “Mechanical Properties and Microstructure of Intraluminal Thrombus From Abdominal Aortic Aneurysm,” J. Biomech. Eng., 123(6), pp. 536–539. 10.1115/1.1411971 [DOI] [PubMed] [Google Scholar]
- [37]. Fontaine, V. , Jacob, M. P. , Houard, X. , Rossignol, P. , Plissonnier, D. , Angles-Cano, E. , and Michel, J. B. , 2002, “Involvement of the Mural Thrombus as a Site of Protease Release and Activation in Human Aortic Aneurysms,” Am. J. Pathol., 161(5), pp. 1701–1710. 10.1016/S0002-9440(10)64447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Whittaker, P. , and Przyklenk, K. , 2009, “Fibrin Architecture in Clots: A Quantitative Polarized Light Microscopy Analysis,” Blood Cells Mol. Dis., 42(1), pp. 51–56. 10.1016/j.bcmd.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Adolph, R. , Vorp, D. A. , Steed, D. L. , Webster, M. W. , Kameneva, M. V. , and Watkins, S. C. , 1997, “Cellular Content and Permeability of Intraluminal Thrombus in Abdominal Aortic Aneurysm,” J. Vasc. Surg., 25(5), pp. 916–926. 10.1016/S0741-5214(97)70223-4 [DOI] [PubMed] [Google Scholar]
- [40]. Di Martino, E. , Mantero, S. , Inzoli, F. , Melissano, G. , Astore, D. , Chiesa, R. , and Fumero, R. , 1998, “Biomechanics of Abdominal Aortic Aneurysm in the Presence of Endoluminal Thrombus: Experimental Characterisation and Structural Static Computational Analysis,” Eur. J. Vasc. Endovasc. Surg., 15(4), pp. 290–299. 10.1016/S1078-5884(98)80031-2 [DOI] [PubMed] [Google Scholar]
- [41]. van Dam, E. A. , Dams, S. D. , Peters, G. W. , Rutten, M. C. , Schurink, G. W. , Buth, J. , and van de Vosse, F. N. , 2008, “Non-Linear Viscoelastic Behavior of Abdominal Aortic Aneurysm Thrombus,” Biomech. Model. Mechanobiol., 7(2), pp. 127–137. 10.1007/s10237-007-0080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Georgakarakos, E. , Ioannou, C. V. , Kamarianakis, Y. , Papaharilaou, Y. , Kostas, T. , Manousaki, E. , and Katsamouris, A. N. , 2010, “The Role of Geometric Parameters in the Prediction of Abdominal Aortic Aneurysm Wall Stress,” Eur. J. Vasc. Endovasc. Surg., 39(1), pp. 42–48. 10.1016/j.ejvs.2009.09.026 [DOI] [PubMed] [Google Scholar]
- [43]. Speelman, L. , Schurink, G. W. , Bosboom, E. M. , Buth, J. , Breeuwer, M. , van de Vosse, F. N. , and Jacobs, M. H. , 2010, “The Mechanical Role of Thrombus on the Growth Rate of an Abdominal Aortic Aneurysm,” J. Vasc. Surg., 51(1), pp. 19–26. 10.1016/j.jvs.2009.08.075 [DOI] [PubMed] [Google Scholar]
- [44]. Vorp, D. A. , Mandarino, W. A. , Webster, M. W. , and Gorcsan, J. , 1996, “Potential Influence of Intraluminal Thrombus on Abdominal Aortic Aneurysm as Assessed by a New Non-Invasive Method,” Cardiovasc. Surg., 4(6), pp. 732–739. 10.1016/S0967-2109(96)00008-7 [DOI] [PubMed] [Google Scholar]
- [45]. Vande Geest, J. P. , Sacks, M. S. , and Vorp, D. A. , 2006, “A Planar Biaxial Constitutive Relation for the Luminal Layer of Intra-Luminal Thrombus in Abdominal Aortic Aneurysms,” J. Biomech., 39(13), pp. 2347–2354. 10.1016/j.jbiomech.2006.05.011 [DOI] [PubMed] [Google Scholar]
- [46]. Ashton, J. H. , Vande Geest, J. P. , Simon, B. R. , and Haskett, D. G. , 2009, “Compressive Mechanical Properties of the Intraluminal Thrombus in Abdominal Aortic Aneurysms and Fibrin-Based Thrombus Mimics,” J. Biomech., 42(3), pp. 197–201. 10.1016/j.jbiomech.2008.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Fontaine, V. , Touat, Z. , Mtairag, E. M. , Vranckx, R. , Louedec, L. , Houard, X. , Andreassian, B. , Sebbag, U. , Palombi, T. , Jacob, M. P. , Meilhac, O. , and Michel, J. B. , 2004, “Role of Leukocyte Elastase in Preventing Cellular Re-Colonization of the Mural Thrombus,” Am. J. Pathol., 164(6), pp. 2077–2087. 10.1016/S0002-9440(10)63766-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Swedenborg, J. , and Eriksson, P. , 2006, “The Intraluminal Thrombus as a Source of Proteolytic Activity,” Ann. N.Y. Acad. Sci., 1085, pp. 133–138. 10.1196/annals.1383.044 [DOI] [PubMed] [Google Scholar]
- [49]. Houard, X. , Touat, Z. , Ollivier, V. , Louedec, L. , Philippe, M. , Sebbag, U. , Meilhac, O. , Rossignol, P. , and Michel, J. B. , 2009, “Mediators of Neutrophil Recruitment in Human Abdominal Aortic Aneurysms,” Cardiovasc. Res., 82(3), pp. 532–541. 10.1093/cvr/cvp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Houard, X. , Ollivier, V. , Louedec, L. , Michel, J. B. , and Bäck, M. , 2009, “Differential Inflammatory Activity Across Human Abdominal Aortic Aneurysms Reveals Neutrophil-Derived Leukotriene B4 as a Major Chemotactic Factor Released From the Intraluminal Thrombus,” FASEB J., 23(5), pp. 1376–1383. 10.1096/fj.08-116202 [DOI] [PubMed] [Google Scholar]
- [51]. Michel, J. B. , Martin-Ventura, J. L. , Egido, J. , Sakalihasan, N. , Treska, V. , Lindholt, J. , Allaire, E. , Thorsteinsdottir, U. , Cockerill, G. , Swedenborg, J. , and FAD EU consortium, 2011, “Novel Aspects of the Pathogenesis of Aneurysms of the Abdominal Aorta in Humans,” Cardiovasc. Res., 90(1), pp. 18–27. 10.1093/cvr/cvq337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Wiernicki, I. , Stachowska, E. , Safranow, K. , Cnotliwy, M. , Rybicka, M. , Kaczmarczyk, M. , and Gutowski, P. , 2010, “Enhanced Matrix-Degrading Proteolytic Activity Within the Thin Thrombus-Covered Wall of Human Abdominal Aortic Aneurysms,” Atherosclerosis, 212(1), pp. 161–165. 10.1016/j.atherosclerosis.2010.04.033 [DOI] [PubMed] [Google Scholar]
- [53]. Touat, Z. , Ollivier, V. , Dai, J. , Huisse, M. G. , Bezeaud, A. , Sebbag, U. , Palombi, T. , Rossignol, P. , Meilhac, O. , Guillin, M. C. , and Michel, J. B. , 2006, “Renewal of Mural Thrombus Releases Plasma Markers and is Involved in Aortic Abdominal Aneurysm Evolution,” Am. J. Pathol., 168(3), pp. 1022–1030. 10.2353/ajpath.2006.050868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Houard, X. , Leclercq, A. , Fontaine, V. , Coutard, M. , Martin-Ventura, J.-L. , Ho-Tin-Noe, B. , Touat, Z. , Meilhac, O. , and Michel, J.-B. , 2006, “Retention and Activation of Blood-Borne Proteases in the Arterial Wall: Implications for Atherothrombosis,” J. Am. Coll. Cardiol., 48(9), pp. A3–A9. 10.1016/j.jacc.2006.04.098 [DOI] [Google Scholar]
- [55]. Khan, J. A. , Abdul Rahman, M. N. , Mazari, F. A. , Shahin, Y. , Smith, G. , Madden, L. , Fagan, M. J. , Greenman, J. , McCollum, P. T. , and Chetter, I. C. , 2012, “Intraluminal Thrombus has a Selective Influence on Matrix Metalloproteinases and Their Inhibitors (Tissue Inhibitors of Matrix Metalloproteinases) in the Wall of Abdominal Aortic Aneurysms,” Ann. Vasc. Surg., 26(3), pp. 322–329. 10.1016/j.avsg.2011.08.015 [DOI] [PubMed] [Google Scholar]
- [56]. Folkesson, M. , Silveira, A. , Eriksson, P. , and Swedenborg, J. , 2011, “Protease Activity in the Multi-Layered Intra-Luminal Thrombus of Abdominal Aortic Aneurysms,” Atherosclerosis, 218(2), pp. 294–299. 10.1016/j.atherosclerosis.2011.05.002 [DOI] [PubMed] [Google Scholar]
- [57]. Freestone, T. , Turner, R. J. , Coady, A. , Higman, D. J. , Greenhalgh, R. M. , and Powell, J. T. , 1995, “Inflammation and Matrix Metalloproteinases in the Enlarging Abdominal Aortic Aneurysm,” Arterioscler., Thromb., Vasc. Biol., 15(8), pp. 1145–1151. 10.1161/01.ATV.15.8.1145 [DOI] [PubMed] [Google Scholar]
- [58]. Curci, J. A. , Liao, S. , Huffman, M. D. , Shapiro, S. D. , and Thompson, R. W. , 1998, “Expression and Localization of Macrophage Elastase (Matrix Metalloproteinase-12) in Abdominal Aortic Aneurysms,” J. Clin. Invest., 102(11), pp. 1900–1910. 10.1172/JCI2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Rizas, K. D. , Ippagunta, N. , and Tilson, M. D. , 2009, “Immune Cells and Molecular Mediators in the Pathogenesis of the Abdominal Aortic Aneurysm,” Cardiol. Rev., 17(5), pp. 201–210. 10.1097/CRD.0b013e3181b04698 [DOI] [PubMed] [Google Scholar]
- [60]. Siegel, C. L. , Cohan, R. H. , Korobkin, M. , Alpern, M. B. , Courneya, D. L. , and Leder, R. A. , 1994, “Abdominal Aortic Aneurysm Morphology: CT Features in Patients With Ruptured and Nonruptured Aneurysms,” AJR, Am. J. Roentgenol., 163(5), pp. 1123–1129. [DOI] [PubMed] [Google Scholar]
- [61]. Schriefl, A. J. , Collins, M. J. , Pierce, D. M. , Holzapfel, G. A. , Niklason, L. E. , and Humphrey, J. D. , 2012, “Remodeling of Intramural Thrombus and Collagen in an Ang-II Infusion ApoE-/- Model of Dissecting Aortic Aneurysms,” Thromb. Res., 130(3), p. pp 139–146. 10.1016/j.thromres.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Houard, X. , Rouzet, F. , Touat, Z. , Philippe, M. , Dominguez, M. , Fontaine, V. , Sarda-Mantel, L. , Meulemans, A. , Le Guludec, D. , Meilhac, O. , and Michel, J. B. , 2007, “Topology of the Fibrinolytic System Within the Mural Thrombus of Human Abdominal Aortic Aneurysms,” J. Pathol., 212(1), pp. 20–28. 10.1002/path.2148 [DOI] [PubMed] [Google Scholar]
- [63]. Carrell, T. W. , Burnand, K. G. , Booth, N. A. , Humphries, J. , and Smith, A. , 2006, “Intraluminal Thrombus Enhances Proteolysis in Abdominal Aortic Aneurysms,” Vascular, 14(1), pp. 9–16. 10.2310/6670.2006.00008 [DOI] [PubMed] [Google Scholar]
- [64]. Knox, J. B. , Sukhova, G. K. , Whittemore, A. D. , and Libby, P. , 1997, “Evidence for Altered Balance Between Matrix Metalloproteinases and Their Inhibitors in Human Aortic Diseases,” Circulation, 95(1), pp. 205–212. 10.1161/01.CIR.95.1.205 [DOI] [PubMed] [Google Scholar]
- [65]. Vorp, D. A. , Lee, P. C. , Wang, D. H. , Makaroun, M. S. , Nemoto, E. M. , Ogawa, S. , and Webster, M. W. , 2001, “Association of Intraluminal Thrombus in Abdominal Aortic Aneurysm With Local Hypoxia and Wall Weakening,” J. Vasc. Surg., 34(2), pp. 291–299. 10.1067/mva.2001.114813 [DOI] [PubMed] [Google Scholar]
- [66]. Diehm, N. , Di Santo, S. , Schaffner, T. , Schmidli, J. , Völzmann, J. , Jüni, P. , Baumgartner, I. , and Kalka, C. , 2008, “Severe Structural Damage of the Seemingly Non-Diseased Infrarenal Aortic Aneurysm Neck,” J. Vasc. Surg., 48(2), pp. 425–434. 10.1016/j.jvs.2008.03.001 [DOI] [PubMed] [Google Scholar]
- [67]. Gong, Y. , Hart, E. , Shchurin, A. , and Hoover-Plow, J. , 2008, “Inflammatory Macrophage Migration Requires MMP-9 Activation by Plasminogen in Mice,” J. Clin. Invest., 118(9), pp. 3012–3024. 10.1172/JCI32750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Plow, E. F. , and Hoover-Plow, J. , 2004, “The Functions of Plasminogen in Cardiovascular Disease,” Trends Cardiovasc. Med., 14(5), pp. 180–186. 10.1016/j.tcm.2004.04.001 [DOI] [PubMed] [Google Scholar]
- [69]. Falcone, D. J. , McCaffrey, T. A. , Haimovitz-Friedman, A. , Vergilio, J. A. , and Nicholson, A. C. , 1993, “Macrophage and Foam Cell Release of Matrix-Bound Growth Factors. Role of Plasminogen Activation,” J. Biol. Chem., 268(16), pp. 11951–11958. [PubMed] [Google Scholar]
- [70]. Meilhac, O. , Ho-Tin-Noé, B. , Houard, X. , Philippe, M. , Michel, J. B. , and Anglés-Cano, E. , 2003, “Pericellular Plasmin Induces Smooth Muscle Cell Anoikis,” FASEB J., 17(10), pp. 1301–1303. 10.1096/fj.02-0687fje [DOI] [PubMed] [Google Scholar]
- [71]. Coutard, M. , Touat, Z. , Houard, X. , Leclercq, A. , and Michel, J. B. , 2010, “Thrombus Versus Wall Biological Activities in Experimental Aortic Aneurysms,” J. Vasc. Res., 47(4), pp. 355–366. 10.1159/000265569 [DOI] [PubMed] [Google Scholar]
- [72]. Antonicelli, F. , Bellon, G. , Debelle, L. , and Hornebeck, W. , 2007, “Elastin-Elastases and Inflamm-Aging,” Curr. Top. Dev. Biol., 79, pp. 99–155. 10.1016/S0070-2153(06)79005-6 [DOI] [PubMed] [Google Scholar]
- [73]. Faisal Khan, K. M. , Laurie, G. W. , McCaffrey, T. A. , and Falcone, D. J. , 2002, “Exposure of Cryptic Domains in the Alpha 1-Chain of Laminin-1 by Elastase Stimulates Macrophages Urokinase and Matrix Metalloproteinase-9 Expression,” J. Biol. Chem., 277(16), pp. 13778–13786. 10.1074/jbc.M111290200 [DOI] [PubMed] [Google Scholar]
- [74]. Weitz, J. I. , Leslie, B. , and Ginsberg, J. , 1991, “Soluble Fibrin Degradation Products Potentiate Tissue Plasminogen Activator-Induced Fibrinogen Proteolysis,” J. Clin. Invest., 87(3), pp. 1082–1090. 10.1172/JCI115069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Nackman, G. B. , Karkowski, F. J. , Halpern, V. J. , Gaetz, H. P. , and Tilson, M. D. , 1997, “Elastin Degradation Products Induce Adventitial Angiogenesis in the Anidjar/Dobrin Rat Aneurysm Model,” Surgery, 122(1), pp. 39–44. 10.1016/S0039-6060(97)90262-2 [DOI] [PubMed] [Google Scholar]
- [76]. Mäyränpää, M. I. , Trosien, J. A. , Fontaine, V. , Folkesson, M. , Kazi, M. , Eriksson, P. , Swedenborg, J. , and Hedin, U. , 2009, “Mast Cells Associate With Neovessels in the Media and Adventitia of Abdominal Aortic Aneurysms,” J. Vasc. Surg., 50(2), pp. 388–395. 10.1016/j.jvs.2009.03.055 [DOI] [PubMed] [Google Scholar]
- [77]. Chen, Z. L. , and Strickland, S. , 1997, “Neuronal Death in the Hippocampus is Promoted by Plasmin-Catalyzed Degradation of Laminin,” Cell, 91(7), pp. 917–925. 10.1016/S0092-8674(00)80483-3 [DOI] [PubMed] [Google Scholar]
- [78]. Michel, J. B. , Thaunat, O. , Houard, X. , Meilhac, O. , Caligiuri, G. , and Nicoletti, A. , 2007, “Topological Determinants and Consequences of Adventitial Responses to Arterial Wall Injury,” Arterioscler., Thromb., Vasc. Biol., 27(6), pp. 1259–1268. 10.1161/ATVBAHA.106.137851 [DOI] [PubMed] [Google Scholar]
- [79]. Dal Canto, A. J. , Swanson, P. E. , O'Guin, A. K. , Speck, S. H. , and Virgin, H. W. , 2001, “IFN-Gamma Action in the Media of the Great Elastic Arteries, a Novel Immunoprivileged Site,” J. Clin. Invest., 107(2), pp. R15–22. 10.1172/JCI11540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Burns, W. R. , Wang, Y. , Tang, P. C. , Ranjbaran, H. , Iakimov, A. , Kim, J. , Cuffy, M. , Bai, Y. , Pober, J. S. , and Tellides, G. , 2005, “Recruitment of CXCR3+ and CCR5+ T Cells and Production of Interferon-Gamma-Inducible Chemokines in Rejecting Human Arteries,” Am. J. Transplant., 5(6), pp. 1226–1236. 10.1111/j.1600-6143.2005.00892.x [DOI] [PubMed] [Google Scholar]
- [81]. Odekon, L. E. , Blasi, F. , and Rifkin, D. B. , 1994, “Requirement for Receptor-Bound Urokinase in Plasmin-Dependent Cellular Conversion of Latent TGF-Beta to TGF-Beta,” J. Cell. Physiol., 158(3), pp. 398–407. 10.1002/jcp.1041580303 [DOI] [PubMed] [Google Scholar]
- [82]. Baxter, B. T. , Davis, V. A. , Minion, D. J. , Wang, Y. P. , Lynch, T. G. , and McManus, B. M. , 1994, “Abdominal Aortic Aneurysms are Associated With Altered Matrix Proteins of the Nonaneurysmal Aortic Segments,” J. Vasc. Surg., 19(5), pp. 797–802. 10.1016/S0741-5214(94)70004-4 [DOI] [PubMed] [Google Scholar]
- [83]. Goodall, S. , Crowther, M. , Hemingway, D. M. , Bell, P. R. , and Thompson, M. M. , 2001, “Ubiquitous Elevation of Matrix Metalloproteinase-2 Expression in the Vasculature of Patients With Abdominal Aneurysms,” Circulation, 104(3), pp. 304–309. 10.1161/01.CIR.104.3.304 [DOI] [PubMed] [Google Scholar]
- [84]. Tilson, M. D. , and Dang, C. , 1981, “Generalized Arteriomegaly. A Possible Predisposition to the Formation of Abdominal Aortic Aneurysms,” Arch. Surg. (Chicago), 116(8), pp. 1030–1032. 10.1001/archsurg.1981.01380200038007 [DOI] [PubMed] [Google Scholar]
- [85]. Iwamoto, T. , Kimura, A. , Nakai, T. , Kanaya, K. , and Ishimaru, S. , 2004, “Implications of Carotid Arteriomegaly in Patients With Aortic Aneurysm,” J. Atheroscler. Thromb., 11(6), pp. 348–353. 10.5551/jat.11.348 [DOI] [PubMed] [Google Scholar]
- [86]. Ayyalasomayajula, A. , Vande Geest, J. P. , and Simon, B. R. , 2010, “Porohyperelastic Finite Element Modeling of Abdominal Aortic Aneurysms,” J. Biomech. Eng., 132(10), p. 104502. 10.1115/1.4002370 [DOI] [PubMed] [Google Scholar]
- [87]. Vande Geest, J. P. , Simon, B. R. , and Mortazavi, A. , 2006, “Toward a Model for Local Drug Delivery in Abdominal Aortic Aneurysms,” Ann. N.Y. Acad. Sci., 1085, pp. 396–399. 10.1196/annals.1383.047 [DOI] [PubMed] [Google Scholar]
- [88]. Daugherty, A. , Manning, M. W. , and Cassis, L. A. , 2000, “Angiotensin II Promotes Atherosclerotic Lesions and Aneurysms in Apolipoprotein E-Deficient Mice,” J. Clin. Invest., 105(11), pp. 1605–1612. 10.1172/JCI7818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Anidjar, S. , Salzmann, J. L. , Gentric, D. , Lagneau, P. , Camilleri, J. P. , and Michel, J. B. , 1990, “Elastase-Induced Experimental Aneurysms in Rats,” Circulation, 82(3), pp. 973–981. 10.1161/01.CIR.82.3.973 [DOI] [PubMed] [Google Scholar]
- [90]. Eliason, J. L. , Hannawa, K. K. , Ailawadi, G. , Sinha, I. , Ford, J. W. , Deogracias, M. P. , Roelofs, K. J. , Woodrum, D. T. , Ennis, T. L. , Henke, P. K. , Stanley, J. C. , Thompson, R. W. , and Upchurch, G. R. , 2005, “Neutrophil Depletion Inhibits Experimental Abdominal Aortic Aneurysm Formation,” Circulation, 112(2), pp. 232–240. 10.1161/CIRCULATIONAHA.104.517391 [DOI] [PubMed] [Google Scholar]
- [91]. Dai, J. , Louedec, L. , Philippe, M. , Michel, J. B. , and Houard, X. , 2009, “Effect of Blocking Platelet Activation With AZD6140 on Development of Abdominal Aortic Aneurysm in a Rat Aneurysmal Model,” J. Vasc. Surg., 49(3), pp. 719–727. 10.1016/j.jvs.2008.09.057 [DOI] [PubMed] [Google Scholar]
- [92]. Hannawa, K. K. , Eliason, J. L. , Woodrum, D. T. , Pearce, C. G. , Roelofs, K. J. , Grigoryants, V. , Eagleton, M. J. , Henke, P. K. , Wakefield, T. W. , Myers, D. D. , Stanley, J. C. , and Upchurch, G. R. , 2005, “L-Selectin-Mediated Neutrophil Recruitment in Experimental Rodent Aneurysm Formation,” Circulation, 112(2), pp. 241–247. 10.1161/CIRCULATIONAHA.105.535625 [DOI] [PubMed] [Google Scholar]
- [93]. Pagano, M. B. , Bartoli, M. A. , Ennis, T. L. , Mao, D. , Simmons, P. M. , Thompson, R. W. , and Pham, C. T. , 2007, “Critical Role of Dipeptidyl Peptidase I in Neutrophil Recruitment During the Development of Experimental Abdominal Aortic Aneurysms,” Proc. Natl. Acad. Sci. U.S.A., 104(8), pp. 2855–2860. 10.1073/pnas.0606091104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Deng, G. G. , Martin-McNulty, B. , Sukovich, D. A. , Freay, A. , Halks-Miller, M. , Thinnes, T. , Loskutoff, D. J. , Carmeliet, P. , Dole, W. P. , and Wang, Y. X. , 2003, “Urokinase-Type Plasminogen Activator Plays a Critical Role in Angiotensin II-Induced Abdominal Aortic Aneurysm,” Circ. Res., 92(5), pp. 510–517. 10.1161/01.RES.0000061571.49375.E1 [DOI] [PubMed] [Google Scholar]
- [95]. Longo, G. M. , Xiong, W. , Greiner, T. C. , Zhao, Y. , Fiotti, N. , and Baxter, B. T. , 2002, “Matrix Metalloproteinases 2 and 9 Work in Concert to Produce Aortic Aneurysms,” J. Clin. Invest., 110(5), pp. 625–632. 10.1172/JCI15334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Longo, G. M. , Buda, S. J. , Fiotta, N. , Xiong, W. , Griener, T. , Shapiro, S. , and Baxter, B. T. , 2005, “MMP-12 has a Role in Abdominal Aortic Aneurysms in Mice,” Surgery, 137(4), pp. 457–462. 10.1016/j.surg.2004.12.004 [DOI] [PubMed] [Google Scholar]
- [97]. Allaire, E. , Hasenstab, D. , Kenagy, R. D. , Starcher, B. , Clowes, M. M. , and Clowes, A. W. , 1998, “Prevention of Aneurysm Development and Rupture by Local Overexpression of Plasminogen Activator Inhibitor-1,” Circulation, 98(3), pp. 249–255. 10.1161/01.CIR.98.3.249 [DOI] [PubMed] [Google Scholar]
- [98]. Allaire, E. , Forough, R. , Clowes, M. , Starcher, B. , and Clowes, A. W. , 1998, “Local Overexpression of TIMP-1 Prevents Aortic Aneurysm Degeneration and Rupture in a Rat Model,” J. Clin. Invest., 102(7), pp. 1413–1420. 10.1172/JCI2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Grigoryants, V. , Hannawa, K. K. , Pearce, C. G. , Sinha, I. , Roelofs, K. J. , Ailawadi, G. , Deatrick, K. B. , Woodrum, D. T. , Cho, B. S. , Henke, P. K. , Stanley, J. C. , Eagleton, M. J. , and Upchurch, G. R. , 2005, “Tamoxifen Up-Regulates Catalase Production, Inhibits Vessel Wall Neutrophil Infiltration, and Attenuates Development of Experimental Abdominal Aortic Aneurysms,” J. Vasc. Surg., 41(1), pp. 108–114. 10.1016/j.jvs.2004.09.033 [DOI] [PubMed] [Google Scholar]
- [100]. Kazi, M. , Thyberg, J. , Religa, P. , Roy, J. , Eriksson, P. , Hedin, U. , and Swedenborg, J. , 2003, “Influence of Intraluminal Thrombus on Structural and Cellular Composition of Abdominal Aortic Aneurysm Wall,” J. Vasc. Surg., 38(6), pp. 1283–1292. 10.1016/S0741-5214(03)00791-2 [DOI] [PubMed] [Google Scholar]
- [101]. Kazi, M. , Zhu, C. , Roy, J. , Paulsson-Berne, G. , Hamsten, A. , Swedenborg, J. , Hedin, U. , and Eriksson, P. , 2005, “Difference in Matrix-Degrading Protease Expression and Activity Between Thrombus-Free and Thrombus-Covered Wall of Abdominal Aortic Aneurysm,” Arterioscler., Thromb., Vasc. Biol., 25(7), pp. 1341–1346. 10.1161/01.ATV.0000166601.49954.21 [DOI] [PubMed] [Google Scholar]
- [102]. Stenbaek, J. , Kalin, B. , and Swedenborg, J. , 2000, “Growth of Thrombus may be a Better Predictor of Rupture Than Diameter in Patients With Abdominal Aortic Aneurysms,” Eur. J. Vasc. Endovasc. Surg., 20(5), pp. 466–469. 10.1053/ejvs.2000.1217 [DOI] [PubMed] [Google Scholar]
- [103]. Hans, S. S. , Jareunpoon, O. , Balasubramaniam, M. , and Zelenock, G. B. , 2005, “Size and Location of Thrombus in Intact and Ruptured Abdominal Aortic Aneurysms,” J. Vasc. Surg., 41(4), pp. 584–588. 10.1016/j.jvs.2005.01.004 [DOI] [PubMed] [Google Scholar]
- [104]. Vorp, D. A. , and Vande Geest, J. P. , 2005, “Biomechanical Determinants of Abdominal Aortic Aneurysm Rupture,” Arterioscler. Thromb. Vasc. Biol., 25(8), pp. 1558–1566. 10.1161/01.ATV.0000174129.77391.55 [DOI] [PubMed] [Google Scholar]
- [105]. Sun, N. , Leung, J. H. , Wood, N. B. , Hughes, A. D. , Thom, S. A. , Cheshire, N. J. , and Xu, X. Y. , 2009, “Computational Analysis of Oxygen Transport in a Patient-Specific Model of Abdominal Aortic Aneurysm With Intraluminal Thrombus,” Br. J. Radiol., 82(1), pp. S18–23. 10.1259/bjr/89466318 [DOI] [PubMed] [Google Scholar]
- [106]. Yoshimura, K. , Ikeda, Y. , and Aoki, H. , 2011, “Innocent Bystander? Intraluminal Thrombus in Abdominal Aortic Aneurysm,” Atherosclerosis, 218(2), pp. 285–286. 10.1016/j.atherosclerosis.2011.06.027 [DOI] [PubMed] [Google Scholar]
- [107]. Satoh, H. , Nakamura, M. , Satoh, M. , Nakajima, T. , Izumoto, H. , Maesawa, C. , Kawazoe, K. , Masuda, T. , and Hiramori, K. , 2004, “Expression and Localization of Tumour Necrosis Factor-Alpha and its Converting Enzyme in Human Abdominal Aortic Aneurysm,” Clin. Sci., 106(3), pp. 301–306. 10.1042/CS20030189 [DOI] [PubMed] [Google Scholar]
- [108]. Li, Z. Y. , Sadat, U. , U-King-Im, J., Tang , T. Y., Bowden , D. J., Hayes , P. D., and Gillard , J. H., 2010, “Association Between Aneurysm Shoulder Stress and Abdominal Aortic Aneurysm Expansion: A Longitudinal Follow-Up Study,” Circulation, 122(18), pp. 1815–1822. 10.1161/CIRCULATIONAHA.110.939819 [DOI] [PubMed] [Google Scholar]
- [109]. Wilson, J. S. , Baek, S. , and Humphrey, J. D. , 2012, “Importance of Initial Aortic Properties on the Evolving Regional Anisotropy, Stiffness and Wall Thickness of Human Abdominal Aortic Aneurysms,” J. R. Soc., Interface, 9(74), pp. 2047–2058. 10.1098/rsif.2012.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Wilson, J. S. , Baek, S. , and Humphrey, J. D. , 2013, “Parametric Study of Effects of Collagen Turnover on the Natural History of Abdominal Aortic Aneurysms,” Proc. R. Soc. London, Ser. A, 469(2150), (ePub ahead of print). 10.1098/rspa.2012.0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Inzoli, F. , Boschetti, F. , Zappa, M. , Longo, T. , and Fumero, R. , 1993, “Biomechanical Factors in Abdominal Aortic Aneurysm Rupture,” Eur. J. Vasc. Surg., 7(6), pp. 667–674. 10.1016/S0950-821X(05)80714-5 [DOI] [PubMed] [Google Scholar]
- [112]. Mower, W. R. , Quiñones, W. J. , and Gambhir, S. S. , 1997, “Effect of Intraluminal Thrombus on Abdominal Aortic Aneurysm Wall Stress,” J. Vasc. Surg., 26(4), pp. 602–608. 10.1016/S0741-5214(97)70058-2 [DOI] [PubMed] [Google Scholar]
- [113]. Wang, D. H. , Makaroun, M. S. , Webster, M. W. , and Vorp, D. A. , 2002, “Effect of Intraluminal Thrombus on Wall Stress in Patient-Specific Models of Abdominal Aortic Aneurysm,” J. Vasc. Surg., 36(3), pp. 598–604. 10.1067/mva.2002.126087 [DOI] [PubMed] [Google Scholar]
- [114]. Maier, A. , Gee, M. W. , Reeps, C. , Pongratz, J. , Eckstein, H. H. , and Wall, W. A. , 2010, “A Comparison of Diameter, Wall Stress, and Rupture Potential Index for Abdominal Aortic Aneurysm Rupture Risk Prediction,” Ann. Biomed. Eng., 38(10), pp. 3124–3134. 10.1007/s10439-010-0067-6 [DOI] [PubMed] [Google Scholar]
- [115]. Gasser, T. C. , Auer, M. , Labruto, F. , Swedenborg, J. , and Roy, J. , 2010, “Biomechanical Rupture Risk Assessment of Abdominal Aortic Aneurysms: Model Complexity Versus Predictability of Finite Element Simulations,” Eur. J. Vasc. Endovasc. Surg., 40(2), pp. 176–185. 10.1016/j.ejvs.2010.04.003 [DOI] [PubMed] [Google Scholar]
- [116]. Larsson, E. , Labruto, F. , Gasser, T. C. , Swedenborg, J. , and Hultgren, R. , 2011, “Analysis of Aortic Wall Stress and Rupture Risk in Patients With Abdominal Aortic Aneurysm With a Gender Perspective,” J. Vasc. Surg., 54(2), pp. 295–299. 10.1016/j.jvs.2010.12.053 [DOI] [PubMed] [Google Scholar]
- [117]. Ene, F. , Gachon, C. , Delassus, P. , Carroll, R. , Stefanov, F. , O'Flynn, P. , and Morris, L. , 2011, “ In Vitro Evaluation of the Effects of Intraluminal Thrombus on Abdominal Aortic Aneurysm Wall Dynamics,” Med. Eng. Phys., 33(8), pp. 957–966. 10.1016/j.medengphy.2011.03.005 [DOI] [PubMed] [Google Scholar]
- [118]. Meyer, C. A. , Guivier-Curien, C. , and Moore, J. E. , 2010, “Trans-Thrombus Blood Pressure Effects in Abdominal Aortic Aneurysms,” J. Biomech. Eng., 132(7), p. 071005. 10.1115/1.4001253 [DOI] [PubMed] [Google Scholar]
- [119]. Schurink, G. W. , van Baalen, J. M. , Visser, M. J. , and van Bockel, J. H. , 2000, “Thrombus Within an Aortic Aneurysm Does not Reduce Pressure on the Aneurysmal Wall,” J. Vasc. Surg., 31(3), pp. 501–506. 10.1016/S0741-5214(00)90311-2 [DOI] [PubMed] [Google Scholar]
- [120]. Brown, A. E. , Litvinov, R. I. , Discher, D. E. , Purohit, P. K. , and Weisel, J. W. , 2009, “Multiscale Mechanics of Fibrin Polymer: Gel Stretching With Protein Unfolding and Loss of Water,” Science, 325(5941), pp. 741–744. 10.1126/science.1172484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Truijers, M. , Fillinger, M. F. , Renema, K. W. , Marra, S. P. , Oostveen, L. J. , Kurvers, H. A. , Schultzekool, L. J. , and Blankensteijn, J. D. , 2009, “ In-Vivo Imaging of Changes in Abdominal Aortic Aneurysm Thrombus Volume During the Cardiac Cycle,” J. Endovasc. Ther., 16(3), pp. 314–319. 10.1583/08-2625.1 [DOI] [PubMed] [Google Scholar]
- [122]. Vande Geest, J. P. , Di Martino, E. S. , Bohra, A. , Makaroun, M. S. , and Vorp, D. A. , 2006, “A Biomechanics-Based Rupture Potential Index for Abdominal Aortic Aneurysm Risk Assessment: Demonstrative Application,” Ann. N.Y. Acad. Sci., 1085, pp. 11–21. 10.1196/annals.1383.046 [DOI] [PubMed] [Google Scholar]
- [123]. Gasser, T. C. , Görgülü, G. , Folkesson, M. , and Swedenborg, J. , 2008, “Failure Properties of Intraluminal Thrombus in Abdominal Aortic Aneurysm Under Static and Pulsating Mechanical Loads,” J. Vasc. Surg., 48(1), pp. 179–188. 10.1016/j.jvs.2008.01.036 [DOI] [PubMed] [Google Scholar]
- [124]. Labruto, F. , Blomqvist, L. , and Swedenborg, J. , 2011, “Imaging the Intraluminal Thrombus of Abdominal Aortic Aneurysms: Techniques, Findings, and Clinical Implications,” J. Vasc. Interv. Radiol., 22(8), pp. 1069–1075. 10.1016/j.jvir.2011.01.454 [DOI] [PubMed] [Google Scholar]
- [125]. Nchimi, A. , Defawe, O. , Brisbois, D. , Broussaud, T. K. , Defraigne, J. O. , Magotteaux, P. , Massart, B. , Serfaty, J. M. , Houard, X. , Michel, J. B. , and Sakalihasan, N. , 2010, “MR Imaging of Iron Phagocytosis in Intraluminal Thrombi of Abdominal Aortic Aneurysms in Humans,” Radiology, 254(3), pp. 973–981. 10.1148/radiol.09090657 [DOI] [PubMed] [Google Scholar]
- [126]. Richards, J. M. , Semple, S. I. , MacGillivray, T. J. , Gray, C. , Langrish, J. P. , Williams, M. , Dweck, M. , Wallace, W. , McKillop, G. , Chalmers, R. T. , Garden, O. J. , and Newby, D. E. , 2011, “Abdominal Aortic Aneurysm Growth Predicted by Uptake of Ultrasmall Superparamagnetic Particles of Iron Oxide: A Pilot Study,” Circ. Cardiovasc. Imaging, 4(3), pp. 274–281. 10.1161/CIRCIMAGING.110.959866 [DOI] [PubMed] [Google Scholar]
- [127]. Watton, P. N. , Hill, N. A. , and Heil, M. , 2004, “A Mathematical Model for the Growth of the Abdominal Aortic Aneurysm,” Biomech. Model. Mechanobiol., 3(2), pp. 98–113. 10.1007/s10237-004-0052-9 [DOI] [PubMed] [Google Scholar]
- [128]. Humphrey, J. D. , and Rajagopal, K. R. , 2003, “A Constrained Mixture Model for Arterial Adaptations to a Sustained Step Change in Blood Flow,” Biomech. Model. Mechanobiol., 2(2), pp. 109–126. 10.1007/s10237-003-0033-4 [DOI] [PubMed] [Google Scholar]
- [129]. Watton, P. N. , Raberger, N. B. , Holzapfel, G. A. , and Ventikos, Y. , 2009, “Coupling the Hemodynamic Environment to the Evolution of Cerebral Aneurysms: Computational Framework and Numerical Examples,” J. Biomech. Eng., 131(10), p. 101003. 10.1115/1.3192141 [DOI] [PubMed] [Google Scholar]
- [130]. Zeinali-Davarani, S. , Sheidaei, A. , and Baek, S. , 2011, “A Finite Element Model of Stress-Mediated Vascular Adaptation: Application to Abdominal Aortic Aneurysms,” Comput. Methods Biomech. Biomed. Eng., 14(9), pp. 803–817. 10.1080/10255842.2010.495344 [DOI] [PubMed] [Google Scholar]
- [131]. Sheidaei, A. , Hunley, S. C. , Zeinali-Davarani, S. , Raguin, L. G. , and Baek, S. , 2011, “Simulation of Abdominal Aortic Aneurysm Growth With Updating Hemodynamic Loads Using a Realistic Geometry,” Med. Eng. Phys., 33(1), pp. 80–88. 10.1016/j.medengphy.2010.09.012 [DOI] [PubMed] [Google Scholar]
- [132]. Karšaj, I. , and Humphrey, J. D. , 2009, “A Mathematical Model of Evolving Mechanical Properties of Intraluminal Thrombus,” Biorheology, 46(6), pp. 509–527. 10.3233/BIR-2009-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]