Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that accounts for the major cause of dementia, and the increasing worldwide prevalence of AD is a major public health concern. Increasing epidemiological studies suggest that diet and nutrition might be important modifiable risk factors for AD. Dietary supplementation of antioxidants, B vitamins, polyphenols, and polyunsaturated fatty acids are beneficial to AD, and consumptions of fish, fruits, vegetables, coffee, and light-to-moderate alcohol reduce the risk of AD. However, many of the results from randomized controlled trials are contradictory to that of epidemiological studies. Dietary patterns summarizing an overall diet are gaining momentum in recent years. Adherence to a healthy diet, the Japanese diet, and the Mediterranean diet is associated with a lower risk of AD. This paper will focus on the evidence linking many nutrients, foods, and dietary patterns to AD.
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that accounts for the major cause of dementia in the world [1, 2]. The number of the disease is projected to reach 106.8 million worldwide by the year 2050; therefore, the disease is a growing public health concern with major socioeconomic burden [3].
Much attention has been paid to disease-modifying factors and risk factors for AD [4]. Cognitive engagement and physical activities have been associated with decreased risk of AD, while diabetes, epsilon 4 allele of the apolipoprotein E gene (APOE ε4), smoking, and depression have been associated with increased risk of AD [5]. In recent years, there has been increasing evidence supporting the role of nutrition in AD [6–8]. A number of dietary factors such as antioxidants, vitamins, polyphenols, and fish have been reported to decrease the risk of AD, while saturated fatty acids, high-calorie intake, and excess alcohol consumption were identified as risk factors [9]. Dietary patterns, which better reflex the complexity of diet, have emerged in recent years to examine the relationship between diet and AD [10]. In this paper, we will investigate the evidence linking nutrients, foods, beverages, and dietary patterns to the risk of AD.
2. AD Is Associated with Both Obesity and Malnutrition
Obesity and overweight seem to be associated with AD [11–13]. However, the evidence relating obesity measured with body mass index (BMI) with AD is conflicting. Obesity (BMI > 30) in midlife has been found to increase the risk of AD, while late-life obesity was found to reduce the risk of AD [11]. Therefore, manipulation of adiposity may provide a means to prevent AD [14].
Malnutrition and weight loss are frequent complications of AD, the mean prevalence of malnutrition in AD patients living at home is 5% as reported by Guigoz et al. [15]. Patients with AD had a worse nutritional status compared to that of controls [16], and a baseline lower nutritional status was reported to indicate the progression of AD [17]. In addition, weight loss was reported to predict rapid cognitive decline in AD patients [18], and treatment of weight loss and malnutrition may also be important in AD patients.
3. The Effects of Nutrients on the Risk of AD
Many nutrients, such as antioxidants, vitamins, fat, and carbohydrates, can affect the risk of AD. Although the mechanisms of these nutrients on AD are not clear, reducing the oxidative stress and amyloid beta-peptide (Aβ) accumulation is considered to play a role in the process of AD [19, 20].
3.1. Antioxidants
The oxidative stress, the undue oxidation of biomolecules leading to cellular damage, promotes many studies of antioxidants in the prevention of AD [21].
3.1.1. Vitamin A and β-Carotene
Vitamin A and β-carotene could be key molecules for the prevention and therapy of AD, due to their ability to inhibit the formation of both Aβ oligomers and fibrils [20]. It has been shown in vitro that vitamin A and β-carotene have antioligomerization effects on Aβ [22]. Low serum and plasma concentrations of vitamin A and β-carotene have been seen in AD patients [23, 24], and a higher β-carotene plasma level was associated with better memory performance [25]. Data on the supplementation of vitamin A alone in AD were not available.
3.1.2. Vitamin C
Vitamin C has been proven to reduce Aβ oligomer formation and oxidative stress in vitro and in vivo studies [26, 27]. Data from cohort studies about the supplement effect of vitamin C on AD are conflicting. A prospective study (n = 980) evaluating the relationship between 4 years of vitamin C and vitamin E intakes and the incidence of AD showed no difference in the incidence of AD during the 4-year followup [28], and the same relationship was also found in another prospective study (n = 5395) showing that vitamin C intake was not associated with AD risk [29]. However, results from mostly published prospective observational studies (n = 4740) suggested that the combined use of vitamin C and vitamin E for at least 3 years was associated with the reduction of AD prevalence and incidence [30]. Overall, there is a large body of evidence that maintaining healthy vitamin C levels can have a protective function against AD, but avoiding vitamin C deficiency is likely to be more beneficial than taking supplements on top of a normal, healthy diet [31].
3.1.3. Vitamin E
Vitamin E is a lipid-soluble antioxidant that has been found to confer neuroprotection by inhibiting oxidative stress [32–34] and scavenging Aβ-associated free radicals [35]. Compared to cognitive normal subjects, AD and mild cognitive impairment (MCI) had lower levels of total tocopherols, total tocotrienols, and total vitamin E [36]. When considered each vitamin E form alone, intake of α-tocopherols and γ-tocopherols was associated with a slower rate of cognitive decline [37]. However, in a double-blind, randomized controlled study with 769 subjects, there were no significant differences in the probability of progression to AD in the vitamin E group compared to the placebo group [38]. There were limitations to the study, for example, the forms of vitamin E were not clarified. In addition, the composition of vitamin E supplement might not reflex the actual composition in the diet. At present, there is no reliable evidence of efficacy of vitamin E in the prevention or treatment of people with AD; thus, more research is needed [39].
3.1.4. Selenium
Current knowledge provides no evidence of a role of selenium (Se) in the treatment of AD but allows speculation on a potential preventive relevance [40], and selenium has been reported to play an important role in the antioxidative defense [41, 42]. AD patients showed a significant lower Se level in plasma, erythrocytes, and nails when compared to controls [43]. Several interventional trials demonstrated that supplementations of selenium-containing mixtures improved cognition [44–46]; however, as the authors did not specify the form of Se, the validity of the interventional trials was limited. In addition, the window of selenium's biological efficacy is a narrow one. The relationship between Se supplementation and AD requires confirmation by randomized trials.
3.1.5. Polyphenols
Polyphenols are natural antioxidants that provide protective effects to AD through a variety of biological actions, such as interaction with transition metals, inactivation of free radicals, inhibition of inflammatory response, modulation in the activity of different enzymes, and effects on intracellular signaling pathways and gene expression [47–49]. Several animal studies have demonstrated that polyphenols inhibited Aβ formation and attenuated cognitive deterioration [50–54]. Data from a randomized, double-blind controlled clinical trial of polyphenols supplementation in 100 subjects showed that polyphenols contained in antioxidant beverages might benefit AD patients by decreasing homocysteine concentrations in AD patients [55].
3.2. B Vitamins (Folic Acid, Vitamin B6, and Vitamin B12)
B vitamins might contribute to AD by inhibiting oxidative stress and lowering the concentrations of homocysteine [56, 57]. Vitamin B6 has been reported to inhibit oxidative stress in AD [56]. High concentrations of homocysteine have been linked to an increased risk of AD [58–60], and homocysteine was significantly elevated in AD patients [61]; high dose supplementation of vitamin B6, B12, and folate lowers plasma homocysteine concentrations in AD patients [62]; homocysteine-lowering treatment might be a therapeutic target for AD.
In a cohort of 816 subjects, low serum folate concentrations were reported to increase the risk of AD [59], and increased dietary intake of folate decreased the risk of AD [72, 73]. A meta-analysis of 9 folic acid supplements versus placebo in 2,835 participants suggested that folic acid, with or without other B vitamins, had no effect on cognitive function within 3 years of the start of treatment [74]. In a randomized controlled trial of homocysteine-lowering treatment with B vitamins, 140 subjects with mild-to-moderate AD or vascular dementia were assigned to take 1 mg methylcobalamin and 5 mg folic acid, or placebo once daily for 24 months; there was no significant group difference in changes in any of the neuropsychological scores [75]. However, in another randomized controlled trial, 266 participants with MCI were randomly assigned to receive a daily dose of 0.8 mg folic acid, 0.5 mg vitamin B12, and 20 mg vitamin B6, or placebo for 2 years; the mean plasma homocysteine concentration was 30% lower in those treated with B vitamins relative to placebo there was significant benefit of B vitamin treatment among participants with baseline homocysteine above the median (11.3 µmol/L) in global cognition, episodic memory and semantic memory [76]. The reasons for the discrepancy in findings for the effect of B vitamins AD and cognition might be the nonuniform choice of regimen, the different pathological courses of the patients, and the diverse measurements of the results. Thus, the available evidence, so far, is insufficient to draw definitive conclusion on the association of B vitamins with cognitive decline [77].
3.3. Vitamin D
Vitamin D might have little association with Aβ mechanisms and its potential association with AD might involve other pathways, such as antioxidative, vascular, anti-inflammatory, or metabolic pathways [78]. Genetic studies have provided the opportunity to determine that vitamin D is associated with AD risk [79, 80].
A meta-analysis of 10 studies showed that AD cases had lower serum vitamin D concentrations than matched controls [81]. In a study with 1,604 men, little evidence of associations between lower 25-hydroxyvitamin D level and cognitive function was found [82]; however, data from another large population-based study with 5,596 community-dwelling women showed that women with inadequate intakes had a lower mean Pfeiffer Short Portable Mental State Questionnaire (SPMSQ) score compared to women with recommended weekly vitamin D dietary intakes [83]. The cross-sectional association between vitamin D and cognition strengthened the hypothesis that correcting hypovitaminosis D among older adults could prevent cognitive decline but prevent the finding of a cause and effect link. Randomized controlled trials testing vitamin D supplements versus placebo should be the next step [84].
3.4. Metals
Dysfunctional homeostasis of transition metals is believed to play a role in the pathogenesis of AD by forming reactive species through metal amyloid complexes [85, 86]. Modulating metals has been proposed as a therapeutic strategy for AD [87]; bivalent cation chelators such as clioquinol and its later derivatives are being developed as a novel AD drug [88].
3.4.1. Copper
Copper is essential for life, but in excess can be toxic. High dietary intake of copper in conjunction with a diet high in saturated and transfats was reported to be associated with cognitive decline [89, 90].
A meta-analysis of 17 studies with 1425 subjects showed that AD patients have higher levels of serum copper than controls [91]. Copper dysfunction is thought to play a role in AD pathology [92]; however, data from a prospective, randomized, placebo-controlled trial with 68 subjects showed that oral copper supplementation had neither a detrimental nor a promoting effect on the progression of AD [93].
3.4.2. Iron
Iron mediates the oxidative stress in AD, and an imbalance in iron homeostasis is thought as a precursor to AD [94, 95]. Diets excessive in Fe together with a high intake of saturated fat acids have been recommended to be avoided in the elderly [89]. However, iron supplementation has been reported to improve attention and concentration irrespective of baseline iron status in older children and adults [96].
3.4.3. Zinc
Zinc supplementation was found to reduce both Aβ and tau pathologies in the hippocampus and to delay hippocampus-dependent memory deficits in AD mouse model [97]. Zinc deficiency was reported to be associated with cognition loss in AD patients [98].
3.5. Fats
Different consumption levels of the major specific fat types, rather than total fat intake itself, appeared to influence cognitive aging. Higher monounsaturated fatty acid was related to better cognitive function, while higher saturated fatty acid was associated with worse cognitive function. Total fat, polyunsaturated fatty acid, transfat intakes were not associated with cognition changes [99].
3.5.1. Monounsaturated Fatty Acids
Monounsaturated fatty acids (MUFAs) and MUFA derivatives have anti-inflammatory effects in vivo [100, 101], and derivatives of MUFA, including low molecular weight phenols, were reported to have antioxidant effects [102]. Data from a prospective study suggested that higher intake of monounsaturated fatty acid is associated with less cognitive decline [103].
3.5.2. Polyunsaturated Fatty Acids (Omega-3 Polyunsaturated Fatty Acids)
Current evidence suggests that elevated intake of polyunsaturated fatty acids might be beneficial to AD [104–106]. Dietary supplementation of omega-3 polyunsaturated fatty acids was reported to affect expression of genes that might influence inflammatory process [107]; however, the protective effects might be limited to APOE epslion4 noncarriers [108]. Dacosahexaenoic acid (DHA), the main form of omega-3 fatty acids, has been demonstrated to reduce Aβ production and pathological changes in AD animal models [109–112]. A meta-analysis of 11 observational studies and 4 clinical trials showed that omega-3 fatty acids slowed cognitive decline in elderly individuals without dementia [113]. However, data from randomized controlled trials showed that supplementation with DHA and eicosapentaenoic acid (EPA), compared with placebo, did not slow the rate of cognitive decline and functional decline [114–116]. The contradictory results between observational studies and randomized controlled trials might be that the duration of randomized controlled trials was often not long enough.
3.5.3. Saturated Fatty Acids
Elevated intake of saturated fatty acids could have negative effects on cognitive functions [117]. In a study of 1,449 participants with an average followup of 21 years, moderate intake of saturated fatty acids was associated with an increased risk of AD and dementia, especially among APOE epslion4 carriers, whereas a higher intake did not affect the risk [118], suggesting that there may be a threshold association.
3.5.4. Transfatty Acid
Transfatty acid might potentially increase AD risk or cause an earlier onset of the disease by increasing the production of Aβ through increase of amyloidogenic and decrease of nonamyloidogenic processing of amyloid precursor protein [119]. However, in a prospective study with 482 women over a followup of 3 years, a validated food frequency was administrated twice to assess dietary intake before cognitive assessment; greater intake of transfat was not associated with cognitive decline [103]. No reliable data from randomized trials on the association of transfatty acids with AD were available.
3.6. Carbohydrates
It has been suggested that patients with T2DM (type 2 diabetes mellitus) are at an increased risk of getting AD [5]. Deficient brain insulin signaling pathway has been proposed as the common mechanism in the two disorders [120]. In AD patient brains, reduced insulin levels, insulin receptor expression, and insulin resistance have been reported [121–123]. With increased exposure to glucose, multiple proteins in neurons are susceptible to glycation, which is viewed as being an important contributor to AD [124]. Therefore, a diet high in carbohydrates may be detrimental to AD [125, 126]. However, in a prospective study with 939 participants over 6.3 years of followup, glycemic load reflexing carbohydrate content in food was not associated with a higher risk of AD [127]. No reliable data from randomized trials on a diet high in carbohydrate and AD were available.
4. The Effects of Foods and Beverages on the Risk of AD
Single nutrients are not consumed in isolation but as a part of diet; examining the role of single nutrients is complicated and difficult due to the interaction between nutrients. Therefore, examining foods rather than single nutrients might be more useful, and many foods and beverages have been reported to affect the risk of AD (Figure 1).
Figure 1.
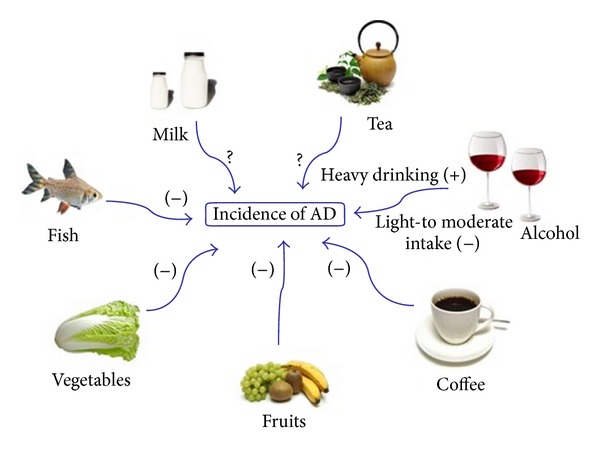
Foods and beverages that influence the incidence of AD. Fish, vegetables, fruits, coffee, and light-to-moderate alcohol intake are reported to reduce AD incidence. Milk and tea are reported to influence cognition, but their influence on AD is not clear.
4.1. Fish
Epidemiological studies suggest that fish consumption can reduce the risk of dementia and AD, especially among APOE epslion4 non-carriers [128–132]. The positive link is thought to be associated with marine long chain omega-3 fatty acids, EPA, and DHA. The belief that consumption of fish as a whole is gaining popularity. In a prospective study with 815 participants aged from 65 to 94 years, consumption of fish more than once a week had 60% less risk of AD compared with those who rarely or never ate fish [131]. Data from randomized trials of the effect of whole-fish consumption on the risk of AD were not available.
4.2. Fruits and Vegetables
Frequent consumption of fruits and vegetables might decrease the risk of AD and dementia [128]. A medium or great proportion of fruits and vegetables in the diet, compared with no or small proportion, was associated with a decreased risk of AD and dementia [133]. If the association between fruits and vegetables intake and AD is validated, the mechanism might be that fruits and vegetables are rich sources of antioxidants and bioactive compounds (e.g., vitamin E, vitamin C, carotenoids, and flavonoids) and also low in saturated fats [134].
Higher vegetable, but not fruit, consumption was reported to be associated with slower rate of cognitive decline in a cohort of 3,718 participants aged 65 years and older; among types of vegetables, green leafy vegetables had the strongest association [134]. The paradoxical results might be due to that vegetables, especially green leafy vegetables, contain more vitamin E than fruits, and some unknown dietary component offsets the protective effects of antioxidants in fruits. In another cohort of 2,613 participants aged 43–70 years old, total intakes of fruits, legumes, and juices were not associated with change in cognitive cognition, while higher intakes of some subgroups (e.g., nuts, cabbage, and root vegetables) may diminish age-related cognitive decline in middle-aged individuals [135]. Data from randomized controlled trials were not available.
4.3. Dairy
A lower consumption of milk or dairy products was found to be associated with poor cognitive function [136, 137]. Dairy, rich in vitamin D, phosphorus, and magnesium may reduce the risk of cognitive impairment by decreasing vascular alterations and structural brain changes that occur with cognitive decline [136]. However, the consumption of whole-fat dairy products may be associated with cognitive decline in the elderly [136]. Moderate intake of unsaturated fats from milk products and spreads at midlife decreased the risk of AD, while saturated fat intake from milk products and spreads at midlife was associated with an increased risk of AD [118, 138]. Unfortunately, the observational studies examined diary as a component of dietary intake not as their primary focus, and there was no evidence available from randomized controlled trials.
4.4. Coffee
Coffee drinking may be associated with a decreased risk of AD [139]. A trend towards a protective effect of caffeine on AD was reported [140, 141]. Coffee may be the best source of caffeine to protect against AD due to a component in coffee that synergizes with caffeine to selectively enhance plasma cytokines [142]. A quantitative review of four studies (two case-control studies and two cohorts) showed that coffee consumption is inversely associated with the risk of AD, compared to nonconsumers; the risk estimate of AD in coffee consumers is 0.70 with 95% confidence interval 0.55–0.90 [143]. However, the four studies had heterogeneous methodologies and results, so further prospective studies evaluating the consumption of coffee and AD are strongly needed.
4.5. Tea
Observational studies suggest that tea drinking was associated with lower risks of cognitive impairment and decline [144, 145], and the protective effect was not limited to a particular type of tea [144]. Black tea was shown to significantly enhance auditory and visual attention compared to placebo [146]. Green tea polyphenols may inhibit cognitive impairment via modulating oxidative stress [147–149], and green tea epigallocatechin-3-gallate (EGCG) has been shown to reduce β-amyloid generation and sarkosyl-soluble phosphorylated tau isoforms in AD mouse models [150, 151]. The neuroprotective effects of tea consumption could be due to catechins, L-theanine, polyphenols, and other compounds in tea leaves [152]. Therefore, tea might be a relevant contributor to AD.
4.6. Alcohol
Epidemiological studies suggest that light-to-moderate alcohol intake was associated with a reduced risk of AD, particularly among APOE epslion4 non-carriers [153–155]. However, heavy drinking (>2 drinks), alongside with heavy smoking and APOE epsilon4, was associated with an earlier onset of AD [156]. The mechanisms by which low-to moderate intake could be protective against AD while heavy intake was detrimental to were unclear.
Different types of alcohol (wine, beer, and mixed alcohol beverages) may have different effects on AD. Resveratrol and other polyphenols in red wine have been found to diminish plaque formation and protect against Aβ-induced neurotoxicity [157–159], and moderate beer consumption was thought to afford a protective factor for AD due to its content in bioavailability silicon [160]. Therefore, alcohol intake might provide benefits to AD, but the quantity and type of alcohol were not clear.
5. The Effects of Dietary Patterns on the Risk of AD (Figure 2)
Figure 2.
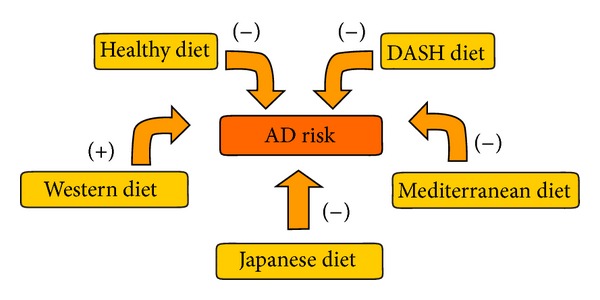
Dietary patterns that influence the risk of AD. Healthy diet, DASH-diet, Mediterranean diet, and Japanese diet might decrease the risk of AD. Western diet might increase the risk of AD. DASH diet: the Dietary Approaches to Stop Hypertension.
Dietary pattern, a combination of food components that summarizes an overall diet for a study population, can have various effects on cognitive function and AD (Table 1). A dietary pattern, characterized by a high intake of meat, butter, high-fat dairy products, eggs, and refined sugar, has been found in AD patients [161].
Table 1.
Summary of studies linking dietary pattern to cognitive function and AD.
| Reference | Participants | Design | Result |
|---|---|---|---|
| Ozawa et al. [63] | 1006 Japanese community | Cohort | A higher adherence to a dietary pattern characterized by a high intake of soybeans and soybean products, vegetables, algae, and milk and dairy products and a low intake of rice is associated with dementia in the general Japanese population. |
|
| |||
| Kesse-Guyot et al. [64] | 3054 participants | Cohort | The healthy pattern was associated with better global cognitive function (50.1 ± 0.7 versus 48.9 ± 0.7; P trend = 0.001) and verbal memory (49.7 ± 0.4 versus 48.7 ± 0.4; P trend = 0.01). |
|
| |||
| Samieri et al. [65] | 1724 elderly community dwellers | Cohort | A “healthy” cluster characterized by higher consumption of fish by men and fruits and vegetables by women had a significantly lower mean number of errors to Mini Mental State score after adjustment for sociodemographic variables (beta = −0.11; 95% confidence interval (CI), −0.22 to −0.004 in men; beta = −0.13; 95% CI, −0.22 to −0.04 in women). |
|
| |||
| Eskelinen et al. [66] | 525 subjects | Cohort | Persons with a healthy diet (healthy-diet index >8 points) had a decreased risk of AD (OR 0.08, 95% CI 0.01–0.09) compared to persons with an unhealthy diet (0–8 points) |
|
| |||
| Smith et al. [67] | 124 participants with elevated blood pressure | Randomized controlled trial | DASH diet combining aerobic exercise and caloric restriction improves neurocognitive function among sedentary and overweight/obese individuals with prehypertension and hypertension. |
|
| |||
| Scarmeas et al. [68] | 2258 nondemented individuals | Case control | Higher adherence to the MeDi was associated with lower risk for AD (odds ratio, 0.76; 95% confidence interval, 0.67–0.87; P < 0.001). Compared with subjects in the lowest MeDi tertile, subjects in the middle MeDi tertile had an odds ratio of 0.47 (95% confidence interval, 0.29–0.76) and those at the highest tertile had an odds ratio of 0.32 (95% confidence interval, 0.17–0.59) for AD (P for trend <0.001). |
|
| |||
| Scarmeas et al. [69] | 1393 cognitively normal participants | Cohort | Higher adherence to the MeDi is associated with a trend for reduced risk of developing MCI and with reduced risk of MCI conversion to AD. |
|
| |||
| Féart et al. [70] | 1410 adults aged over 65 y | Cohort | Higher adherence to a Mediterranean diet was associated with slower MMSE cognitive decline but not consistently with other cognitive tests. Higher adherence was not associated with risk for incident dementia. |
|
| |||
| Tangney et al. [71] | 3790 participants aged over 65 | Cohort | The Mediterranean dietary pattern may reduce the rate of cognitive decline with older age. |
5.1. Western Diet
A Western diet is characterized by higher intake of red and processed meats, refined grains, sweets, and desserts [162]. A high-fat Western diet may contribute to the development of AD by impacting Aβ deposition and oxidative stress [163, 164]. Data from epidemiological studies exploring Western diet and the risk of AD were not available.
5.2. Japanese Diet
Traditional Japanese diet is characterized by increased intake of fish and plant foods (soybean products, seaweeds, vegetables, and fruits) and decreased intake of refined carbohydrates and animal fats (meat) [165]. In a population-based study with a total of 1006 Japanese subjects followed by 15 years, a dietary characterized by a high intake of soybeans and soybean products, vegetables, algae, and milk and dairy products and a low intake of rice was associated with a reduced risk of AD [63].
5.3. Health Diets
In a cohort of 3054 participants, a healthy diet was defined as one positively correlated with consumption of fruit, whole grains, fresh dairy products, vegetables, breakfast cereal, tea, vegetable fat, nuts, and fish and negatively correlated with meat, poultry, refined grains, animal fat, and processed meat. Participants with the highest compared with lowest adherence to the health diet had a better cognitive function [64]. A healthy diet, characterized by higher consumption of fish by men and fruits and vegetables by women, was also reported to be associated with better cognitive performance [65]. In another study with 525 subjects, a healthy diet index was constructed to assess healthy and unhealthy diet components; persons with a healthy diet (healthy-diet index >8 points) had a decreased risk of AD [66].
5.4. DASH-Style Diets
The Dietary Approaches to Stop Hypertension (DASH) diet contains a high intake of plant foods, fruits, vegetables, fish, poultry, whole grains, low-fat dairy products, and nuts, while minimizing intake of red meat, sodium, sweets, and sugar-sweetened beverages [165]. In a randomized clinical trial of 124 participants with elevated blood pressure, subjects on the DASH diet exhibited greater neurocognitive improvements when compared to normal subjects [67]. As hypertension is associated with increased risk for AD [166], it is biologically plausible that DASH could reduce the risk of AD.
5.5. Mediterranean Diets
The Mediterranean diet, a typical diet of the Mediterranean region, is characterized by a high consumption of fruits, vegetables, cereals, bread, potatoes, poultry, beans, nuts, olive oil, and fish; a moderate consumption of alcohol; a lower consumption of red meat and dairy products.
Adherence to the Mediterranean diet may not only affect the risk of AD but also mortality in AD [167]. A meta-analysis of eighteen cohort studies with 2,190,627 subjects showed that adherence to the Mediterranean diet was associated with a significant reduction of overall mortality and neurodegenerative diseases [168]. Several researches supported a beneficial association between adherence to a Mediterranean diet and AD [68, 69]. As fruits, and vegetables, fish, and moderate alcohol reduced the risk of AD [128, 130, 131, 153–155], in spite of lack of data from randomized controlled trials, the Mediterranean diet can be thought to be beneficial to AD.
6. Conclusions and Future Directions
In this paper, we searched PubMed articles published from 2000 to 2013, using the search terms “Alzheimer's disease,” “nutrition,” “nutrients,” “food,” “diet,” “dietary patterns,” “overweight,” “obesity,” “prospective cohort studies,” “randomized controlled trials,” “systematic review,” and “meta-analysis.” Articles were also identified through searches of lists. Studies were selected for inclusion on the basis of a judgment about the quality of the evidence according to four key elements: study design, study quality, consistency, and directness, as proposed by the Grading of Recommendations Assessment, Development and Evaluating (GRADE) working group. For each nutrient, food, or dietary pattern, only the studies with the highest level of evidence were included. If randomized trials had not been undertaken and only observational data were available, studies were included if they were prospective, population-based, and large, with standardized diagnostic criteria for AD. Studies were excluded if serious limitations to study quality and major uncertainty about directness existed. Only articles published in English were included.
Epidemiological studies suggest antioxidants, vitamins, polyphenols, polyunsaturated fatty acids, fish, fruits, vegetables, tea, and light-to moderate consumption of alcohol are beneficial for AD, while trans-fatty acids, saturated fatty acids, carbohydrates, and whole-fat dairy are detrimental to AD. However, epidemiological studies cannot eliminate bias and confounding in any association between a risk factor and AD [169]; the results of such studies should be interpreted with caution. In addition, it is difficult to examine the individual effects of nutrients and foods because they are correlated with each other; therefore, the idea of focusing on diet as a whole is gaining momentum.
Randomization is the best method to minimize bias and confounding and establish causality. However, randomized trials are not always feasible, and the few randomized controlled trials that have been undertaken provide conclusions that dietary supplementation of vitamin E, B vitamins, and polyunsaturated fatty acids does not reduce cognitive decline and the risk of AD. Several reasons might be responsible for the discrepancy between observational studies and randomized controlled trials. First, nutrients might be useful only for primary prevention of AD, and not protective once the pathological process started. Second, the dose of nutrients might not be equivalent to levels seen in the epidemiological studies. Third, the duration of most trials has been suggested to be inadequate to show benefits. Besides, the association between nutrients and epidemiological studies is confounded by social and behavioural factors acting across the life course [170].
Dietary pattern, which better reflexes the complexity of diet, has emerged in recent years to examine the relationship between diet and AD. Adherence to the Mediterranean diet, the Japanese diet, and the healthy diet has been reported to be associated with decreased risk of AD. Given that the studies relating dietary patterns to AD are very few, further studies are needed. In general, the very few studies that have been done suggested that higher intake of fruits, vegetables, fish, nuts, legumes, cereal, lower intake of meats, high fat diary, sodium, sweets, and refined grains seemed to be associated with reduced risk of AD.
Further researches are needed to improve the quality of evidence relating to the association of many nutrients, foods, and dietary patterns with AD. To establish a causative role for specific nutrients, foods, and dietary patterns in the pathogenesis of AD, adequately powered, large randomized trials are needed in which the patient population and intervention are carefully described.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81000544, 81171209), the Shandong Provincial Natural Science Foundation, China, (ZR2010HQ004, ZR2011HZ001), and the Shandong Provincial Outstanding Medical Academic Professional Program.
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. The Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Jicha GA, Carr SA. Conceptual evolution in Alzheimer’s disease: implications for understanding the clinical phenotype of progressive neurodegenerative disease. Journal of Alzheimer’s Disease. 2010;19(1):253–272. doi: 10.3233/JAD-2010-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s and Dementia. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 4.Jiang T, Yu JT, Tan L. Novel disease-modifying therapies for Alzheimer’s disease. Journal of Alzheimer's Disease. 2012;31(3):475–492. doi: 10.3233/JAD-2012-120640. [DOI] [PubMed] [Google Scholar]
- 5.Williams JW, Plassman BL, Burke J, Benjamin S. Preventing Alzheimer’s disease and cognitive decline. Evidence Report/Technology Assessment. 2010;(193):1–727. [PMC free article] [PubMed] [Google Scholar]
- 6.Dosunmu R, Wu J, Basha MR, Zawia NH. Environmental and dietary risk factors in Alzheimer’s disease. Expert Review of Neurotherapeutics. 2007;7(7):887–900. doi: 10.1586/14737175.7.7.887. [DOI] [PubMed] [Google Scholar]
- 7.Lau FC, Shukitt-Hale B, Joseph JA. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. Sub-cellular biochemistry. 2007;42:299–318. [PubMed] [Google Scholar]
- 8.Shah R. The role of nutrition and diet in Alzheimer disease: a systematic review. Journal of the American Medical Directors Association. 2013 doi: 10.1016/j.jamda.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Ramassamy C, Belkacémi A. Nutrition and alzheimer’s disease: is there any connection? Current Alzheimer Research. 2011;8(5):443–444. doi: 10.2174/156720511796391890. [DOI] [PubMed] [Google Scholar]
- 10.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Current Opinion in Lipidology. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Archives of Neurology. 2009;66(3):336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 13.Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central obesity in the elderly is related to late-onset Alzheimer disease. Alzheimer Disease & Associated Disorders. 2012;26(2):101–105. doi: 10.1097/WAD.0b013e318222f0d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luchsinger JA, Gustafson DR. Adiposity and Alzheimer’s disease. Current Opinion in Clinical Nutrition & Metabolic Care. 2009;12(1):15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition the mini nutritional assessment. Clinics in Geriatric Medicine. 2002;18(4):737–757. doi: 10.1016/s0749-0690(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 16.Saragat B, Buffa R, Mereu E, et al. Nutritional and psycho-functional status in elderly patients with Alzheimer’s disease. Journal of Nutrition, Health and Aging. 2012;16(3):231–236. doi: 10.1007/s12603-011-0347-3. [DOI] [PubMed] [Google Scholar]
- 17.Ousset P-J, Nourhashemi F, Reynish E, Vellas B. Nutritional status is associated with disease progression in very mild Alzheimer disease. Alzheimer Disease and Associated Disorders. 2008;22(1):66–71. doi: 10.1097/WAD.0b013e31815a9dbb. [DOI] [PubMed] [Google Scholar]
- 18.Soto ME, Secher M, Gillette-Guyonnet S, et al. Weight loss and rapid cognitive decline in community-dwelling patients with Alzheimer’s disease. Journal of Alzheimer’s Disease. 2012;28(3):647–654. doi: 10.3233/JAD-2011-110713. [DOI] [PubMed] [Google Scholar]
- 19.Clark TA, Lee HP, Rolston RK, et al. Oxidative stress and its implications for future treatments and management of alzheimer disease. International Journal of Biomedical Science. 2010;6(3):225–227. [PMC free article] [PubMed] [Google Scholar]
- 20.Ono K, Yamada M. Vitamin A and Alzheimer’s disease. Geriatrics and Gerontology International. 2012;12(2):180–188. doi: 10.1111/j.1447-0594.2011.00786.x. [DOI] [PubMed] [Google Scholar]
- 21.Viña J, Lloret A, Giraldo E, Badia MC, Alonso MD. Antioxidant pathways in Alzheimer’s disease: possibilities of intervention. Current Pharmaceutical Design. 2011;17(35):3861–3864. doi: 10.2174/138161211798357755. [DOI] [PubMed] [Google Scholar]
- 22.Takasaki J, Ono K, Yoshiike Y, et al. Vitamin A has anti-oligomerization effects on amyloid-β in vitro. Journal of Alzheimer’s Disease. 2011;27(2):271–280. doi: 10.3233/JAD-2011-110455. [DOI] [PubMed] [Google Scholar]
- 23.Bourdel-Marchasson I, Delmas-Beauviex M-C, Peuchant E, et al. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age and Ageing. 2001;30(3):235–241. doi: 10.1093/ageing/30.3.235. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez-Jimenez FJ, Molina JA, de Bustos F, et al. Serum levels of beta-carotene, alpha-carotene and vitamin A in patients with Alzheimer’s disease. European Journal of Neurology. 1999;6(4):495–497. doi: 10.1046/j.1468-1331.1999.640495.x. [DOI] [PubMed] [Google Scholar]
- 25.Perrig WJ, Perrig P, Stähelin HB. The relation between antioxidants and memory performance in the old and very old. Journal of the American Geriatrics Society. 1997;45(6):718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 26.Montilla-López P, Muoz-Águeda MC, Feijóo López M, Muoz-Castaeda JR, Bujalance-Arenas I, Túnez-Fiana I. Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer’s disease induced by okadaic acid in neuroblastoma cells. European Journal of Pharmacology. 2002;451(3):237–243. doi: 10.1016/s0014-2999(02)02151-9. [DOI] [PubMed] [Google Scholar]
- 27.Murakami K, Murata N, Ozawa Y, et al. Vitamin C restores behavioral deficits and amyloid-β oligomerization without affecting plaque formation in a mouse model of alzheimer’s disease. Journal of Alzheimer’s Disease. 2011;26(1):7–18. doi: 10.3233/JAD-2011-101971. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Tang M-X, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Archives of Neurology. 2003;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 29.Devore EE, Grodstein F, van Rooij FJA, et al. Dietary antioxidants and long-term risk of dementia. Archives of Neurology. 2010;67(7):819–825. doi: 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Archives of Neurology. 2004;61(1):82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 31.Harrison FE. A critical review of vitamin C for the prevention of age-related cognitive decline and alzheimer’s disease. Journal of Alzheimer’s Disease. 2012;29(4):711–726. doi: 10.3233/JAD-2012-111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan J-Z, Guan W-P, Maeda T, Makino N. Effect of vitamin E administration on the elevated oxygen stress and the telomeric and subtelomeric status in Alzheimer’s disease. Gerontology. 2011;58(1):62–69. doi: 10.1159/000327821. [DOI] [PubMed] [Google Scholar]
- 33.Kaneai N, Arai M, Takatsu H, Fukui K, Urano S. Vitamin E inhibits oxidative stress-induced denaturation of nerve terminal proteins involved in neurotransmission. Journal of Alzheimer’s Disease. 2012;28(1):183–189. doi: 10.3233/JAD-2011-111133. [DOI] [PubMed] [Google Scholar]
- 34.Khanna S, Parinandi NL, Kotha SR, et al. Nanomolar vitamin e α-tocotrienol inhibits glutamate-induced activation of phospholipase A2 and causes neuroprotection. Journal of Neurochemistry. 2010;112(5):1249–1260. doi: 10.1111/j.1471-4159.2009.06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yatin SM, Varadarajan S, Butterfield DA. Vitamin E prevents Alzheimer’s amyloid β-peptide (1-42)-induced neuronal protein oxidation and reactive oxygen species production. Journal of Alzheimer’s Disease. 2000;2(2):123–131. doi: 10.3233/jad-2000-2212. [DOI] [PubMed] [Google Scholar]
- 36.Mangialasche F, Xu W, Kivipelto M, et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiology of Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Morris MC, Evans DA, Tangney CC, et al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. American Journal of Clinical Nutrition. 2005;81(2):508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 38.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Archives of Internal Medicine. 2006;166(22):2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 39.Isaac MGEKN, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database of Systematic Reviews. 2008;(3) doi: 10.1002/14651858.CD002854.pub2.CD002854 [DOI] [PubMed] [Google Scholar]
- 40.Loef M, Schrauzer GN, Walach H. Selenium and alzheimer’s disease: a systematic review. Journal of Alzheimer’s Disease. 2011;26(1):81–104. doi: 10.3233/JAD-2011-110414. [DOI] [PubMed] [Google Scholar]
- 41.Ishrat T, Parveen K, Khan MM, et al. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Research. 2009;1281:117–127. doi: 10.1016/j.brainres.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed J, Wei WL, Husin NNA, Alwahaibi NY, Budin SB. Selenium supplementation reduced oxidative stress in diethylnitrosamine-induced hepatocellular carcinoma in rats. Pakistan Journal of Biological Sciences. 2011;14(23):1055–1060. doi: 10.3923/pjbs.2011.1055.1060. [DOI] [PubMed] [Google Scholar]
- 43.Cardoso BR, Ong TP, Jacob-Filho W, Jaluul O, Freitas MID, Cozzolino SMF. Nutritional status of selenium in Alzheimer’s disease patients. British Journal of Nutrition. 2010;103(6):803–806. doi: 10.1017/S0007114509992832. [DOI] [PubMed] [Google Scholar]
- 44.Chandra RK. Effect of vitamin and trace-element supplementation on cognitive function in elderly subjects. Nutrition. 2001;17(9):709–712. doi: 10.1016/s0899-9007(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 45.Cornelli U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegenerative Diseases. 2010;7(1–3):193–202. doi: 10.1159/000295663. [DOI] [PubMed] [Google Scholar]
- 46.Scheltens P, Kamphuis PJGH, Verhey FRJ, et al. Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimer’s and Dementia. 2010;6(1):1.e1–10.e1. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Choi D-Y, Lee Y-J, Hong JT, Lee H-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Research Bulletin. 2012;87(2-3):144–153. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Obrenovich ME, Nair NG, Beyaz A, Aliev G, Reddy VP. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Research. 2010;13(6):631–643. doi: 10.1089/rej.2010.1043. [DOI] [PubMed] [Google Scholar]
- 49.Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. Journal of Nutrition. 2013;143(5):597–605. doi: 10.3945/jn.112.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartman RE, Shah A, Fagan AM, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiology of Disease. 2006;24(3):506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Ho L, Chen LH, Wang J, et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. Journal of Alzheimer’s Disease. 2009;16(1):59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mori T, Rezai-Zadeh K, Koyama N, et al. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. Journal of Biological Chemistry. 2012;287(9):6912–6927. doi: 10.1074/jbc.M111.294025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Ho L, Zhao W, et al. Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. Journal of Neuroscience. 2008;28(25):6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Ho L, Zhao Z, et al. Moderate consumption of Cabernet Sauvignon attenuates Aβ neuropathology in a mouse model of Alzheimer’s disease. The FASEB Journal. 2006;20(13):2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 55.Galasko DR, Peskind E, Clark CM, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Archives of Neurology. 2012;69(7):836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashim A, Wanga L, Junej K, Yeb Y, Zhao Y, Ming L-J. Vitamin b6s inhibit oxidative stress caused by Alzheimer’s disease-related cuII-b-amyloid complexes-cooperative action of phospho-moiety. Bioorganic and Medicinal Chemistry Letters. 2011;21(21):6430–6432. doi: 10.1016/j.bmcl.2011.08.123. [DOI] [PubMed] [Google Scholar]
- 57.Nilforooshan R, Broadbent D, Weaving G, et al. Homocysteine in Alzheimer’s disease: role of dietary folate, vitamin B6 and B12. International Journal of Geriatric Psychiatry. 2011;26(8):876–877. doi: 10.1002/gps.2666. [DOI] [PubMed] [Google Scholar]
- 58.Cito A, Porcelli B, Coppola MG, Mangiavacchi P, Cortelazzo A, Terzuoli L. Analysis of serum levels of homocysteine and oxidative stress markers in patients with Alzheimer disease. Biomedicine & Pharmacotherapy. 2010 doi: 10.1016/j.biopha.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. American Journal of Clinical Nutrition. 2005;82(3):636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 60.van Dam F, van Gool WA. Hyperhomocysteinemia and Alzheimer’s disease: a systematic review. Archives of Gerontology and Geriatrics. 2009;48(3):425–430. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Ho RCM, Cheung MWL, Fu E, et al. Is high homocysteine level a risk factor for cognitive decline in elderly? a systematic review, meta-analysis, and meta-regression. American Journal of Geriatric Psychiatry. 2011;19(7):607–617. doi: 10.1097/JGP.0b013e3181f17eed. [DOI] [PubMed] [Google Scholar]
- 62.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. Journal of the American Medical Association. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozawa M, Ninomiya T, Ohara T, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama study. The American Journal of Clinical Nutrition. 2013;97(5):1076–1082. doi: 10.3945/ajcn.112.045575. [DOI] [PubMed] [Google Scholar]
- 64.Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Hercberg S, Galan P. A healthy dietary pattern at midlife is associated with subsequent cognitive performance. Journal of Nutrition. 2012;142(5):909–915. doi: 10.3945/jn.111.156257. [DOI] [PubMed] [Google Scholar]
- 65.Samieri C, Jutand M-A, Féart C, Capuron L, Letenneur L, Barberger-Gateau P. Dietary patterns derived by hybrid clustering method in older people: association with cognition, mood, and self-rated health. Journal of the American Dietetic Association. 2008;108(9):1461–1471. doi: 10.1016/j.jada.2008.06.437. [DOI] [PubMed] [Google Scholar]
- 66.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. Journal of Alzheimer’s Disease. 2009;16(1):85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- 67.Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331–1338. doi: 10.1161/HYPERTENSIONAHA.109.146795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, alzheimer disease, and vascular mediation. Archives of Neurology. 2006;63(12):1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Archives of Neurology. 2009;66(2):216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Féart C, Samieri C, Rondeau V, et al. Adherence to a mediterranean diet, cognitive decline, and risk of dementia. Journal of the American Medical Association. 2009;302(6):638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. American Journal of Clinical Nutrition. 2011;93(3):601–607. doi: 10.3945/ajcn.110.007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corrada MM, Kawas CH, Hallfrisch J, Muller D, Brookmeyer R. Reduced risk of Alzheimer’s disease with high folate intake: the Baltimore Longitudinal Study of Aging. Alzheimer’s and Dementia. 2005;1(1):11–18. doi: 10.1016/j.jalz.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luchsinger JA, Tang M-X, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Archives of Neurology. 2007;64(1):86–92. doi: 10.1001/archneur.64.1.86. [DOI] [PubMed] [Google Scholar]
- 74.Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. American Journal of Medicine. 2010;123(6):522–527.e2. doi: 10.1016/j.amjmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Kwok T, Lee J, Law CB, et al. A randomized placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clinical Nutrition. 2011;30(3):297–302. doi: 10.1016/j.clnu.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 76.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. International Journal of Geriatric Psychiatry. 2012;27(6):592–600. doi: 10.1002/gps.2758. [DOI] [PubMed] [Google Scholar]
- 77.Dangour AD, Whitehouse PJ, Rafferty K, et al. B-vitamins and fatty acids in the prevention and treatment of Alzheimer’s disease and dementia: a systematic review. Journal of Alzheimer’s Disease. 2010;22(1):205–224. doi: 10.3233/JAD-2010-090940. [DOI] [PubMed] [Google Scholar]
- 78.Gu Y, Schupf N, Cosentino SA, Luchsinger JA, Scarmeas N. Nutrient intake and plasma beta-amyloid. Neurology. 78(23):1832–1840. doi: 10.1212/WNL.0b013e318258f7c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith AD. The vitamin D receptor gene is associated with Alzheimer’s disease. Neuroscience Letters. 2011;504(2):79–82. doi: 10.1016/j.neulet.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 80.Wang L, Hara K, van Baaren JM, et al. Vitamin D receptor and Alzheimer's disease: a genetic and functional study. Neurobiol Aging. 2012;33(8):1844.e1–1844.e9. doi: 10.1016/j.neurobiolaging.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 81.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. Journal of Alzheimer's Disease. 2013;33(3):659–674. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- 82.Slinin Y, Paudel ML, Taylor BC, et al. 25-hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74(1):33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Annweiler C, Schott AM, Rolland Y, Blain H, Herrmann FR, Beauchet O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology. 2010;75(20):1810–1816. doi: 10.1212/WNL.0b013e3181fd6352. [DOI] [PubMed] [Google Scholar]
- 84.Annweiler C, Beauchet O. Vitamin D-Mentia: randomized clinical trials should be the next step. Neuroepidemiology. 2011;37(3-4):249–258. doi: 10.1159/000334177. [DOI] [PubMed] [Google Scholar]
- 85.Craddock TJA, Tuszynski JA, Chopra D, et al. The zinc dyshomeostasis hypothesis of Alzheimer’s disease. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033552.e33552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schrag M, Mueller C, Oyoyo U, Smith MA, Kirsch WM. Iron, zinc and copper in the Alzheimer’s disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Progress in Neurobiology. 2011;94(3):296–306. doi: 10.1016/j.pneurobio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hung LW, Barnham KJ. Modulating metals as a therapeutic strategy for Alzheimer’s disease. Future Medicinal Chemistry. 2012;4(8):955–969. doi: 10.4155/fmc.12.32. [DOI] [PubMed] [Google Scholar]
- 88.Bush AI. Drug development based on the metals hypothesis of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2008;15(2):223–240. doi: 10.3233/jad-2008-15208. [DOI] [PubMed] [Google Scholar]
- 89.Loef M, Walach H. Copper and iron in Alzheimer’s disease: a systematic review and its dietary implications. British Journal of Nutrition. 2012;107(1):7–19. doi: 10.1017/S000711451100376X. [DOI] [PubMed] [Google Scholar]
- 90.Morris MC, Evans DA, Tangney CC, et al. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Archives of Neurology. 2006;63(8):1085–1088. doi: 10.1001/archneur.63.8.1085. [DOI] [PubMed] [Google Scholar]
- 91.Bucossi S, Ventriglia M, Panetta V, et al. Copper in alzheimer’s disease: a meta-analysis of serum,plasma, and cerebrospinal fluid studies. Journal of Alzheimer’s Disease. 2011;24(1):175–185. doi: 10.3233/JAD-2010-101473. [DOI] [PubMed] [Google Scholar]
- 92.Squitti R. Copper dysfunction in Alzheimer’s disease: from meta-analysis of biochemical studies to new insight into genetics. Journal of Trace Elements in Medicine and Biology. 2012 doi: 10.1016/j.jtemb.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 93.Kessler H, Bayer TA, Bach D, et al. Intake of copper has no effect on cognition in patients with mild Alzheimer’s disease: a pilot phase 2 clinical trial. Journal of Neural Transmission. 2008;115(8):1181–1187. doi: 10.1007/s00702-008-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castellani RJ, Moreira PI, Perry G, Zhu X. The role of iron as a mediator of oxidative stress in Alzheimer disease. BioFactors. 2012;38(2):133–138. doi: 10.1002/biof.1010. [DOI] [PubMed] [Google Scholar]
- 95.Smith MA, Zhu X, Tabaton M, et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. Journal of Alzheimer’s Disease. 2010;19(1):353–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutrition Journal. 2010;9(1, article 4) doi: 10.1186/1475-2891-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corona C, Masciopinto F, Silvestri E, et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death & Disease. 2010;1:p. e91. doi: 10.1038/cddis.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. BioFactors. 2012;38(2):107–113. doi: 10.1002/biof.1005. [DOI] [PubMed] [Google Scholar]
- 99.Okereke OI, Rosner BA, Kim DH, et al. Dietary fat types and 4-year cognitive change in community-dwelling older women. Annals of Neurology. 2012;72(1):124–134. doi: 10.1002/ana.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Borniquel S, Jansson EÅ, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARγ and attenuates experimental inflammatory bowel disease. Free Radical Biology and Medicine. 2010;48(4):499–505. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vassiliou EK, Gonzalez A, Garcia C, Tadros JH, Chakraborty G, Toney JH. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids in Health and Disease. 2009;8, article 25 doi: 10.1186/1476-511X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Briante R, Febbraio F, Nucci R. Antioxidant properties of low molecular weight phenols present in the mediterranean diet. Journal of Agricultural and Food Chemistry. 2003;51(24):6975–6981. doi: 10.1021/jf034471r. [DOI] [PubMed] [Google Scholar]
- 103.Naqvi AZ, Harty B, Mukamal KJ, Stoddard AM, Vitolins M, Dunn JE. Monounsaturated, trans, and saturated fatty acids and cognitive decline in women. Journal of the American Geriatrics Society. 2011;59(5):837–843. doi: 10.1111/j.1532-5415.2011.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boudrault C, Bazinet RP, Ma DWL. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. Journal of Nutritional Biochemistry. 2009;20(1):1–10. doi: 10.1016/j.jnutbio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 105.Jicha GA, Markesbery WR. Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clinical Interventions in Aging. 2010;5(1):45–61. doi: 10.2147/cia.s5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Advances in Nutrition. 2012;3(1):1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vedin I, Cederholm T, Freund-Levi Y, et al. Effects of DHA-rich n-3 fatty acid supplementation on gene expression in blood mononuclear leukocytes: the omegAD study. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035425.e35425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barberger-Gateau P, Lambert JC, Feart C, et al. From genetics to dietetics: the contribution of epidemiology to understanding Alzheimer’s disease. Journal of Alzheimer's Disease. 2013;33(supplement 1):457–463. doi: 10.3233/JAD-2012-129019. [DOI] [PubMed] [Google Scholar]
- 109.Calon F, Lim GP, Yang F, et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43(5):633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hooijmans CR, van der Zee CEEM, Dederen PJ, et al. DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiology of Disease. 2009;33(3):482–498. doi: 10.1016/j.nbd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Lim GP, Calon F, Morihara T, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. Journal of Neuroscience. 2005;25(12):3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perez SE, Berg BM, Moore KA, et al. DHA diet reduces AD pathology in young APPswe/PS1ΔE9 transgenic mice: possible gender effects. Journal of Neuroscience Research. 2010;88(5):1026–1040. doi: 10.1002/jnr.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nature Clinical Practice Neurology. 2009;5(3):140–152. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- 114.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. Journal of the American Medical Association. 2010;304(17):1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 116.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. American Journal of Clinical Nutrition. 2008;88(3):706–713. doi: 10.1093/ajcn/88.3.706. [DOI] [PubMed] [Google Scholar]
- 117.Solfrizzi V, Frisardi V, Capurso C, et al. Dietary fatty acids in dementia and predementia syndromes: epidemiological evidence and possible underlying mechanisms. Ageing Research Reviews. 2010;9(2):184–199. doi: 10.1016/j.arr.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Laitinen MH, Ngandu T, Rovio S, et al. Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dementia and Geriatric Cognitive Disorders. 2006;22(1):99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 119.Grimm MO, Rothhaar TL, Grosgen S, et al. Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP) The Journal of Nutritional Biochemistry. 2012;23(10):1214–1223. doi: 10.1016/j.jnutbio.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 120.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong C-X. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. Journal of Pathology. 2011;225(1):54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Current Alzheimer Research. 2007;4(2):147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 122.Frölich L, Blum-Degen D, Bernstein H-G, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. Journal of Neural Transmission. 1998;105(4-5):423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 123.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? Journal of Alzheimer’s Disease. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 124.Kikuchi S, Shinpo K, Takeuchi M, et al. Glycation—a sweet tempter for neuronal death. Brain Research Reviews. 2003;41(2-3):306–323. doi: 10.1016/s0165-0173(02)00273-4. [DOI] [PubMed] [Google Scholar]
- 125.Henderson ST. High carbohydrate diets and Alzheimer’s disease. Medical Hypotheses. 2004;62(5):689–700. doi: 10.1016/j.mehy.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 126.Seneff S, Wainwright G, Mascitelli L. Nutrition and Alzheimer’s disease: the detrimental role of a high carbohydrate diet. European Journal of Internal Medicine. 2011;22(2):134–140. doi: 10.1016/j.ejim.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 127.Luchsinger JA, Tang M-X, Mayeux R. Glycemic load and risk of Alzheimer’s disease. Journal of Nutrition, Health and Aging. 2007;11(3):238–241. [PubMed] [Google Scholar]
- 128.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69(20):1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 129.Dangour AD, Allen E, Elbourne D, Fletcher A, Richards M, Uauy R. Fish consumption and cognitive function among older people in the UK: baseline data from the OPAL study. Journal of Nutrition, Health and Aging. 2009;13(3):198–203. doi: 10.1007/s12603-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 130.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE ε4. Neurology. 2005;65(9):1409–1414. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 131.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Archives of Neurology. 2003;60(7):940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 132.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Archives of Neurology. 2005;62(12):1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 133.Hughes TF, Andel R, Small BJ, et al. Midlife fruit and vegetable consumption and risk of dementia in later life in swedish twins. American Journal of Geriatric Psychiatry. 2010;18(5):413–420. doi: 10.1097/JGP.0b013e3181c65250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nooyens ACJ, Bueno-De-Mesquita HB, van Boxtel MPJ, van Gelder BM, Verhagen H, Verschuren WMM. Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. British Journal of Nutrition. 2011;106(5):752–761. doi: 10.1017/S0007114511001024. [DOI] [PubMed] [Google Scholar]
- 136.Crichton GE, Bryan J, Murphy KJ, Buckley J. Review of dairy consumption and cognitive performance in adults: findings and methodological issues. Dementia and Geriatric Cognitive Disorders. 2010;30(4):352–361. doi: 10.1159/000320987. [DOI] [PubMed] [Google Scholar]
- 137.Lee L, Kang SA, Lee HO, et al. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health. 2001;115(2):133–138. doi: 10.1038/sj/ph/1900729. [DOI] [PubMed] [Google Scholar]
- 138.Eskelinen MH, Ngandu T, Helkala E-L, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. International Journal of Geriatric Psychiatry. 2008;23(7):741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 139.Eskelinen MH, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;20(1):S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- 140.Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;20(1):S117–S126. doi: 10.3233/JAD-2010-091249. [DOI] [PubMed] [Google Scholar]
- 141.Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: systematic review and meta-analysis. Journal of Alzheimer’s Disease. 2010;20(1):S187–S204. doi: 10.3233/JAD-2010-091387. [DOI] [PubMed] [Google Scholar]
- 142.Cao C, Wang L, Lin X, et al. Caffeine synergizes with another coffee component to increase plasma GCSF: linkage to cognitive benefits in Alzheimer’s mice. Journal of Alzheimer’s Disease. 2011;25(2):323–335. doi: 10.3233/JAD-2011-110110. [DOI] [PubMed] [Google Scholar]
- 143.Barranco Quintana JL, Allam MF, Del Castillo AS, Navajas RF-C. Alzheimer’s disease and coffee: a quantitative review. Neurological Research. 2007;29(1):91–95. doi: 10.1179/174313206X152546. [DOI] [PubMed] [Google Scholar]
- 144.Feng L, Gwee X, Kua E-H, Ng T-P. Cognitive function and tea consumption in community dwelling older Chinese in Singapore. Journal of Nutrition, Health and Aging. 2010;14(6):433–438. doi: 10.1007/s12603-010-0095-9. [DOI] [PubMed] [Google Scholar]
- 145.Ng T-P, Feng L, Niti M, Kua E-H, Yap K-B. Tea consumption and cognitive impairment and decline in older Chinese adults. American Journal of Clinical Nutrition. 2008;88(1):224–231. doi: 10.1093/ajcn/88.1.224. [DOI] [PubMed] [Google Scholar]
- 146.De Bruin EA, Rowson MJ, van Buren L, Rycroft JA, Owen GN. Black tea improves attention and self-reported alertness. Appetite. 2011;56(2):235–240. doi: 10.1016/j.appet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 147.Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MBH. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. Journal of Nutrition. 2008;138(8):1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- 148.Weinreb O, Mandel S, Amit T, Youdim MBH. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. Journal of Nutritional Biochemistry. 2004;15(9):506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 149.Xu Y, Zhang J-J, Xiong L, Zhang L, Sun D, Liu H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. Journal of Nutritional Biochemistry. 2010;21(8):741–748. doi: 10.1016/j.jnutbio.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 150.Rezai-Zadeh K, Arendash GW, Hou H, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Research. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 151.Rezai-Zadeh K, Shytle D, Sun N, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. Journal of Neuroscience. 2005;25(38):8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song J, Xu H, Liu F, Feng L. Tea and cognitive health in late life: current evidence and future directions. Journal of Nutrition, Health and Aging. 2012;16(1):31–34. doi: 10.1007/s12603-011-0139-9. [DOI] [PubMed] [Google Scholar]
- 153.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. American Journal of Geriatric Psychiatry. 2009;17(7):542–555. doi: 10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- 154.Panza F, Capurso C, D’Introno A, et al. Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. Journal of Alzheimer’s Disease. 2009;17(1):7–31. doi: 10.3233/JAD-2009-1009. [DOI] [PubMed] [Google Scholar]
- 155.Weyerer S, Schäufele M, Wiese B, et al. Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age and Ageing. 2011;40(4):456–463. doi: 10.1093/ageing/afr007. [DOI] [PubMed] [Google Scholar]
- 156.Harwood DG, Kalechstein A, Barker WW, et al. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2010;25(5):511–518. doi: 10.1002/gps.2372. [DOI] [PubMed] [Google Scholar]
- 157.Huang T-C, Lu K-T, Wo Y-YP, Wu Y-J, Yang Y-L. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0029102.e29102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karuppagounder SS, Pinto JT, Xu H, Chen H-L, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochemistry International. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ho L, Ferruzzi MG, Janle EM, et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. The FASEB Journal. 2013;27(2):769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.González-Muñoz MJ, Peña A, Meseguer I. Role of beer as a possible protective factor in preventing Alzheimer’s disease. Food and Chemical Toxicology. 2008;46(1):49–56. doi: 10.1016/j.fct.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 161.Gustaw-Rothenberg K. Dietary patterns associated with alzheimer’s disease: population based study. International Journal of Environmental Research and Public Health. 2009;6(4):1335–1340. doi: 10.3390/ijerph6041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fung TT, Stampfer MJ, Manson JE, Rexrode KM, Willett WC, Hu FB. Prospective study of major dietary patterns and stroke risk in women. Stroke. 2004;35(9):2014–2019. doi: 10.1161/01.STR.0000135762.89154.92. [DOI] [PubMed] [Google Scholar]
- 163.Hooijmans CR, Rutters F, Dederen PJ, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD) Neurobiology of Disease. 2007;28(1):16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 164.Studzinski CM, Li F, Bruce-Keller AJ, et al. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP x PS1 knock-in mice. Journal of Neurochemistry. 2009;108(4):860–866. doi: 10.1111/j.1471-4159.2008.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hankey GJ. Nutrition and the risk of stroke. The Lancet Neurology. 2012;11(1):66–81. doi: 10.1016/S1474-4422(11)70265-4. [DOI] [PubMed] [Google Scholar]
- 166.Birkenhäger WH, Staessen JA, Casserly IP, et al. Convergence of atherosclerosis and Alzheimer’s disease. The Lancet. 2004;363(9426):2091–2092. doi: 10.1016/S0140-6736(04)16471-4. [DOI] [PubMed] [Google Scholar]
- 167.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69(11):1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. American Journal of Clinical Nutrition. 2010;92(5):1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 169.Lawlor DA, Smith GD, Kundu D, Bruckdorfer KR, Ebrahim S. Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? The Lancet. 2004;363(9422):1724–1727. doi: 10.1016/S0140-6736(04)16260-0. [DOI] [PubMed] [Google Scholar]
- 170.Smith GD. Reflections on the limitations to epidemiology. Journal of Clinical Epidemiology. 2001;54(4):325–331. doi: 10.1016/s0895-4356(00)00334-6. [DOI] [PubMed] [Google Scholar]


