Short abstract
Pulmonary arterial hypertension (PAH) is a rapidly fatal disease in which mortality is typically due to right ventricular (RV) failure. An excellent predictor of mortality in PAH is proximal pulmonary artery stiffening, which is mediated by collagen accumulation in hypoxia-induced pulmonary hypertension (HPH) in mice. We sought to investigate the impact of limiting vascular and ventricular collagen accumulation on RV function and the hemodynamic coupling efficiency between the RV and pulmonary vasculature. Inbred mice were exposed to chronic hypoxia for 10 days with either no treatment (HPH) or with treatment with a proline analog that impairs collagen synthesis (CHOP-PEG; HPH + CP). Both groups were compared to control mice (CTL) exposed only to normoxia (no treatment). An admittance catheter was used to measure pressure-volume loops at baseline and during vena cava occlusion, with mice ventilated with either room air or 8% oxygen, from which pulmonary hemodynamics, RV function, and ventricular-vascular coupling efficiency (ηvvc) were calculated. Proline analog treatment limited increases in RV afterload (neither effective arterial elastance Ea nor total pulmonary vascular resistance significantly increased compared to CTL with CHOP-PEG), limited the development of pulmonary hypertension (CHOP-PEG reduced right ventricular systolic pressure by 10% compared to HPH, p < 0.05), and limited RV hypertrophy (CHOP-PEG reduced RV mass by 18% compared to HPH, p < 0.005). In an acutely hypoxic state, treatment improved RV function (CHOP-PEG increased end-systolic elastance Ees by 43%, p < 0.05) and maintained ηvvc at control, room air levels. CHOP-PEG also decreased lung collagen content by 12% measured biochemically compared to HPH (p < 0.01), with differences evident in large and small pulmonary arteries by histology. Our results demonstrate that preventing new collagen synthesis limits pulmonary hypertension development by reducing collagen accumulation in the pulmonary arteries that affect RV afterload. In particular, the proline analog limited structural and functional changes in distal pulmonary arteries in this model of early and somewhat mild pulmonary hypertension. We conclude that collagen plays an important role in small pulmonary artery remodeling and, thereby, affects RV structure and function changes induced by chronic hypoxia.
Keywords: cardiopulmonary hemodynamics, chronic hypoxia, acute hypoxia, collagen, ventricular-vascular coupling
Introduction
Pulmonary hypertension is a debilitating disease that affects millions of Americans. The most severe form of pulmonary hypertension is pulmonary arterial hypertension, which is rare (∼5000 people/year) but deadly, with a median survival of 2.8 years [1,2]. PAH is characterized by remodeling throughout the pulmonary vasculature including distal arterial narrowing and proximal and distal pulmonary artery stiffening. A common model of PAH is hypoxic pulmonary hypertension, which acutely causes vasoconstriction of distal arterioles in the lungs and chronically causes proximal and distal pulmonary artery remodeling as well as right ventricular hypertrophy, analogous to PAH. While distal arterial narrowing largely determines the increase in pulmonary vascular resistance, by which pulmonary hypertension severity is typically gauged, proximal pulmonary artery stiffening is a better predictor of mortality [3], which is most often caused by RV failure [4].
Previous work has demonstrated that collagen accumulation is a key contributor to proximal pulmonary artery stiffening in HPH [5–7] and may play a role in distal arterial remodeling as well [8]. Agents that impair the synthesis of new collagen have been shown to limit the severity of HPH and RV hypertrophy [9–12], but their effects on RV contractility, RV filling, and the efficiency of hemodynamic coupling between the RV and the pulmonary vasculature remain unknown. Overall, the role of collagen in the progression of PAH remains largely unknown.
Pulmonary hypertension progression is typically assessed via pulmonary vascular resistance (PVR) and RV function parameters such as RV stroke volume (SV), RV ejection fraction (EF), and RV cardiac output (CO). A more comprehensive approach may be the ventricular-vascular coupling efficiency ηvvc, which is the ratio of the preload independent contractility measured by end-systolic elastance (Ees) to the right ventricular afterload determined by the effective arterial elastance (Ea) [13,14]. Both Ees and Ea can be measured directly from simultaneous pressure-volume curves obtained at baseline and during a brief vena cava occlusion [15]. Previous research by our group has demonstrated that ηvvc can be reliably measured in mice using admittance catheter technology [16].
Here, we sought to investigate the role of collagen in ventricular and vascular changes in response to HPH. Our goal was to determine if a proline analog, which inhibits new collagen synthesis, could preserve ventricular and vascular function as well as ηvvc during HPH progression. We hypothesized that preventing new collagen synthesis would limit pulmonary hypertension, prevent RV hypertrophy, and preserve ηvvc. To test our hypotheses, we measured Ees, Ea, and other hemodynamic parameters in mice exposed to chronic hypoxia, ventilated with either room air or acute hypoxia (8% oxygen) as well as mice treated with or without a proline analog and control mice exposed only to normoxia (no treatment).
Methods
Materials.
Male C57BL6/J mice, 12–13 weeks old, with a body weight of 25.0 ± 0.4 g, were obtained from Jackson Laboratory (Bar Harbor, ME). Two groups of mice were exposed to 10 days of chronic, normobaric hypoxia (10% oxygen) created in an environmentally controlled chamber in which nitrogen was mixed with room air. Oxygen levels were measured with a sensor in the chamber (Servoflo, Lexington, MA) that controlled a relay valve on the nitrogen gas inflow line via a custom-built closed loop control system. Of these two groups, one was treated with the proline analog cis–hydroxy-L-proline poly (ethylene glycol)-lysine (CHOP-PEG), which interferes with new collagen synthesis via an osmotic pump (HPH + CP, n = 10) while the other was untreated (HPH, n = 13). A third group was neither exposed to hypoxia nor treated with CHOP-PEG (CTL, n = 10). Both the CTL and HPH groups underwent sham surgery of the osmotic pump insertion. The hypoxia chamber was opened for 10–20 min three times per week to clean cages and replenish food and water. Control mice were housed in room air. All mice were exposed to a 12 h light-dark cycle. The University of Wisconsin Institutional Animal Care and Use Committee approved all procedures.
Anesthesia, Ventilation and Ventricular Exposure.
Mice were anesthetized with an intraperitoneal injection of urethane solution (1000 mg/kg body weight, intraperitoneally), intubated, and placed on a ventilator (Harvard Apparatus, Holliston, MA) using a tidal volume of ∼225 μl and respiratory rate of ∼125 breaths/min. They were then placed supine on a heated pad to maintain body temperature at 38 °C to 39 °C. A ventral midline skin incision was made from the lower mandible inferior to the xiphoid process. The thoracic cavity was entered through the sternum. The chest wall and lungs were carefully retracted to expose the right ventricle. Hydroxyethyl starch (∼24 μg) (6%; 2 mg/g body weight) was injected intravenously to restore vascular volumes as previously reported [3,4].
Instrumentation and Hemodynamic Measurements.
The left carotid artery was cannulated with a 1.2 F catheter tipped pressure transducer (Scisense, London, Ontario, Canada) and advanced into the ascending aorta to measure systemic blood pressure. Subsequently, the apex of the RV was localized and a 1.2 F admittance pressure-volume catheter (Scisense) was introduced using a 20 gauge needle, leaving the pericardium intact. After instrumentation was established and initial RV pressure-volume measurements were obtained, the inferior vena cava was isolated and briefly occluded to obtain alterations in venous return for determination of end-systolic and end-diastolic pressure relations. This vena cava occlusion (VCO) was limited to a few seconds in duration to avoid reflex responses. VCO was performed at least three times. Measurements were obtained under normoxic ventilation conditions initially and, subsequently, under acutely hypoxic ventilation conditions (8% O2) after 5 min of exposure. One underweight (17 g) CTL mouse died unexpectedly during acute hypoxia; no data from this mouse are presented herein.
The magnitude and phase of the electrical admittance as well as the RV pressure were continuously recorded at 1000 Hz and analyzed on commercially available software (Notocord Systems, Croissy Sur Seine, France). Except for the measurements obtained under acutely hypoxic conditions, these methods are identical to those previously established by our group [16].
Blood Gas and Hematocrit Measurements.
To ensure our acute low oxygen ventilation condition resulted in hypoxemia, we tested blood oxygen saturation in additional CTL mice (n = 4). Prior to euthanasia, a sample of arterial blood was taken and oxygen levels were measured with an I-STAT portable analyzer and CG4 + cartridge. After euthanasia, another blood sample was taken for measurement of hematocrit (via centrifugation).
Heart and Lung Morphometry and Histology.
After euthanasia, for all CTL, HPH, and HPH + CP mice (used for either hemodynamic or blood gas measurements), the heart and lungs were harvested and a blood sample was taken for hematocrit measurement. The RV free wall and left ventricular (LV) free wall plus septum (S) were weighed to calculate the Fulton index, a measure of RV hypertrophy. Then portions of the RV, the left extralobar pulmonary artery (LPA), and the whole right lung (RL) were frozen for analysis of collagen content with a hydroxyproline (OHP) assay as described previously [6]. Portions of the RV and the right extralobar pulmonary artery (RPA) were fixed in formalin (without prior pressurization) for histology. In particular, the tissue was embedded in paraffin, cross-sectioned, and stained with picosirius red for collagen.
In addition, in a separate group of mice (CTL, HPH, and HPH + CP; n = 3 each), whole lungs were excised for histology. Fixation was performed via formalin perfused through the trachea for 20 min at an approximate pressure of 18 mm Hg. Lungs were stored in formalin for 48 h and then transferred to 70% ethanol for sectioning and staining with picosirius red.
Hemodynamic Data Analysis.
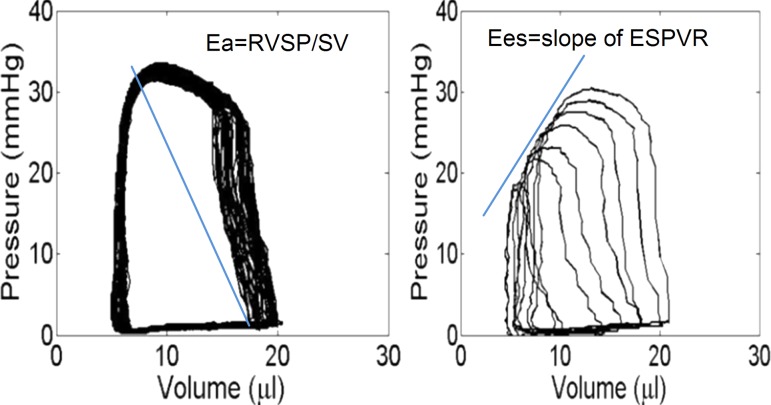
The pressure and volume signals were visually checked for quality and recorded for later analysis. At least ten consecutive cardiac cycles free of extra systolic beats were selected and used for the analysis. Standard hemodynamic variables including heart rate (HR), RV peak systolic pressure (RVSP), and RV end-diastolic pressure (RVEDP), total PVR (TPVR, estimated as RVSP/CO), and RV function parameters such as SV, EF, CO, chamber compliance, and Ea (SV/PP) were calculated. RV contractile function was quantified in three ways: as the slope of the ESPVR (Ees) (Fig. 1), preload-recruitable stroke work (PRSW), and dP/dtmax. Finally, ηvvc was calculated as Ees/Ea [16].
Fig. 1.
Pressure-volume loops obtained in a representative (CTL) mouse right ventricle during vena cava occlusion. Ees is obtained graphically as shown during VCO in the right panel.
Statistical Analysis.
Statistical analysis was performed using a one-way ANOVA for condition (CTL versus HPH versus HPH + CP) or generalized least squares with multiple comparisons for condition and ventilation (room air versus acute hypoxia). All values are given as mean ±SE.
Results
Morphometric and Tissue Effects of Chronic Hypoxia.
The average body weights of the CTL and HPH were similar to one another while the HPH + CP group weighed less on average (Table 1). The Fulton index (RV/LV + S) increased with HPH and the CHOP-PEG treatment limited this increase (Table 1).
Table 1.
Body weight, Fulton index, RV collagen content, and hematocrit for CTL, HPH, and HPH + CP mice
| CTL | HPH | HPH + CP | |
|---|---|---|---|
| BW (g) | 26.8 ± 0.7 | 25.5 ± 0.4 | 23.3 ± 0.6 a , b |
| Fulton index (RV/LV + S) | 0.26 ± 0.01 | 0.40 ± 0.02 b | 0.35 ± 0.01 a , b |
| Relative RV OHP (μg/mg tissue) | 2.40 ± 0.50 (n = 8) | 3.59 ± 0.37 b (n = 5) | 3.30 ± 0.20 b (n = 7) |
| Total RV OHP (μg/heart) | 53 ± 4 (n = 8) | 120 ± 16 b (n = 5) | 92 ± 7 b (n = 7) |
| Hematocrit (%) | 40 ± 1 | 69 ± 3 b | 71 ± 3 b |
Note: Values are means ± SE; n = 9 for CTL, n = 13 for HPH and n = 10 for HPH + CP. BW, body weight; RV, right ventricular; LV, left ventricular; S, septum.
P < 0.05 versus CTL;
P < 0.05 versus HPH.
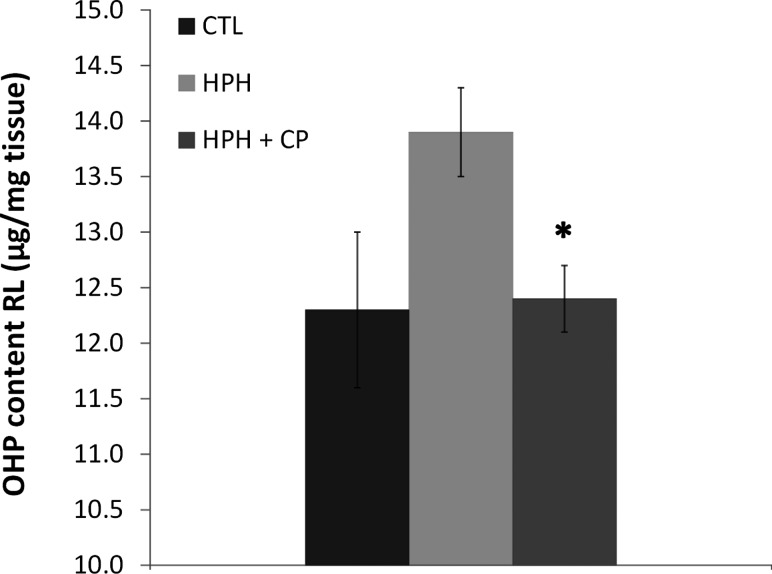
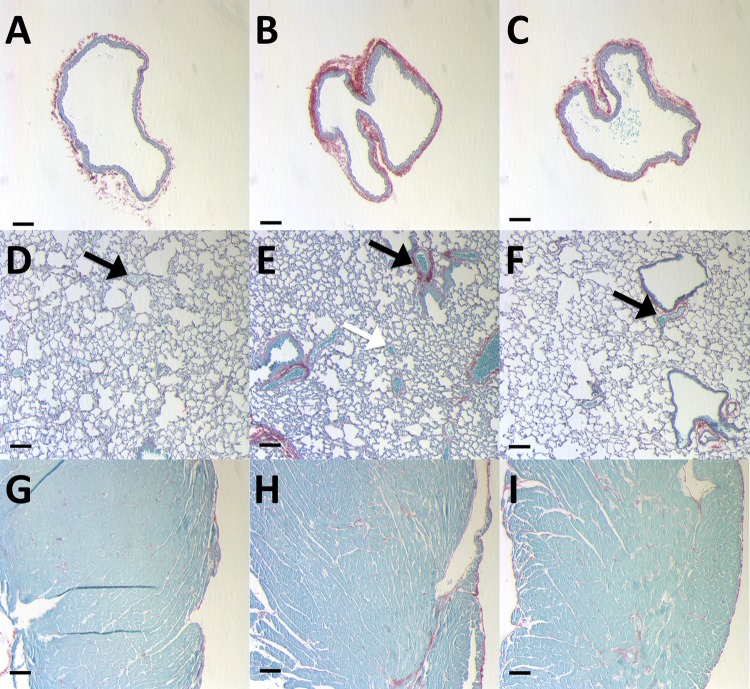
Collagen content in the RV as measured by the hydroxyproline assay increased with chronic hypoxia, and CHOP-PEG treatment limited this increase, although not significantly either as a relative amount (per mg tissue) or total amount (per RV) (Table 1). In contrast, increases in pulmonary artery collagen content with chronic hypoxia were almost entirely prevented by CHOP-PEG treatment, as evidenced by both biochemical and histological assays. In particular, total right lung collagen content measured by the hydroxyproline assay increased with chronic hypoxia, and CHOP-PEG treatment returned collagen content to control levels (Fig. 2). Furthermore, histology showed that RPA wall thickening and accumulation of collagen with chronic hypoxia was prevented by the CHOP-PEG treatment (Figs. 3(a)–3(c)), and similar changes could be observed in distal pulmonary arteries, which can be differentiated from pulmonary veins by their close proximity to airways (Figs. 3(d)–3(f)). Some accumulation of collagen around large airways with HPH was also evident, which was prevented by CHOP-PEG. Hematocrit increased with chronic hypoxia as expected; CHOP-PEG did not alter hematocrit (Table 1).
Fig. 2.
Hydroxyproline content of right lungs from CTL, HPH, and HPH + CP groups. *P < 0.05 versus HPH
Fig. 3.
Representative histology images of picosirius red stain for collagen in (a)–(c) RPA for CTL (a), HPH (b), HPH + CP (c); (d)–(f) lung for CTL (d), HPH (e), HPH + CP (f); and (g)–(i) RV for CTL (g), HPH (h), HPH + CP (i). Black arrows indicate pulmonary arteries in close proximity to large airways, and white arrow indicates a pulmonary vein surrounded by alveoli. Note, only the black arrow in panel (e) demonstrates significant collagen accumulation. Scale bar is 0.1 mm for all images.
Hemodynamic Effects of Chronic Hypoxia.
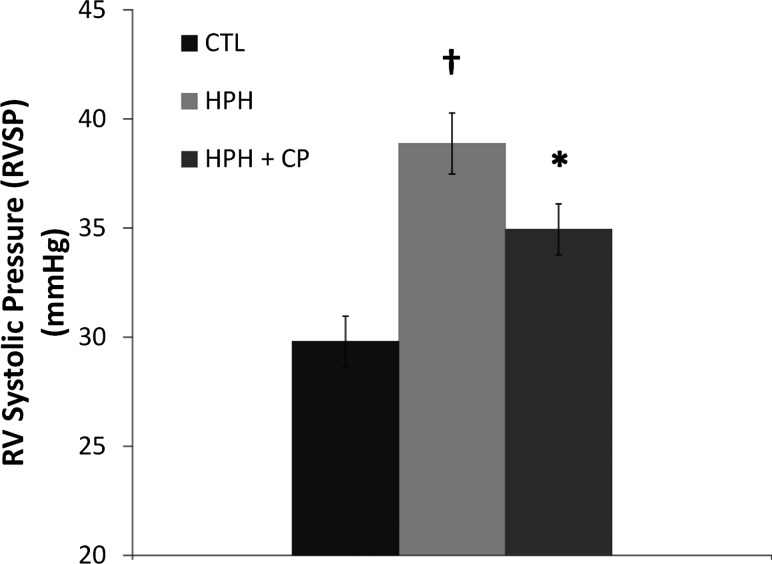
RVSP increased with chronic hypoxia as expected, and CHOP-PEG treatment limited this increase (Fig. 4). No differences in CO, SV, or EF between groups were found with room air ventilation. Acutely hypoxic ventilation did not change CO or SV in any group (Table 2). However, with acute hypoxia, both untreated groups had severely compromised EF, while the CHOP-PEG group maintained normal values (Table 2). With room air ventilation, no significant differences in the heart rate between groups were evident. Acute hypoxia tended to decrease the heart rate in all groups but not significantly. Systemic arterial pressures were not different between groups (Table 2).
Fig. 4.
Right ventricular systolic pressure for CTL, HPH, and HPH + CP groups during room air ventilation. † P < 0.05 versus CTL; *P < 0.05 versus HPH.
Table 2.
Hemodynamic parameters and indexes of systolic and diastolic function derived from right ventricular pressure-volume relationships in CTL, HPH, and HPH + CP mice
|
Room Air 21% O2 |
Acute Hypoxia 8% O2 |
|||||
|---|---|---|---|---|---|---|
| CTL | HPH | CP | CTL | HPH | CP | |
| Heart rate (beats/min) | 598 ± 8 | 598 ± 10 | 572 ± 12 | 573 ± 9 | 575 ± 10 | 552 ± 14 |
| Aortic peak systolic pressure (mm Hg) | 78 ± 4 | 81 ± 5 | 76 ± 3 | 72 ± 9 | 71 ± 12 | 73 ± 6 |
| RV peak systolic pressure (mm Hg) | 29.8 ± 1.2 | 38.9 ± 1.0 b | 34.9 ± 1.2 a | 28.6 ± 1.8 | 41.0 ± 1.5 b | 34.5 ± 1.2 b , a |
| RV end-diastolic volume (μl) | 24.6 ± 0.9 | 24.9 ± 1.8 | 23.7 ± 1.9 | 33.8 ± 6.6 | 24.9 ± 3.3 b | 17.6 ± 1.6 b |
| Cardiac output (ml/min) | 8.5 ± 0.3 | 8.0 ± 0.6 | 7.8 ± 0.4 | 4.6 ± 0.7 c | 5.2 ± 0.5 c | 5.1 ± 0.5 c |
| RV ejection fraction (%) | 58.0 ± 2.5 | 54.8 ± 3.3 | 60.2 ± 4.5 | 27.9 ± 5.4 c | 38.2 ± 3.6 c | 54.8 ± 4.6 b , a |
| Total pulmonary vascular resistance (mm Hg-min/ml) | 3.5 ± 0.2 | 5.3 ± 0.4 b | 4.6 ± 0.4 | 6.4 ± 0.9 c | 9.7 ± 0.8 b , c | 5.7 ± 1.0 a , c |
| RV dP/dtmax (mm Hg/s) | 2720 ± 141 | 3638 ± 78 b | 3103 ± 97 a | 2080 ± 165 | 3279 ± 158 b | 3193 ± 197 b |
| RV preload recruitable stroke work (mm Hg) | 20.5 ± 0.9 | 31.2 ± 1.9 b | 25.4 ± 1.3 | 15.1 ± 2.0 | 27.1 ± 2.4 b | 28.5 ± 2.0 b |
| RV chamber compliance (μl/mm Hg) | 0.57 ± 0.03 | 0.41 ± 0.03 b | 0.44 ± 0.03 b | 0.46 ± 0.06 | 0.30 ± 0.02 b | 0.30 ± 0.02 b |
| RV dP/dtmin (mm Hg/s) | –2039 ± 100 | –2892 ± 98 b | –2566 ± 67 b | –1636 ± 133 | –2657 ± 111 b | –2554 ± 143 b |
| RV relaxation factor τ (ms) | 6.7 ± 0.4 | 5.2 ± 0.2 | 4.9 ± 0.3 | 10.1 ± 1.4 | 6.3 ± 0.4 b | 5.1 ± 0.3 b |
Note: Values are means ± SE; n = 9 for CTL, n = 13 for HPH, and n = 10 for CP. BW, body weight; RV, right ventricular; LV, left ventricular; S, septum.
P < 0.05 versus CTL
P < 0.05 versus HPH
P < 0.05 versus room air.
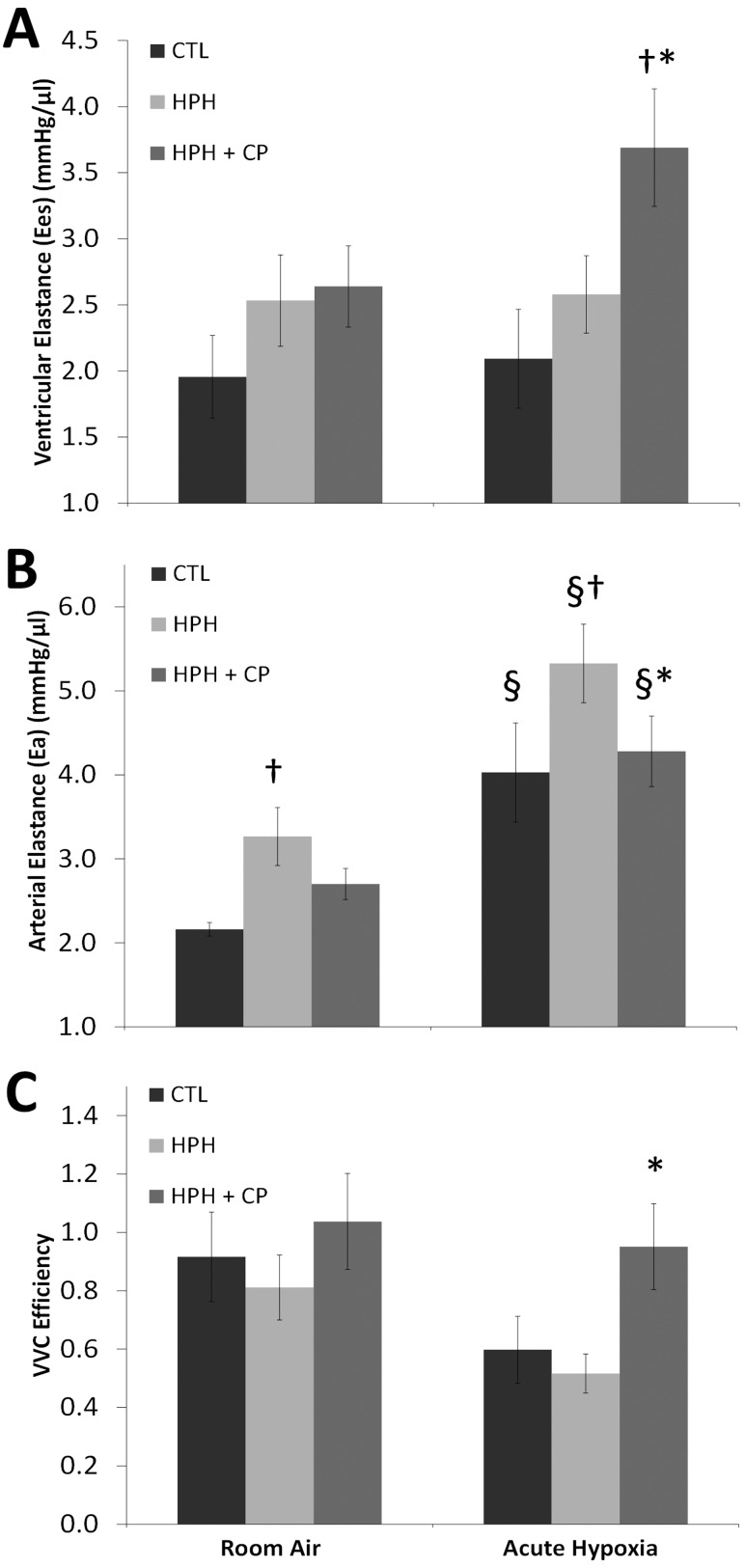
RV function quantified by dP/dtmax and preload recruitable stroke work increased with HPH in mice ventilated with room air, and CHOP-PEG had no effect on these changes. Acute hypoxia ventilation did not significantly alter dP/dtmax or PRSW in any of the three groups. RV contractility measured by Ees increased with HPH and HPH + CP in both ventilation conditions; during acutely hypoxic ventilation, the increase in the HPH + CP group was both significant and dramatic (Fig. 5(a)).
Fig. 5.
(a) Ees, (b) Ea, and (c) VVC efficiency for CTL, HPH, and HPH + CP groups. † P < 0.05 versus CTL; *P < 0.05 versus HPH, § P < 0.05 versus room air.
Similar to pulmonary vascular structure, pulmonary vascular function was affected by CHOP-PEG treatment. In particular, Ea (Fig. 5(b)) and TPVR (Table 2) increased with HPH, and CHOP-PEG treatment prevented these increases. Under the stress of acute hypoxia, all groups had significantly increased Ea and TPVR, and CHOP-PEG treatment limited these additional increases.
The ventricular-vascular coupling efficiency ηvvc was not significantly altered by HPH or HPH + CP with room air ventilation. However, in acute hypoxia, only the HPH + CP mice maintained ηvvc at control room air levels (Fig. 5(c)).
Blood Gases.
Blood gas measurements confirmed that acute low oxygen creates hypoxemia in mice. Blood oxygen saturation sO2% decreased from 95.3 ± 1.0 in room air conditions to 78.4 ± 7.2 with acutely hypoxic ventilation (Table 3). In addition, the partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), and bicarbonate concentration decreased significantly during exposure to acute hypoxia whereas blood pH did not change (Table 3).
Table 3.
Arterial blood gas measurements in CTL mice after exposure to room air and after exposure to acute hypoxia (8%) for five minutes
| Room Air | Acute hypoxia | |
|---|---|---|
| pH | 7.35 ± 0.03 | 7.38 ± 0.12 |
| pO2 (mm Hg) | 80.8 ± 4.0 | 46.4 ± 8.3 a |
| pCO2 (mm Hg) | 32.9 ± 5.7 | 15.3 ± 6.8 a |
| HCO3 (mmol/liter) | 18.1 ± 1.8 | 8.7 ± 3.5 a |
| sO2 (%) | 95.3 ± 1.0 | 78.4 ± 7.2 a |
Note: Values are means ±SD; n = 4 for CTL mice ventilated with room air and n = 9 for CTL mice ventilated with acute hypoxia. pO2 is partial pressure of arterial oxygen, pCO2 denotes partial pressure of arterial carbon dioxide, HCO3 is the bicarbonate level in arterial blood, and sO2% is arterial oxygen saturation.
P < 0.05 versus CTL normoxic.
Discussion
The major finding of this study is that preventing new collagen synthesis has a more dramatic effect on pulmonary vascular remodeling than right ventricular remodeling in HPH. Pulmonary vascular remodeling is a critical component of PAH progression because it determines the afterload of the RV and thus contributes to RV remodeling [17]. We had hypothesized that preventing new collagen synthesis would impact both the pulmonary vascular and RV response to chronic hypoxia. Here we found that the CHOP-PEG treatment maintained lung collagen at control levels, prevented large increases in effective arterial elastance and limited the increase in right ventricular systolic pressure; in contrast, the CHOP-PEG treatment only tended to prevent RV collagen accumulation and did not affect RV function in room air conditions.
It is worth noting that hypoxia alone creates a somewhat mild form of PAH in rodents [18,19] and that 10 days is not a long exposure time. A more severe form of PAH in mice can be generated with the addition of other drugs such as the VEGF inhibitor SUGEN [20]. With 21 days of hypoxia plus SUGEN, RVSP increased to nearly 50 mm Hg in a closed chest preparation in mice and led to incipient RV failure [20]. Excessive collagen accumulation is a hallmark of severe and chronic RV dysfunction [21], but we did not observe either dramatic changes in RV collagen content or RV dysfunction. We speculate that the conditions imposed here led to adaptive (physiological) RV remodeling, not maladaptive (pathological) remodeling. Limiting new collagen synthesis would likely have more significant effects on RV structure and function in a later stage of disease.
Our results also demonstrate, for the first time, that the CHOP-PEG treatment led to improved RV function under the stress of acute hypoxia, with Ees greater than control levels in either ventilation condition and ηvvc preserved at control, room air levels. The mechanisms of this improved RV adaptation to chronic hypoxia with the CHOP-PEG treatment remain to be elucidated.
The pulmonary vascular hemodynamics and right ventricular function data obtained in CTL mice under normoxic conditions agree well with the prior literature. In particular, RVSP and hematocrit in CTL mice were similar to several prior studies (∼25 mm Hg and ∼40, respectively) [16,22–26]. TPVR in CTL mice was also comparable to previous results [27] as were CO (∼8 ml/min) and EF (∼50%) [16,28]. The effective arterial elastance Ea (∼2.5 mm Hg/μl), ventricular Ees (∼2 mm Hg/μl), and ηvvc (Ees/Ea) (˜.8), were similar to previous values obtained by our group, which was the first report of Ea and ηvvc in mice [16].
With chronic hypoxia, hematocrit, TPVR, and RVSP increased in agreement with the prior literature for mice exposed to hypoxia alone [16,27,29]. A significant increase in Ea (to ∼3 mm Hg/μl) and trend of increasing in Ees (to ∼2.5 mm Hg/μl) were apparent with chronic hypoxia as well. EF (∼50%), CO (∼8 ml/min), and SV (∼14 μl) did not change with chronic hypoxia, which is consistent with prior findings [30]. Our group previously found that EF, CO, and SV decreased with 10 days of chronic hypoxia [16], but we attribute this discrepancy to the cumulative effects of small improvements in technique including reduced blood loss, better catheter placement and better maintenance of systemic hemodynamics.
Mice ventilated on 8% oxygen for 5 min showed a drop in oxygen saturation and pO2. This additional stressor did not change RVSP for any of the groups but did decrease CO, SV, and EF. Consequently and as expected, Ea and TPVR increased with acute hypoxic exposure, presumably due to hypoxic pulmonary vasoconstriction in the distal vessels. The CTL group did not demonstrate increased RV contractility during acute hypoxia (Table 2), likely due to the low hematocrit and thus inability to increase RV oxygen supply to meet demand. While both the HPH and HPH + CP groups had elevated hematocrit, developed in adaptation to the chronic hypoxia to increase oxygen supply to tissues, only the CP group was able to increase Ees and maintain EF as well as ηvvc in acutely hypoxic conditions. It is unclear why the HPH untreated group did not have sufficient RV reserve to maintain contractility during the stress of acute hypoxia. Our findings suggest that the CHOP-PEG treatment increases the cardiac reserve such that contractility is improved, and coupling efficiency is maintained during the stress of acute hypoxia.
The CHOP-PEG treatment reduced RVSP in agreement with previous results [9], which is the likely mechanism for the reduction in RV hypertrophy as measured by the Fulton index. The treatment also limited the HPH-induced increase in lung collagen content, which appeared localized to the distal pulmonary arteries by histology. We speculate that this effect on the distal pulmonary arteries is the mechanism by which the treatment limited the increase in TPVR with chronic hypoxia. Indeed our prior results indicate that pulmonary arterial collagen accumulation is an important component of HPH-induced pulmonary vascular remodeling in distal arteries [8] as well as large proximal arteries [6]. In proximal arteries, collagen accumulation is largely responsible for an increase in stiffness that may impair RV function [6,31]. Some collagen accumulation was also evident in large airways, which was prevented by the CHOP-PEG treatment. Collagen accumulation in large and small airways may also have contributed to decreased airway compliance [32,33]. While neither proximal PA stiffening nor airway stiffening likely contribute to the increase in TPVR, they may contribute RV afterload and ηvvc. Thus, the ability of CHOP-PEG to limit proximal PA and airway collagen accumulation and subsequent stiffening may contribute to increased RV cardiac reserve in the CHOP-PEG treated mice.
A limitation of this work is that a control group treated only with CHOP-PEG and without exposure to chronic hypoxia was not used. However, prior work in rats demonstrated that CHOP-PEG alone had no effect on mean RV pressure or CO [9,12]. It remains unknown if the treatment was responsible for the lower body weights of the mice in our study; in the prior work in rats, hypoxic and hypoxic-treated animals were pair-fed with control (normoxic and normoxic-treated) animals to maintain similar body weights [9,12]. It is also unclear why CHOP-PEG impaired new collagen synthesis differently in the lung compared to the RV; differences in uptake and clearance may be responsible. Biodistribution experiments [34] could shed light on these potential mechanisms.
Conclusions
The results presented here confirm prior work in rats demonstrating that preventing new collagen synthesis limits both the severity of pulmonary hypertension and RV hypertrophy in response to chronic hypoxia [9] and further suggest that the mechanism of action is reduced pulmonary vascular remodeling that limits the increase in total pulmonary vascular resistance in this model of early and somewhat mild pulmonary hypertension. In addition, limiting new collagen synthesis increased right ventricular contractility reserve in response to chronic hypoxia, as evidenced in acutely hypoxic ventilation conditions, which warrants additional investigation.
Acknowledgment
This study was supported in part by NIH R01HL086939 and an ARRA supplement for undergraduate research (3R01HL86939-3S1). CHOP-PEG was generously donated by VectraMed, Inc. and Ben Belinka. The authors would like to thank Zhijie Wang for helpful discussions.
Glossary
Nomenclature
- ηVVC =
ventricular-vascular coupling efficiency
- CO =
cardiac output
- CP =
CHOP-PEG
- CTL =
control
- Ea =
effective arterial elastance
- Ees =
end-systolic elastance
- EF =
ejection fraction
- HPH =
hypoxic pulmonary hypertension
- HR =
heart rate
- LPA =
left pulmonary artery
- LV =
left ventricle
- OHP =
hydroxyproline
- PA =
pulmonary artery
- PAH =
pulmonary arterial hypertension
- PP =
pulse pressure
- PRSW =
preload recruitable stroke work
- PVR =
pulmonary vascular resistance
- pCO2 =
partial pressure of carbon dioxide
- pO2 =
partial pressure of oxygen
- RPA =
right pulmonary artery
- RV =
right ventricle or right ventricular
- RVEDP =
right ventricular end-diastolic pressure
- RVSP =
right ventricular peak systolic pressure
- S =
intraventricular septum
- SO2% =
systemic blood oxygen saturation
- SV =
stroke volume
- TPVR =
total pulmonary vascular resistance
- VCO =
vena cava occlusion
Contributor Information
David Schreier, Department of Biomedical Engineering, University of Wisconsin, 2145 ECB, 1550 Engineering Drive, Madison, WI 53706.
Gouqing Song, Department of Medicine, Medical Science Center, University of Wisconsin, 1300 University Avenue, Madison, WI 53706.
Naomi Chesler, Department of Biomedical Engineering, University of Wisconsin, 2146 ECB, 1550 Engineering Drive, Madison, WI 53706; Department of Medicine, Medical Science Center, University of Wisconsin, 1300 University Avenue, Madison, WI 53706, e-mail: chesler@engr.wisc.edu.
References
- [1]. D'Alonzo, G. E. , Barst, R. J. , Ayres, S. M. , Bergofsky, E. H. , Brundage, B. H. , Detre, K. M. , Fishman, A. P. , Goldring, R. M. , Groves, B. M. , Kernis, J. T. , Levy, P. S. , Pietra, G. G. , Reid, L. M. , Reeves, J. T. , Rich, S. , Vreim, C. E. , Williams, G. W. , and Wu, M. , 1991, “Survival in Patients With Primary Pulmonary Hypertension. Results From a National Prospective Registry,” Ann. Intern. Med., 115(5), pp. 343–349. [DOI] [PubMed] [Google Scholar]
- [2]. Humbert, M. , Sitbon, O. , Chaouat, A. , Bertocchi, M. , Habib, G. , Gressin, V. , Yaici, A. , Weitzenblum, E. , Cordier, J. F. , Chabot, F. , Dromer, C. , Pison, C. , Reynaud-Gaubert, M. , Haloun, A. , Laurent, M. , Hachulla, E. , Cottin, V. , Degano, B. , Jais, X. , Montani, D. , Souza, R. , and Simonneau, G. , 2010, “Survival in Patients With Idiopathic, Familial, and Anorexigen-Associated Pulmonary Arterial Hypertension in the Modern Management Era,” Circulation, 122(2), pp. 156–163. 10.1161/CIRCULATIONAHA.109.911818 [DOI] [PubMed] [Google Scholar]
- [3]. Pacher, P. N. T. , Mukhopadhyay, P. , Batkai, S. , and Kass, D. A. , 2008, “Measurement of Cardiac Function Using Pressure-Volume Conductance Catheter Technique in Mice and Rats,” Nat. Protoc., 3, pp. 1422–1434. 10.1038/nprot.2008.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Porterfield, J. E. K. A. , Raghavan, K. , Escobedo, D. , Jenkins, J. T. , Larson, E. R. , Trevino, R. J. , Valvano, J. W. , Pearce, J. A. , and Feldman, M. D. , 2009, “Dynamic Correction for Parallel Conductance, GP, and Gain Factor, Alpha, in Invasive Murine Left Ventricular Volume Measurements,” J. Appl. Phys., 107, pp. 1864–1869. 10.1152/japplphysiol.00392.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Tozzi, C. A. , Christiansen, D. L. , Poiani, G. J. , and Riley, D. J. , 1994, “Excess Collagen in Hypertensive Pulmonary Arteries Decreases Vascular Distensibility,” Am. J. Respir. Crit. Care Med., 149(5), pp. 1317–1326. [DOI] [PubMed] [Google Scholar]
- [6]. Ooi, C. Y. , Wang, Z. , Tabima, D. M. , Eickhoff, J. C. , and Chesler, N. C. , 2010, “The Role of Collagen in Extralobar Pulmonary Artery Stiffening in Response to Hypoxia-Induced Pulmonary Hypertension,” Am. J. Physiol. Heart Circ. Physiol., 299(6), pp. H1823–1831. 10.1152/ajpheart.00493.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Wang, Z. , and Chesler, N. C. , 2012, “Role of Collagen Content and Cross-Linking in Large Pulmonary Arterial Stiffening After Chronic Hypoxia,” Biomech. Model. Mechanobiol., 11(1–2), pp. 279–289. 10.1007/s10237-011-0309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Estrada, K. D. , and Chesler, N. C. , 2009, “Collagen-Related Gene and Protein Expression Changes in the Lung in Response to Chronic Hypoxia,” Biomech. Model. Mechanobiol., 8(4), pp. 263–272. 10.1007/s10237-008-0133-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Simon, P. M. , Pachence, J. , Belinka, B. , Poiani, G. J. , Lu, S. E. , Tozzi, C. A. , and Riley, D. J. , 2006, “Prodrug of Proline Analogue Reduces Hypoxic Pulmonary Hypertension in Rats,” Pulm. Pharmacol. Ther., 19(4), pp. 242–250. 10.1016/j.pupt.2005.07.001 [DOI] [PubMed] [Google Scholar]
- [10]. Poiani, G. J. , Riley, D. J. , Fox, J. D. , Kemnitzer, J. E. , Gean, K. F. , and Kohn, J. , 1994, “Conjugates of Cis-4-Hydroxy-L-Proline and Poly(PEG-Lys), a Water Soluble Poly(Ether Urethane): Synthesis and Evaluation of Antifibrotic Effects In Vitro and In Vivo ,” Bioconjugate Chem., 5(6), pp. 621–630. 10.1021/bc00030a018 [DOI] [PubMed] [Google Scholar]
- [11]. Poiani, G. J. , Tozzi, C. A. , Choe, J. K. , Yohn, S. E. , and Riley, D. J. , 1990, “An Antifibrotic Agent Reduces Blood Pressure in Established Pulmonary Hypertension in the Rat,” J. Appl. Physiol., 68(4), pp. 1542–1547. [DOI] [PubMed] [Google Scholar]
- [12]. Kerr, J. S. , Ruppert, C. L. , Tozzi, C. A. , Neubauer, J. A. , Frankel, H. M. , Yu, S. Y. , and Riley, D. J. , 1987, “Reduction of Chronic Hypoxic Pulmonary Hypertension in the Rat by an Inhibitor of Collagen Production,” Am. Rev. Respir. Dis., 135(2), pp. 300–306. [DOI] [PubMed] [Google Scholar]
- [13]. Sagawa, K. M. L. , Maughan, L. , Suga, H. , and Sunagawa, K. , 1988, Cardiac Contraction and the Pressure-Volume Relationship, Oxford University Press, London. [Google Scholar]
- [14]. Sagawa, K. , 1981, “The End-Systolic Pressure-Volume Relation of the Ventricle: Definition, Modifications and Clinical Use,” Circulation, 63(6), pp. 1223–1227. 10.1161/01.CIR.63.6.1223 [DOI] [PubMed] [Google Scholar]
- [15]. Burkhoff, D. , Mirsky, I. , and Suga, H. , 2005, “Assessment of Systolic and Diastolic Ventricular Properties via Pressure-Volume Analysis: A Guide for Clinical, Translational, and Basic Researchers,” Am. J. Physiol. Heart Circ. Physiol., 289(2), pp. H501–512. 10.1152/ajpheart.00138.2005 [DOI] [PubMed] [Google Scholar]
- [16]. Tabima, D. M. , Hacker, T. A. , and Chesler, N. C. , 2010, “Measuring Right Ventricular Function in the Normal and Hypertensive Mouse Hearts Using Admittance-Derived Pressure-Volume Loops,” Am. J. Physiol. Heart Circ. Physiol., 299(6), pp. H2069–2075. 10.1152/ajpheart.00805.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Rubin, L. J. , 1997, “Primary Pulmonary Hypertension,” N. Engl. J. Med., 336(2), pp. 111–117. 10.1056/NEJM199701093360207 [DOI] [PubMed] [Google Scholar]
- [18]. Gomez-Arroyo, J. , Saleem, S. J. , Mizuno, S. , Syed, A. A. , Bogaard, H. J. , Abbate, A. , Taraseviciene-Stewart, L. , Sung, Y. , Kraskauskas, D. , Farkas, D. , Conrad, D. H. , Nicolls, M. R. , and Voelkel, N. F. , 2012, “A Brief Overview of Mouse Models of Pulmonary Arterial Hypertension: Problems and Prospects,” Am. J. Physiol. Lung Cell. Mol. Physiol., 302(10), pp. L977–991. 10.1152/ajplung.00362.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Nicolls, M. R. , Mizuno, S. , Taraseviciene-Stewart, L. , Farkas, L. , Drake, J. I. , Husseini, A. A. , Gomez-Arroyo, J. , Voelkel, N. , and Bogaard, H. , 2012, “New Models of Pulmonary Hypertension Based on VEGF Receptor Blockage-Induced Endothelial Cell Apoptosis,” Pulm. Circ., 2(4), pp. 434–442. 10.4103/2045-8932.105031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Ciuclan, L. , Bonneau, O. , Hussey, M. , Duggan, N. , Holmes, A. M. , Good, R. , Stringer, R. , Jones, P. , Morrell, N. W. , Jarai, G. , Walker, C. , Westwick, J. , and Thomas, M. , 2011, “A Novel Murine Model of Severe Pulmonary Arterial Hypertension,” Am. J. Respir. Crit Care Med., 184(10), pp. 1171–1182. 10.1164/rccm.201103-0412OC [DOI] [PubMed] [Google Scholar]
- [21]. Bernardo, B. C. , Weeks, K. L. , Pretorius, L. , and McMullen, J. R. , 2010, “Molecular Distinction Between Physiological and Pathological Cardiac Hypertrophy: Experimental Findings and Therapeutic Strategies,” Pharmacol. Ther., 128(1), pp. 191–227. 10.1016/j.pharmthera.2010.04.005 [DOI] [PubMed] [Google Scholar]
- [22]. Fujita, M. , Mason, R. J. , Cool, C. , Shannon, J. M. , Hara, N. , and Fagan, K. A. , 2002, “Pulmonary Hypertension in TNF-Alpha-Overexpressing Mice is Associated With Decreased VEGF Gene Expression,” J. Appl. Physiol., 93(6), pp. 2162–2170. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12391106 [DOI] [PubMed] [Google Scholar]
- [23]. Nikam, V. S. , Schermuly, R. T. , Dumitrascu, R. , Weissmann, N. , Kwapiszewska, G. , Morrell, N. , Klepetko, W. , Fink, L. , Seeger, W. , and Voswinckel, R. , 2010, “Treprostinil Inhibits the Recruitment of Bone Marrow-Derived Circulating Fibrocytes in Chronic Hypoxic Pulmonary Hypertension,” Eur. Respir. J., 36(6), pp. 1302–1314. 10.1183/09031936.00028009 [DOI] [PubMed] [Google Scholar]
- [24]. Ochoa, C. D. , Yu, L. , Al-Ansari, E. , Hales, C. A. , and Quinn, D. A. , 2010, “Thrombospondin-1 Null Mice are Resistant to Hypoxia-Induced Pulmonary Hypertension,” J. Card. Surg., 5, pp. 32–38. 10.1186/1749-8090-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Schermuly, R. T. , Dony, E. , Ghofrani, H. A. , Pullamsetti, S. , Savai, R. , Roth, M. , Sydykov, A. , Lai, Y. J. , Weissmann, N. , Seeger, W. , and Grimminger, F. , 2005, “Reversal of Experimental Pulmonary Hypertension by PDGF Inhibition,” J. Clin. Invest., 115(10), pp. 2811–2821. 10.1172/JCI24838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Scherrer-Crosbie, M. , Steudel, W. , Hunziker, P. R. , Foster, G. P. , Garrido, L. , Liel-Cohen, N. , Zapol, W. M. , and Picard, M. H. , 1998, “Determination of Right Ventricular Structure and Function in Normoxic and Hypoxic Mice: A Transesophageal Echocardiographic Study,” Circulation, 98(10), pp. 1015–1021. 10.1161/01.CIR.98.10.1015 [DOI] [PubMed] [Google Scholar]
- [27]. Beppu, H. , Ichinose, F. , Kawai, N. , Jones, R. C. , Yu, P. B. , Zapol, W. M. , Miyazono, K. , Li, E. , and Bloch, K. D. , 2004, “BMPR-II Heterozygous Mice Have Mild Pulmonary Hypertension and an Impaired Pulmonary Vascular Remodeling Response to Prolonged Hypoxia,” Am. J. Physiol. Lung Cell. Mol. Physiol., 287(6), pp. L1241–1247. 10.1152/ajplung.00239.2004 [DOI] [PubMed] [Google Scholar]
- [28]. Champion, H. C. , Villnave, D. J. , Tower, A. , Kadowitz, P. J. , and Hyman, A. L. , 2000, “A Novel Right-Heart Catheterization Technique for In Vivo Measurement of Vascular Responses in Lungs of Intact Mice,” Am. J. Physiol. Heart Circ. Physiol., 278(1), pp. H8–H15. [DOI] [PubMed] [Google Scholar]
- [29]. Zhao, L. , Long, L. , Morrell, N. W. , and Wilkins, M. R. , 1999, “NPR-A-Deficient Mice Show Increased Susceptibility to Hypoxia-Induced Pulmonary Hypertension,” Circulation, 99(5), pp. 605–607. 10.1161/01.CIR.99.5.605 [DOI] [PubMed] [Google Scholar]
- [30]. Steudel, W. , Scherrer-Crosbie, M. , Bloch, K. D. , Weimann, J. , Huang, P. L. , Jones, R. C. , Picard, M. H. , and Zapol, W. M. , 1998, “Sustained Pulmonary Hypertension and Right Ventricular Hypertrophy After Chronic Hypoxia in Mice With Congenital Deficiency of Nitric Oxide Synthase 3,” J. Clin. Invest., 101(11), pp. 2468–2477. 10.1172/JCI2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Wang, Z. , and Chesler, N. C. , 2011, “Pulmonary Vascular Wall Stiffness: An Important Contributor to the Increased Right Ventricular Afterload With Pulmonary Hypertension,” Pulm. Circ., 1(2), pp. 212–223. 10.4103/2045-8932.83453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Habre, W. , Janosi, T. Z. , Fontao, F. , Meyers, C. , Albu, G. , Pache, J. C. , and Petak, F. , 2010, “Mechanisms for Lung Function Impairment and Airway Hyperresponsiveness Following Chronic Hypoxia in Rats,” Am. J. Physiol. Lung Cell. Mol. Physiol., 298(4), pp. L607–614. 10.1152/ajplung.00222.2009 [DOI] [PubMed] [Google Scholar]
- [33]. Inscore, S. C. , Stenmark, K. R. , Orton, C. , and Irvin, C. G. , 1991, “Neonatal Calves Develop Airflow Limitation due to Chronic Hypobaric Hypoxia,” J. Appl. Physiol., 70(1), pp. 384–390. [DOI] [PubMed] [Google Scholar]
- [34]. Lammers, T. , Subr, V. , Peschke, P. , Kuhnlein, R. , Hennink, W. E. , Ulbrich, K. , Kiessling, F. , Heilmann, M. , Debus, J. , Huber, P. E. , and Storm, G. , 2008, “Image-Guided and Passively Tumour-Targeted Polymeric Nanomedicines for Radiochemotherapy,” Br. J. Cancer, 99(6), pp. 900–910. 10.1038/sj.bjc.6604561 [DOI] [PMC free article] [PubMed] [Google Scholar]