Abstract
Pesticides are normally used to control specific pests and to increase the productivity in crops; as a result, soils are contaminated with mixtures of pesticides. In this work, the ability of Streptomyces strains (either as pure or mixed cultures) to remove pentachlorophenol and chlorpyrifos was studied. The antagonism among the strains and their tolerance to the toxic mixture was evaluated. Results revealed that the strains did not have any antagonistic effects and showed tolerance against the pesticides mixture. In fact, the growth of mixed cultures was significantly higher than in pure cultures. Moreover, a pure culture (Streptomyces sp. A5) and a quadruple culture had the highest pentachlorophenol removal percentages (10.6% and 10.1%, resp.), while Streptomyces sp. M7 presented the best chlorpyrifos removal (99.2%). Mixed culture of all Streptomyces spp. when assayed either as free or immobilized cells showed chlorpyrifos removal percentages of 40.17% and 71.05%, respectively, and for pentachlorophenol 5.24% and 14.72%, respectively, suggesting better removal of both pesticides by using immobilized cells. These results reveal that environments contaminated with mixtures of xenobiotics could be successfully cleaned up by using either free or immobilized cultures of Streptomyces, through in situ or ex situ remediation techniques.
1. Introduction
The agriculture sector is a very important part of the economy and provides foods and raw materials needed for a sustainable development. For this reason, this sector uses different resources as pesticides, chemical fertilizers, equipment, and machines [1]. Pesticides are usually applied simultaneously or one after another for crop protection, and this type of pesticide application often leads to a combined contamination of these compound residues in the soil environment [2]. Among the pesticides, compounds such as organochlorines, organophosphates, carbamates, and pyrethroids are commonly used in vegetables and other crops, in order to increase the productivity [3]. As a result, an increase in the pesticide application translates into an increase in their residues in all spheres of environment [4], especially in agricultural soils.
Because of the restriction imposed on toxic organophosphate compounds, chlorpyrifos (CP), a broad-spectrum and moderately toxic organophosphate insecticide, has gained the status of one of the most widely used commercial compounds [5]. The widespread use of this pesticide and its resulting residues, which accumulates on agricultural crops, produces an increase of not only environmental contamination but also health hazards to consumers [6]. Moreover, it has been determined that CP resistance to biodegradation is increased due to the accumulation of one of its main byproducts known as 3,5,6-trichloro-2-pyridinol (TCP). TCP is listed as a persistent and mobile pollutant by the US Environmental Protection Agency [7] and shows relatively high antimicrobial effects on microorganisms, which prevents its own degradation [8]. The contamination of soil by CP can be caused by the handling of the pesticide in the farmyard and by the rinsing of containers [6]. CP, among others pesticides, has been detected in groundwater in Greece: 0.005–0.01 μg L−1, and in Brazil in superficial water, rivers, and lakes: 0.001–0.174 μg L−1 [9].
Other substances widely used in both agricultural and industrial sector are chlorophenolic compounds, which are applied as broad spectrum biocides [10]. Among them, pentachlorophenol (PCP) and its sodium salt have been used as wood and leather preservatives [11]. PCP is toxic to all life forms since it inhibits oxidative phosphorylation [12]. Furthermore, it can accumulate in living organisms and thus produce adverse effect as carcinogenicity and acute toxicity [13]. In addition, this compound is highly recalcitrant due to the stability of the aromatic ring and its high degree of chlorination [14], whereby it can be considered as a hazardous pollutant for the environment. In surface waters from different countries, PCP was detected at concentrations ranging from trace levels to 10,500 μg L−1 [13]. In Chile, it was found in water samples of the Limari river basin [9].
Thus, remediation of CP and PCP-contaminated sites is urgently required. A number of methods, including chemical treatment, volatilization, photodecomposition, incineration, and stockpiling, can be applied for the detoxification of these xenobiotic compounds [15–17]. However, most of them are not applicable for diffused contamination at low concentration making it expensive, slow, inefficient, and not always environmental friendly. Thus, biotic degradation is one of the most viable options for the remediation of CP and PCP in soil and water.
In some early studies, CP was reported to be resistant to biodegradation due to accumulation of the antimicrobial degradation products in soil [18]. However, subsequent studies have revealed that many microorganisms are capable of degrading CP efficiently [19–22]. Moreover, different researchers have reported PCP degrading microorganisms from the natural environment. Several bacterial strains such as Arthrobacter, Pseudomonas, Sphingobium chlorophenolicum, and Serratia marcescens capable of PCP degradation have been reported [23–26]. Among Gram-positive microorganisms, actinobacteria have a great potential for biodegradation of organic and inorganic toxic compounds, and previous studies demonstrated the ability of different genera of actinobacteria to degrade pesticides such as lindane, chlordane, methoxychlor, CP, diuron, and PCP [6, 27, 28].
Complete mineralization of pesticides or their transformation to nontoxic products is desirable in bioremediation process, which is more reasonable with the use of microbial consortia rather than single cultures [29]. Microbial consortia have been shown to be more suitable for bioremediation of recalcitrant compounds as their biodiversity supports environmental survival and increase the number of catabolic pathways available for contaminant biodegradation [30]. However, there are no reports about simultaneous bioremediation of CP and PCP, particularly by actinobacteria consortia. Thus, the aim of this work was to evaluate the ability of pure as well as mixed actinobacteria cultures isolated from different contaminated environments, to degrade a pentachlorophenol and chlorpyrifos mixture, using free and immobilized microbial cells.
2. Materials and Methods
2.1. Chemicals
Chlorpyrifos (CP) (99% pure) and pentachlorophenol (PCP) (98% pure) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals used throughout the study were of analytical grade and were purchased from standard manufacturers.
2.2. Microorganisms and Culture Media
Four Streptomyces spp. (A2, A5, A11, and M7) isolated from organochlorine pesticides contaminated Argentinian soils and sediments [27, 31], and other two strains (Streptomyces spp. AC5 and AC7) isolated from a Chilean soil contaminated with organophosphorus pesticides [6], were used in this study. These actinobacteria were grown as single cultures and combined as different microbial consortia. The first mixed culture was an Argentinian microbial consortium (Streptomyces spp. A2-A5-A11-M7), known for its lindane biodegradation potential [32]; the second one was a combination of the two Chilean strains (Streptomyces spp. AC5 and AC7), and the third consortium consisted of the six actinobacteria strains together.
Starch-casein medium (SC) was used for antagonism assays, consisting of (g L−1): starch, 10.0; casein, 1.0; K2HPO4, 0.5; agar, 15.0. The pH was adjusted to 7.0 prior to sterilization.
Minimal medium (MM) was used for growth of the microorganisms and pollutants removal assays. It consisted of (g L−1): L-asparagine, 0.5; K2HPO4, 0.5; MgSO4·7H2O, 0.2; FeSO4·7H2O, 0.01 [33]. The same medium was added with agar, 15.0 g L−1, for tolerance to toxicity of CP and PCP assay. The pH was adjusted to 7.0 prior to sterilization.
Tryptic Soy Broth (TSB), containing (g L−1): trypticase, 15.0; soy peptone, 3.0; NaCl, 5.0; K2HPO4, 2.5; glucose, 2.5, was used for the inocula preparation for the immobilization technique. The pH was adjusted to 7.3 ± 0.2 prior to sterilization.
All media were sterilized by autoclaving at 121°C for 15 min.
2.3. Antagonism and Tolerance Assays
In order to determine the potential presence of antagonistic effects among the Streptomyces spp. strains studied, a modification of Bell et al. [34] technique was used. One Streptomyces strain was spread in the center of a Petri dish containing SC medium and faced transversely with the other Streptomyces spp. strains. One strain was considered to be antagonistic to the other when a growth inhibition was observed. Thereby, the presence of antagonism among the six studied strains was assessed by considering all possible combinations [32].
To evaluate the tolerance of Streptomyces spp. strains to the pesticide mixture (CP and PCP), a qualitative assay was performed using MM agar plates. Rectangular troughs were cut in the centre of the plate and then filled with a filter-sterilized solution of the mixed pesticides (CP and PCP, each at a concentration of 1.66 mg L−1). The actinobacteria strains were inoculated by streaking perpendicularly to the troughs. Microbial growth was used as a qualitative parameter of the tolerance to the pesticides mixture. The Petri dishes were incubated at 30°C for seven days. Growth controls were performed using plates without pesticides [35].
2.4. Study of the Ability of Pure and Mixed Actinobacteria Cultures to Grow and Remove the Pesticide Mixture
2 g L−1 of biomass (wet weight) of the microbial consortia Streptomyces spp. A2-A5-A11-M7 and Streptomyces spp. AC5 and AC7 and the six pure actinobacteria strains were inoculated in different Erlenmeyer flasks containing 30 mL MM spiked with the pesticides mixture (1.66 mg L−1 of each one). Cultures were incubated at 30°C for 72 h on a rotary shaker at 200 rpm and then centrifuged (8,500 ×g, 10 min and 4°C). Ten milliliters of the supernatant were aseptically taken out in each case for residual pesticides determination. Microbial growth was measured as dry weight at 105°C. All experiments were carried out in triplicate, and the results are given as the means.
2.5. Removal of Pesticides Mixture by a Free and Immobilized Consortium of Six Streptomyces spp. Strains
All Streptomyces spp. strains were individually cultured in TSB for 72 h at 30°C and 200 rpm. The cultures were centrifuged at 8,500 ×g for 10 min, and the pellets obtained were then washed with sterile distilled water for the immobilization. For this, the actinobacteria consortium (Streptomyces spp. A2-A5-A11-M7-AC5-AC7, each strain at equal proportion), was mixed with a sodium alginate solution, obtaining a final concentration of 7.5% of biomass in the support (w/v, wet weight) of the mixed culture [36]. This mixture was poured into 2% CaCl2·2H2O solution and incubated at room temperature for 1 h. Then, the beads (3–5 mm diameter) were washed three times with sterile distilled water [37]. All the materials used for the preparation of the entrapped cells were sterilized, and the operations were carried out under sterile conditions.
The immobilized consortium was inoculated into MM containing mixed pesticides (CP and PCP, 1.66 mg L−1 of each one) and subsequently incubated for 72 h at 30°C. Samples collected every 24 h were analyzed for residual pesticides concentrations.
For free cell culture assays with all the Streptomyces spp. strains together, the methodology used was the same described above (see Section 2.4).
2.6. Analysis of Pentachlorophenol and Chlorpyrifos
Supernatant samples of the centrifuged cultures were used to determine residual CP and PCP concentrations. For residual CP concentration determination, 1 mL of each sample was diluted to a volume of 10 mL with distilled water, and then it was extracted twice with 10 mL of hexane. The organic extracts were combined and dehydrated with Na2SO4. The samples were stored at −20°C before chromatographic analysis. A Shimadzu gas chromatograph GC-2014 equipped with an RTX-5 capillary column (crossbond 5% diphenyl/95% dimethyl polysiloxane, 30 m, 0.32 mm i.d., film thickness 0.25 μm), and NPD detector was used. The injection and detector temperatures were set at 280°C and 300°C, respectively. The oven temperature program began at 90°C for 1 min, increased to 180°C at 15°C/min, then increased to 240°C at 5°C/min, and finally increased to 280°C at 15°C/min. The obtained data were analyzed with the program GC Solution Version 2.30.00 (GC Solution Analysis Copyright 2000–2004 Shimadzu) [6]. The retention time for CP was 12.5 min. The recovery of CP in the liquid medium was 90%.
Residual PCP concentration was determined with an HPLC equipped with a Merck-Hitachi L-7100 pump, a Rheodyne 7725 injector with a 20-μL loop, a Merck-Hitachi L-7455 diode array detector operating at 215 nm and a Hitachi D-7000 data processor. A LiCHrospher 60 RP select B 250 × 4 mm column of 5 μm particle size with a LichroCART 4-4 guard column (Merck) was used. The mobile phase consisted of acetonitrile and phosphoric acid (1% aqueous solution) 1 : 1 (v/v) with a flow rate of 1 mL min−1. In these operative conditions, PCP retention time was 12 min [38]. Method calibration and quantification was performed by the pure reference standard (0.05–5 mg L−1). The recovery of PCP ranged from 97% to 100%.
2.7. Statistical Analysis
All the results are the average of three replicates per sample. One-way analysis of variance (ANOVA) and Tukey test were performed to test the significant differences among treatments. When significant differences were found, Tukey post-test was used to separate the effects among treatments. Tests were considered significantly different at P < 0.05. Professional versions of Infostat and Statistic 6.0 software were used.
3. Results and Discussion
3.1. Antagonism and Tolerance Assays
In order to formulate mixed cultures, all the studied actinobacteria were assayed to determine the presence of antagonistic effects among them. The antagonistic phenomenon is a common event showed in a mixed microbial population [39]. In case of Streptomyces spp. strains isolated from Argentinean and Chilean environments, no antagonistic effect on their individual growth was observed (Figure 1), which suggests that all the strains could be cultured together as a consortium. In contrast, Thouand et al. [40] observed the presence of antagonistic relations in a mixed microbial population, which exerted a negative impact on the ability of oil degradation in liquid systems. In fact, van Hamme et al. [41] showed the presence of antagonistic relations in bacterial populations within a mixed community, due to the metabolites production capable of killing or inhibiting the growth of other populations.
Figure 1.
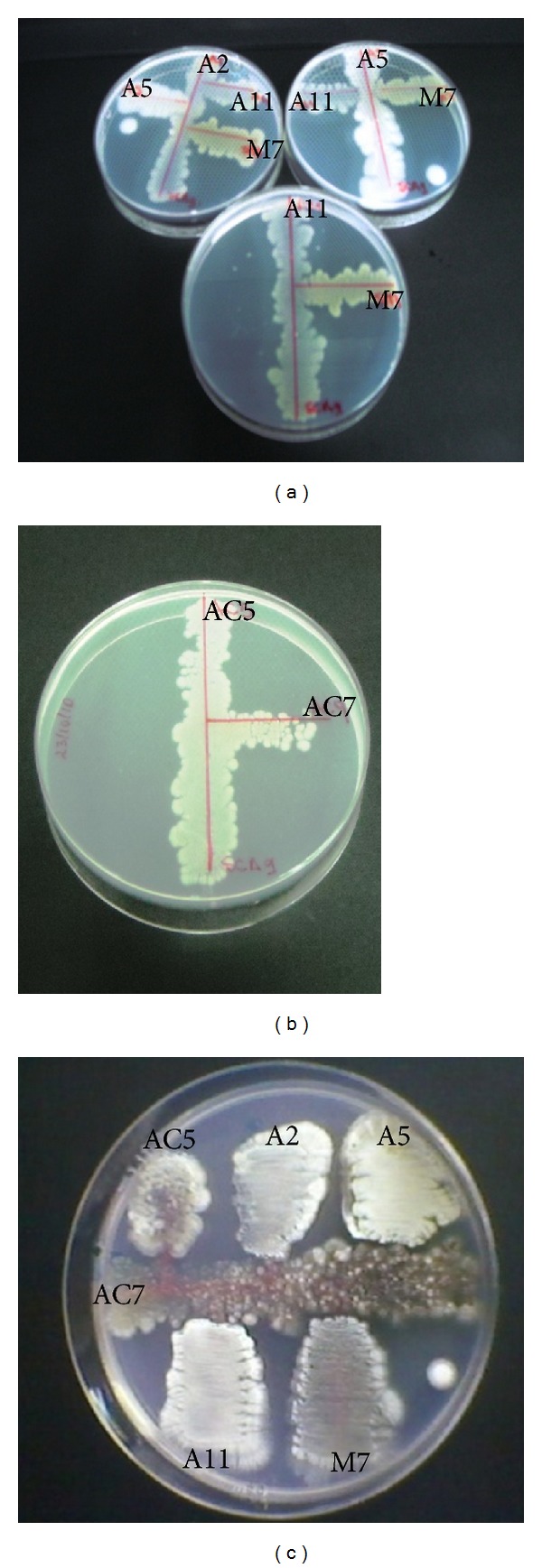
Antagonism assays among: (a) Streptomyces spp. A2, A5, A11, and M7; (b) Streptomyces spp. AC5 and AC7; and (c) the six Streptomyces spp. strains.
Although it have been described actinobacteria capable of tolerating and/or degrading CP or PCP [6, 28, 42, 43], it was necessary to evaluate the ability of the actinobacteria to tolerate the pesticides mixture. Thereby, when the actinobacteria tolerance to the mixed pesticides was tested, it was observed that four of them (Streptomyces spp. A2, A11, M7 and AC7) showed a high degree of tolerance to the toxic mixture, while the other two strains (Streptomyces spp. A5 and AC5) presented moderate tolerance, based on qualitative analyses taking into account the degree of growth of each strain in the surroundings of the mixture of pesticides (Figure 2). This experimental technique was used previously for systematic screening on heavy metals resistance and organochlorine pesticides tolerance by actinobacteria [31, 44]. The results presented here would indicate that the pesticides mixture concentration used were not toxic for these actinobacteria strains under the evaluated experimental conditions.
Figure 2.
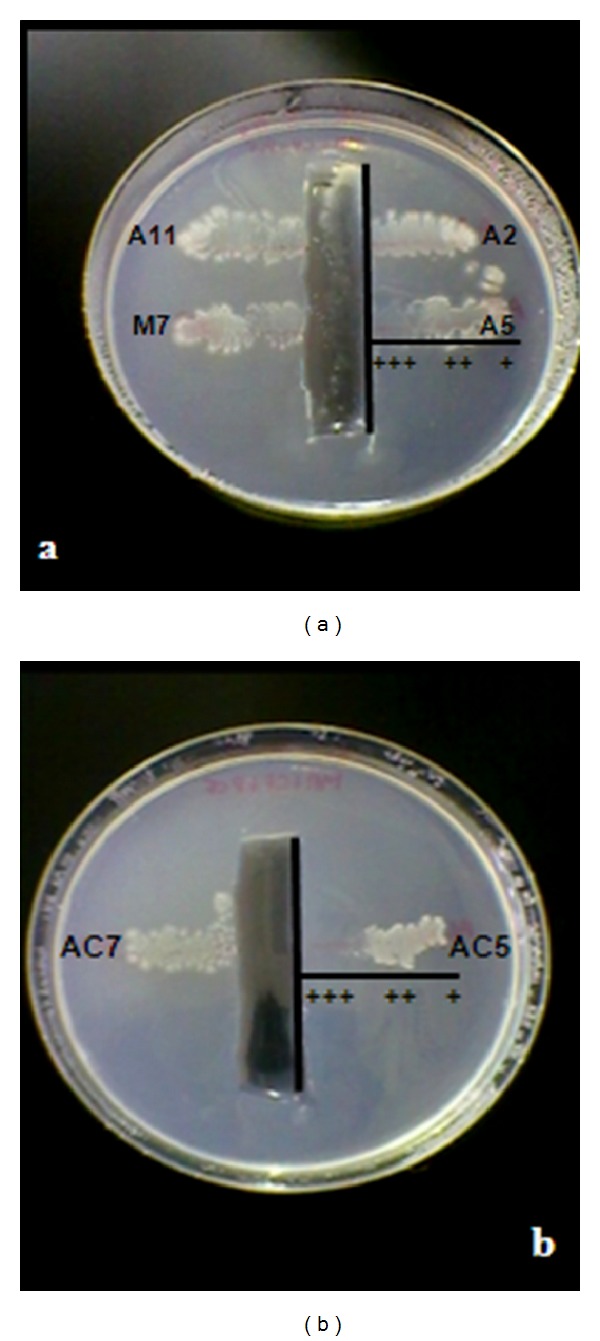
Tolerance assays of the studied actinobacteria to the mixture of CP and PCP (1.66 mg L−1 of each one). (a) Streptomyces spp. A2, A5, A11, and M7. (b) Streptomyces spp. AC5 and AC7. (+++) Abundant growth: highly tolerant, (++) moderate growth: tolerant, (+) scarce growth: lowly tolerant, and (−) no growth: not tolerant.
3.2. Microbial Growth and Removal of Pesticides Mixture by Pure and Mixed Actinobacteria Cultures
Based on obtained results (Section 3.1) and previous studies, which describe the actinobacteria abilities to use compounds with chlorine atoms in their molecules as carbon source [6, 27, 28, 43, 45], the growth of the strains as pure and mixed cultures in liquid MM supplemented with the pesticides mix (CP + PCP) was evaluated. Microbial biomass (dry weight) of the pure cultures ranged between 5 and 21.7 mg L−1, whereas the growth of the mixed cultures was significantly higher (P < 0.05), reaching a biomass of 98.3 mg L−1 for the mixed culture of Streptomyces spp. AC5-AC7 and 101.67 mg L−1 for Streptomyces spp. A2-A5-A11-M7 (Figure 3). In absence of the pesticides mixture in the culture medium, no growth was detected. The highest biomass production of mixed cultures could be explained due to a metabolic action complementary among actinobacteria in the consortia, which make them capable of allowing the most efficient use of these pesticides as carbon source. In previous studies, Yang et al. [46] observed a high atrazine mineralizing efficiency when a mixed culture of Klebsiella sp. A1 and Comamonas sp. A2 was used. However, when these authors used pure cultures, they obtained no or poor growth and no or less atrazine degrading ability. In the present study, the results showed an increase of the biomass when increasing the number of strains in the culture medium, demonstrating that there is no inhibition of growth by the presence of pesticides or antagonism among strains.
Figure 3.
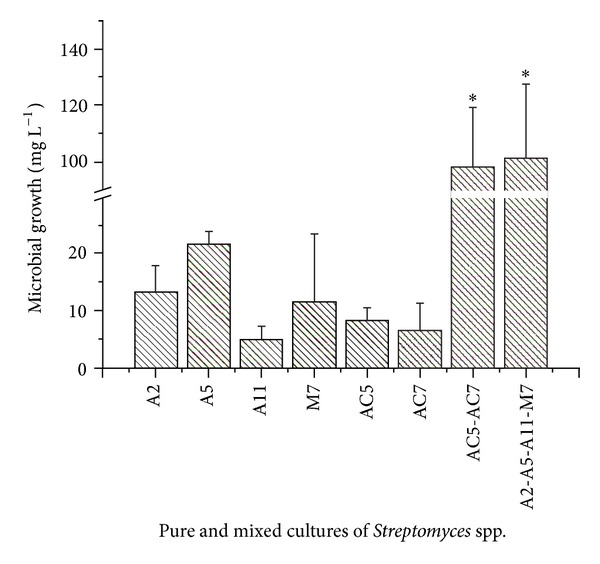
Microbial growth of pure and mixed cultures of Streptomyces spp. in MM contaminated with a mixture of chlorpyrifos (CP) and pentachlorophenol (PCP). Bars showing asterisk indicate they were significantly different to all others (P < 0.05, Tukey post-test).
The pesticide removal abilities of the pure and mixed cultures were determined by analyzing CP and PCP residual concentrations. It was observed that Streptomyces sp. A5 and the mixed culture Streptomyces spp. A2-A5-A11-M7 presented similar PCP removal percentages (10.6 and 10.1%, resp.); the mixed culture Streptomyces spp. AC5-AC7 only removed 6% of PCP; instead Streptomyces spp. A2, AC5, and AC7 in pure cultures did not show the ability to remove PCP (Figure 4). Compared to most of the pure cultures, removal of PCP was significantly enhanced (P < 0.05) when these strains were grown in a coculture. Similar enhanced degradation has been observed in many studies of different pesticide-degrading consortia. For instance, Sørensen et al. [47] reported an enhancement of 59% in the mineralization of isoproturon when Sphingomonas sp. SRS2 was grown in coculture with SRS1 strain, rather than pure. Other coculture was able to mineralize a 62% of the added diuron (10 mg L−1) in a mineral medium due to the cooperative degradative capacities of Arthrobacter globiformis strain D47 and Variovorax sp. strain SRS16, since neither strain D47 nor strain SRS16 was capable of performing extensive mineralization of the herbicide in pure culture [48]. In natural environments, microorganisms are heterogeneously distributed and possibly occur in multispecies rather than single-species communities [47]. Close proximity within the community may synergistically improve the metabolism of organic pollutants introduced into the environment [47]. In these studies, neither strain Streptomyces sp. AC5 nor strain Streptomyces sp AC7 conclusively removed PCP in MM but combined the constructed two-member consortium removed approximately 6% of PCP, demonstrating that synergistic interactions between both strains may be involved in the degradation of PCP.
Figure 4.
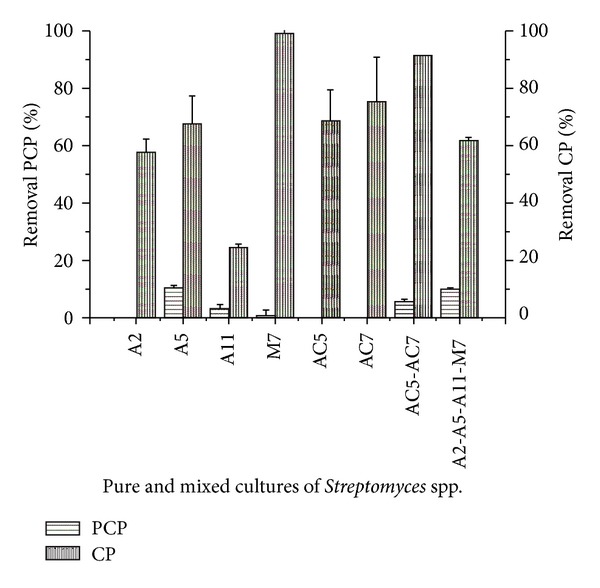
Removal percentages of pentachlorophenol (PCP) and chlorpyrifos (CP) in pure and mixed cultures of Streptomyces spp. in minimal medium contaminated with the pesticides mixture.
All the pure and mixed actinobacteria cultures were able to remove CP, reaching removal percentages greater than PCP removal. The strain Streptomyces sp. M7 in pure culture showed the best CP removal capability (99.2%) but the mixed culture Streptomyces spp. AC5-AC7 also showed high ability for CP removal (91.52%) (Figure 4). In previous studies, Krishna and Philip [3] observed great differences in the removal efficiency of three toxic compounds in a submerged soil system contaminated with a mixture of pesticides (carbofuran, lindane, and methyl parathion at a final concentration of 2 mg g−1 of soil), where carbofuran degradation was maximum whereas minimum degradation was observed for lindane, both in the soil phase and in the liquid phase. They also found that in the mixture of these pesticides, the degradation efficiency was minor than the efficiency detected in systems contaminated with one pesticide at a time. This phenomenon was attributed to the lower number of microorganisms available to degrade specific individual pesticides. In the present work, this phenomenon could also explain the low PCP removal obtained in comparison to CP removal, although further studies are required to prove it. On the other hand, Buono et al. [49], who studied the toxic effects of pesticides of current use (PCP, azinphos-methyl (AZM) and CP) on the development of Paracentrotus lividus embryos, demonstrated that the most toxic pesticides were PCP and AZM at EC50 (median toxic effect concentration 50%) level. They also observed that PCP toxic effects were not significant at concentrations below 0.03 mg L−1, but at higher concentrations, such as 0.3 mg L−1; the effects were significant. In this work, the PCP concentration was approximately 5.5 times higher than 0.3 mg L−1, which could be another reason for the minor removal of this compound. On the contrary, Matamoros et al. [50], in a study pertaining to behavior of organic pollutants in constructed wetlands, found that PCP removal efficiency was higher (>90%) than CP removal (80%–90%), starting with an initial concentration of 2.5 mg L−1 of each pollutant. Although in the present study, the CP was the pesticide with the highest removal percentages from the mixture.
All Streptomyces strains studied at the present work have been previously exposed to different chlorinated pesticides. Thus, these microorganisms could have the enzymatic ability to release chloride ions, favoring its degradation. The cross-adaptation phenomenon suggests that one pesticide may be rapidly degraded in soil in which it has never been applied; provided that the same soil had been previously exposed to a pesticide belonging to the same chemical group [51]. This could explain removal percentages obtained for both pesticides, PCP and CP, for strains as Streptomyces sp. A5 or Streptomyces sp. M7.
Another actinobacteria strain, Kocuria sp. CL2 isolated from secondary sludge of pulp and paper mill, able to use PCP as the sole source of carbon and degrade this pesticide, had been reported [28].
3.3. Removal of Pesticides Mixture by a Consortium of Six Streptomyces spp. Strains Free and Immobilized
Different researchers have demonstrated that cell immobilization techniques can significantly increase the removal efficiency of different pesticides compared with free cells [36, 52]. Thus, removal of a pesticides mixture (CP and PCP) by a six actinobacteria consortium, either free or immobilized in alginate beads, was compared.
The results revealed that CP removal was higher than PCP removal, following the same trend observed in previous assays (see Section 3.2), both in free and immobilized mixed cultures. CP removal percentages were 40.17 and 71.05 for free and immobilized cells, respectively. For PCP removal, the obtained values were 5.24% and 14.74% for the free and immobilized systems, respectively (Table 1). Thus, it is evident the increase of the removal of both pesticides by using the microbial immobilization technique. A possible reason for this could be that the alginate beads allow the optimal diffusion of contaminants [53–55] and besides; the support could be also acting as a protection for the cells against the detrimental effects of the surrounding medium such as pH and toxic substances, also enhancing the degrading ability of the cells [56, 57]. In fact, several researchers have demonstrated that calcium alginate immobilization improved the removal of toxic compounds. For instance, a Pseudomonas strain immobilized in calcium alginate mineralized a 50% more of phenol than free cells under the same conditions [58]. Also, Yañez-Ocampo et al. [52] studied the removal of two organophosphate pesticides by a bacterial consortium, and they obtained a percentage of methyl parathion removed 31% higher when the consortium was immobilized in alginate beads, compared with a suspension culture.
Table 1.
Pesticides removal percentages of the six actinobacteria strains culture in free and immobilized cells systems.
| Sixfold culture | PCP removal (%) | CP removal (%) |
|---|---|---|
| Free cells | 5.24 ± 0.56 | 40.17 ± 1.79 |
| Immobilized cells | 14.74 ± 4.73 | 71.05 ± 0.88 |
On the other hand, the pesticide sorption phenomenon to the alginate support was registered for both compounds. However, analysis of CP removal showed that the phenomenon of sorption was gradual, increasing up to 72 h, whereas for PCP it remained almost stable from 24 h until the end of the assay (data not shown). The sorption percentage was higher for CP (60.34% ± 0.42%) than for PCP (5.97% ± 2.41%) (data not shown), although the pesticides removal percentages observed by using alginate beads with or without microorganisms were significantly different (P < 0.05), evidencing the microbial activity. The sorption of different compounds, such as dyes and pesticides, on different immobilization supports was also reported by other researchers [36, 59].
Furthermore, when comparing the ability of removing the pesticides among all the free cell cultures, it might be concluded that the use of the six Streptomyces strains together did not present the best percentages of removal of the pesticides. These results are similar to those obtained by Fuentes et al. [32] in which mixed cultures consisting in two, three, and four strains improved the lindane removal compared with pure cultures, whereas combinations of five and six strains were not efficient for the removal of the pesticide from the culture medium.
Moreover, the analysis of the removal percentage of the pesticides mixture, calculated as the average between the removal percentages of CP and PCP for the mixed cultures, showed that the double consortium (Streptomyces spp. AC5-AC7) and the mixed culture of the six strains immobilized (Streptomyces spp. A2-A5-A11-M7-AC5-AC7) were the consortia with higher ability to removal the toxic mixture (48.64% and 42.90%, resp.) (Figure 5).
Figure 5.
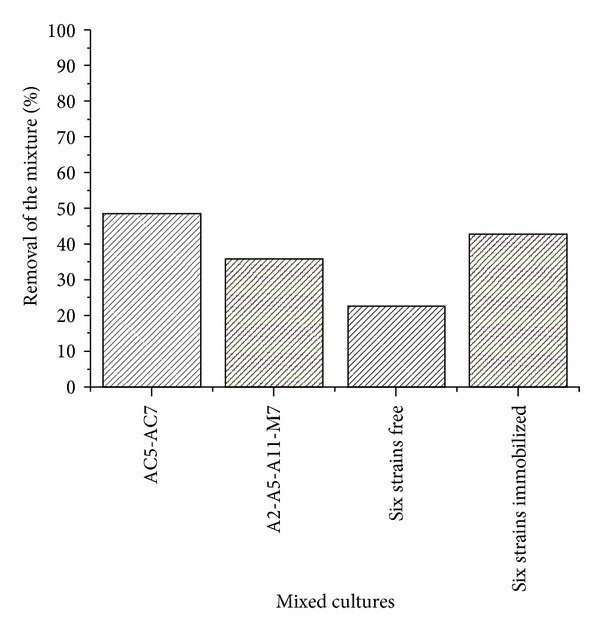
Removal percentages of the mixture (CP + PCP) for mixed cultures of Streptomyces spp. strains (A2, A5, A11, M7, AC5, AC7) as free and immobilized cells.
4. Conclusions
Six Streptomyces spp. strains were able to tolerate a mixture of PCP and CP and did not show antagonistic effects among them. These strains were also able to grow and remove mixed pesticides, in pure as well as in mixed cultures. The immobilization of the cells allowed an increase of the removal of both pesticides. Our results reveal that Streptomyces strains could be used in mixed cultures and in immobilized systems as a potential tool for remediation of environments contaminated with multiple xenobiotics.
Conflict of Interests
This research was not influenced by any conflict of interests.
Acknowledgments
This work was financially supported by Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), FONDECYT Postdoctoral Project no. 3100118, and Program of Scientific International Cooperation CONYCYT/MINCYT 2009-111. The authors gratefully acknowledge Mr. G. Borchia for his technical assistance.
References
- 1.Kumari B, Madan VK, Kathpal TS. Status of insecticide contamination of soil and water in Haryana, India. Environmental Monitoring and Assessment. 2008;136(1–3):239–244. doi: 10.1007/s10661-007-9679-1. [DOI] [PubMed] [Google Scholar]
- 2.Chu X, Fang H, Pan X, et al. Degradation of chlorpyrifos alone and in combination with chlorothalonil and their effects on soil microbial populations. Journal of Environmental Sciences. 2008;20(4):464–469. doi: 10.1016/s1001-0742(08)62080-x. [DOI] [PubMed] [Google Scholar]
- 3.Krishna KR, Philip L. Bioremediation of single and mixture of pesticide-contaminated soils by mixed pesticide-enriched cultures. Applied Biochemistry and Biotechnology. 2011;164(8):1257–1277. doi: 10.1007/s12010-011-9211-5. [DOI] [PubMed] [Google Scholar]
- 4.Stocka J, Tankiewicz M, Biziuk M, Namieśnik J. Green aspects of techniques for the determination of currently used pesticides in environmental samples. International Journal of Molecular Sciences. 2011;12(11):7785–7805. doi: 10.3390/ijms12117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuperberg JM, Soliman KFA, Stino FKR, Kolta MG. Effects of time of day on chlorpyrifos-induced alterations in body temperature. Life Sciences. 2000;67(16):2001–2009. doi: 10.1016/s0024-3205(00)00785-2. [DOI] [PubMed] [Google Scholar]
- 6.Briceño G, Fuentes MS, Palma G, Jorquera MA, Amoroso MJ, Diez MC. Chlorpyrifos biodegradation and 3,5,6-trichloro-2-pyridinol production by actinobacteria isolated from soil. International Biodeterioration & Biodegradation. 2012;73:1–7. [Google Scholar]
- 7.Armbrust KL. Chlorothalonil and chlorpyrifos degradation products in golf course leachate. Pest Management Science. 2001;57(9):797–802. doi: 10.1002/ps.361. [DOI] [PubMed] [Google Scholar]
- 8.Cáceres T, He W, Naidu R, Megharaj M. Toxicity of chlorpyrifos and TCP alone and in combination to Daphnia carinata: the influence of microbial degradation in natural water. Water Research. 2007;41(19):4497–4503. doi: 10.1016/j.watres.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Diez MC. Biological aspects involves in the degradation of organic pollutants. Journal of Soil Science and Plant Nutrition. 2010;10:244–267. [Google Scholar]
- 10.Vallecillo A, Garcia-Encina PA, Peña M. Anaerobic biodegradability and toxicity of chlorophenols. Water Science and Technology. 1999;40(8):161–168. [Google Scholar]
- 11.Kaoa CM, Chaib CT, Liub JK, Yehc TY, Chena KF, Chend SC. Evaluation of natural and enhanced PCP biodegradation at a former pesticide manufacturing plant. Water Research. 2004;38:663–672. doi: 10.1016/j.watres.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Yang C-F, Lee C-M, Wang C-C. Isolation and physiological characterization of the pentachlorophenol degrading bacterium Sphingomonas chlorophenolica . Chemosphere. 2006;62(5):709–714. doi: 10.1016/j.chemosphere.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Remes A, Pop A, Manea F, Baciu A, Picken SJ, Schoonman J. Electrochemical determination of pentachlorophenol in water on a multi-wall carbon nanotubes-epoxy composite electrode. Sensors. 2012;12:7033–7046. doi: 10.3390/s120607033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copley SD. Evolution of a metabolic pathway for degradation of a toxic xenobiotic: the patchwork approach. Trends in Biochemical Sciences. 2000;25(6):261–265. doi: 10.1016/s0968-0004(00)01562-0. [DOI] [PubMed] [Google Scholar]
- 15.Muhamad SG. Kinetic studies of catalytic photodegradation of chlorpyrifos insecticide in various natural waters. Arabian Journal of Chemistry. 2010;3(2):127–133. [Google Scholar]
- 16.B. Carvalho M, Tavares S, Medeiros J, et al. Degradation pathway of pentachlorophenol by Mucor plumbeus involves phase II conjugation and oxidation-reduction reactions. Journal of Hazardous Materials. 2011;198:133–142. doi: 10.1016/j.jhazmat.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Chen S, Hu M, Hu Q, Luo J, Li Y. Purification and characterization of a novel chlorpyrifos hydrolase from Cladosporium cladosporioides Hu-01. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038137.e38137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racke KD, Laskowski DA, Schultz MR. Resistance of chlorpyrifos to enhanced biodegradation in soil. Journal of Agricultural and Food Chemistry. 1990;38(6):1430–1436. [Google Scholar]
- 19.Singh BK, Walker A. Microbial degradation of organophosphorus compounds. FEMS Microbiology Reviews. 2006;30(3):428–471. doi: 10.1111/j.1574-6976.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Zhao Y, Qiu J. Isolation and application of a chlorpyrifos-degrading Bacillus licheniformis ZHU-1. African Journal of Microbiology Research. 2010;4(22):2410–2413. [Google Scholar]
- 21.Kulshrestha G, Kumari A. Fungal degradation of chlorpyrifos by Acremonium sp. strain (GFRC-1) isolated from a laboratory-enriched red agricultural soil. Biology and Fertility of Soils. 2011;47(2):219–225. [Google Scholar]
- 22.Liu Z, Chen X, Shi Y, Su ZC. Bacterial degradation of chlorpyrifos by Bacillus cereus . Advanced Materials Research. 2012;356–360:676–680. [Google Scholar]
- 23.Edgehill RU. Pentachlorophenol removal from slightly acidic mineral salts, commercial sand, and clay soil by recovered Arthrobacter strain ATCC 33790. Applied Microbiology and Biotechnology. 1994;41(1):142–148. [Google Scholar]
- 24.Thakur IS, Verma P, Upadhayaya K. Molecular cloning and characterization of pentachlorophenol-degrading monooxygenase genes of Pseudomonas sp. from the chemostat. Biochemical and Biophysical Research Communications. 2002;290(2):770–774. doi: 10.1006/bbrc.2001.6239. [DOI] [PubMed] [Google Scholar]
- 25.Dams RI, Paton GI, Killham K. Rhizoremediation of pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Chemosphere. 2007;68(5):864–870. doi: 10.1016/j.chemosphere.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Chandra R, Patel DK, Rai V. Isolation and characterization of novel Serratia marcescens (AY927692) for pentachlorophenol degradation from pulp and paper mill waste. World Journal of Microbiology and Biotechnology. 2007;23(12):1747–1754. doi: 10.1007/s11274-007-9424-5. [DOI] [PubMed] [Google Scholar]
- 27.Fuentes MS, Benimeli CS, Cuozzo SA, Amoroso MJ. Isolation of pesticide-degrading actinomycetes from a contaminated site: bacterial growth, removal and dechlorination of organochlorine pesticides. International Biodeterioration and Biodegradation. 2010;64(6):434–441. [Google Scholar]
- 28.Karn SK, Chakrabarti SK, Reddy MS. Degradation of pentachlorophenol by Kocuria sp. CL2 isolated from secondary sludge of pulp and paper mill. Biodegradation. 2011;22(1):63–69. doi: 10.1007/s10532-010-9376-6. [DOI] [PubMed] [Google Scholar]
- 29.Castillo MA, Felis N, Aragón P, Cuesta G, Sabater C. Biodegradation of the herbicide diuron by streptomycetes isolated from soil. International Biodeterioration and Biodegradation. 2006;58(3-4):196–202. [Google Scholar]
- 30.Smith D, Alvey S, Crowley DE. Cooperative catabolic pathways within an atrazine-degrading enrichment culture isolated from soil. FEMS Microbiology Ecology. 2005;53(2):265–273. doi: 10.1016/j.femsec.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Benimeli CS, Amoroso MJ, Chaile AP, Castro GR. Isolation of four aquatic streptomycetes strains capable of growth on organochlorine pesticides. Bioresource Technology. 2003;89(2):133–138. doi: 10.1016/s0960-8524(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 32.Fuentes MS, Sáez JM, Benimeli CS, Amoroso MJ. Lindane biodegradation by defined consortia of indigenous Streptomyces strains. Water, Air, and Soil Pollution. 2011;222(1–4):217–231. [Google Scholar]
- 33.Hopwood DA. Genetic analysis and genome structure in Streptomyces coelicolor . Bacteriological reviews. 1967;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell DK, Wells HD, Markham CR. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology. 1980;72:379–382. [Google Scholar]
- 35.Amoroso MJ, Castro GR, Carlino FJ, Romero NC, Hill RT, Oliver G. Screening of heavy metal-tolerant actinomycetes isolated from the Sali River. Journal of General and Applied Microbiology. 1998;44(2):129–132. doi: 10.2323/jgam.44.129. [DOI] [PubMed] [Google Scholar]
- 36.Saez JM, Benimeli CS, Amoroso MJ. Lindane removal by pure and mixed cultures of immobilized actinobacteria. Chemosphere. 2012;89:982–987. doi: 10.1016/j.chemosphere.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 37.Pattanapipitpaisal P, Brown NL, Macaskie LE. Chromate reduction by Microbacterium liquefaciens immobilised in polyvinyl alcohol. Biotechnology Letters. 2001;23(1):61–65. [Google Scholar]
- 38.Rubilar O, Tortella GR, Cuevas R, Cea M, Rodríguez-Couto S, Diez MC. Adsorptive removal of pentachlorophenol by anthracophyllum discolor in a fixed-bed column reactor. Water, Air and Soil Pollution. 2012;223(5):2463–2472. [Google Scholar]
- 39.Odjadjare EEO, Ajisebutu SO, Igbinosa EO, Aiyegoro OA, Trejo-Hernandez MR, Okoh AI. Escravos light crude oil degrading potentials of axenic and mixed bacterial cultures. Journal of General and Applied Microbiology. 2008;54(5):277–284. doi: 10.2323/jgam.54.277. [DOI] [PubMed] [Google Scholar]
- 40.Thouand G, Bauda P, Oudot J, Kirsch G, Sutton C, Vidalie JF. Laboratory evaluation of crude oil biodegradation with commercial or natural microbial inocula. Canadian Journal of Microbiology. 1999;45(2):106–115. [PubMed] [Google Scholar]
- 41.van Hamme JD, Singh A, Ward OP. Recent advances in petroleum microbiology. Microbiology and Molecular Biology Reviews B. 2003;67(4):503–549. doi: 10.1128/MMBR.67.4.503-549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaborina O, Baskunov B, Baryshnikova L, Golovleva L. Degradation of pentachlorophenol in soil by Streptomyces rochei 303. Journal of Environmental Science and Health. 1997;32(1):55–70. doi: 10.1080/03601239709373076. [DOI] [PubMed] [Google Scholar]
- 43.Sasikala CH, Jiwal S, Rout P, Ramya M. Biodegradation of chlorpyrifos by bacterial consortium isolated from agriculture soil. World Journal of Microbiology and Biotechnology. 2012;28(3):1301–1308. doi: 10.1007/s11274-011-0879-z. [DOI] [PubMed] [Google Scholar]
- 44.Polti MA, Amoroso MJ, Abate CM. Chromium(VI) resistance and removal by actinomycete strains isolated from sediments. Chemosphere. 2007;67(4):660–667. doi: 10.1016/j.chemosphere.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Fuentes MS, Alvarez A, Saez JM, Benimeli CS, Amoroso MJ. Methoxychlor bioremediation by defined consortium of environmental Streptomyces strains. International Journal of Environmental Science and Technology. 2013 [Google Scholar]
- 46.Yang C, Li Y, Zhang K, et al. Atrazine degradation by a simple consortium of Klebsiella sp. A1 and Comamonas sp. A2 in nitrogen enriched medium. Biodegradation. 2010;21(1):97–105. doi: 10.1007/s10532-009-9284-9. [DOI] [PubMed] [Google Scholar]
- 47.Sørensen SR, Ronen Z, Aamand J. Growth in coculture stimulates metabolism of the phenylurea herbicide isoproturon by Sphingomonas sp. strain SRS2. Applied and Environmental Microbiology. 2002;68(7):3478–3485. doi: 10.1128/AEM.68.7.3478-3485.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sørensen SR, Albers CN, Aamand J. Rapid mineralization of the phenylurea herbicide diuron by Variovorax sp. strain SRS16 in pure culture and within a two-member consortium. Applied and Environmental Microbiology. 2008;74(8):2332–2340. doi: 10.1128/AEM.02687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buono S, Manzo S, Maria G, Sansone G. Toxic effects of pentachlorophenol, azinphos-methyl and chlorpyrifos on the development of Paracentrotus lividus embryos. Ecotoxicology. 2012;21(3):688–697. doi: 10.1007/s10646-011-0827-6. [DOI] [PubMed] [Google Scholar]
- 50.Matamoros V, Puigagut J, García J, Bayona JM. Behavior of selected priority organic pollutants in horizontal subsurface flow constructed wetlands: a preliminary screening. Chemosphere. 2007;69(9):1374–1380. doi: 10.1016/j.chemosphere.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Prakash D, Chauhan A, Jain RK. Plasmid-encoded degradation of p-nitrophenol by Pseudomonas cepacia . Biochemical and Biophysical Research Communications. 1996;224(2):375–381. doi: 10.1006/bbrc.1996.1036. [DOI] [PubMed] [Google Scholar]
- 52.Yañez-Ocampo G, Sanchez-Salinas E, Jimenez-Tobon GA, Penninckx M, Ortiz-Hernández ML. Removal of two organophosphate pesticides by a bacterial consortium immobilized in alginate or tezontle. Journal of Hazardous Materials. 2009;168(2-3):1554–1561. doi: 10.1016/j.jhazmat.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 53.Wu J-Y, Chen K-C, Chen C-T, Hwang S-CJ. Hydrodynamic characteristics of immobilized cell beads in a liquid-solid fluidized-bed bioreactor. Biotechnology and Bioengineering. 2003;83(5):583–594. doi: 10.1002/bit.10710. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee I, Modak JM, Bandopadhyay K, Das D, Maiti BR. Mathematical model for evaluation of mass transfer limitations in phenol biodegradation by immobilized Pseudomonas putida . Journal of Biotechnology. 2001;87(3):211–223. doi: 10.1016/s0168-1656(01)00235-8. [DOI] [PubMed] [Google Scholar]
- 55.Niazi JH, Karegoudar TB. Degradation of dimethylphthalate by cells of Bacillus sp. immobilized in calcium alginate and polyurethane foam. Journal of Environmental Science and Health A. 2001;36(6):1135–1144. doi: 10.1081/ese-100104137. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L, Li G, An T, Fu J, Sheng G. Recent patents on immobilized microoganism technology and its engineering application in wastewater treatment. Recent Patents on Engineering. 2008;2(1):28–35. [Google Scholar]
- 57.Pannier A, Mkandawire M, Soltmann U, Pompe W, Böttcher H. Biological activity and mechanical stability of sol-gel-based biofilters using the freeze-gelation technique for immobilization of Rhodococcus ruber. Applied Microbiology and Biotechnology. 2012;93(4):1755–1767. doi: 10.1007/s00253-011-3489-7. [DOI] [PubMed] [Google Scholar]
- 58.Ahamad PYA, Kunhi AAM. Enhanced degradation of phenol by Pseudomonas sp. CP4 entrapped in agar and calcium alginate beads in batch and continuous processes. Biodegradation. 2011;22(2):253–265. doi: 10.1007/s10532-010-9392-6. [DOI] [PubMed] [Google Scholar]
- 59.Wang B-E, Hu Y-Y. Comparison of four supports for adsorption of reactive dyes by immobilized Aspergillus fumigatus beads. Journal of Environmental Sciences. 2007;19(4):451–457. doi: 10.1016/s1001-0742(07)60075-8. [DOI] [PubMed] [Google Scholar]


