Abstract
Purpose
To investigate and integrate anatomic and physiologic findings from a group of patients who present retinitis pigmentosa affecting just one eye and use this information to propose mechanisms of disease pathogenesis.
Methods
This prospective cross-sectional study examined 5 patients, all female, from 8 to 60 years old. The study was conducted in 4 university hospitals. The patients were selected according to the characteristics of ocular involvement, notably unilateral presentation of similar anatomic and functional abnormalities. Full-field electroretinogram, fundus photography, fundus autofluorescence, infrared imaging, optical coherence tomography, and genetic testing were performed.
Results
Full-field electroretinogram showed unilateral decrease in amplitude and increase in implicit time; autofluorescence showed unilateral areas of decreased intensity. The USH2AW4149R mutation was confirmed in one patient.
Conclusions
Imaging and functional testing are important in elucidating the unilateral pattern of the disease and in monitoring these individuals. Mosaicism or somatic mutation may cause unilateral genetic disease presentation.
Keywords: Electroretinogram, Full-field electroretinography, Fundus autofluorescence, Retina degeneration, Retinitis pigmentosa, Unilateral retinitis pigmentosa
Introduction
Retinitis pigmentosa (RP) differential diagnosis is important because of the prognostic implications and psychological impact of the disease and of the failure to recognize a treatable entity.
According to Sharma et al, different etiologic entities with fundus appearance resembling RP are called pseudoretinitis pigmentosa (Retinitis pigmentosa and allied disorders; available at: http://www.jkscience.org/archive/volume63/retini.pdf). Non-hereditary phenodeviants and other inherited disorders that closely mimic known RP mutant phenotypes are called phenocopies. They often appear in response to a trigger such as trauma or inflammation, but vary with multiple factors including hereditary constitution, duration of exposure to the trigger, and developmental stage at time of exposure (1).
Uniocular pigmentary retinopathy is usually caused by either inflammation or trauma. Differential diagnoses of uniocular pigmentary retinopathy and other RP phenocopies are required to ensure an accurate diagnosis. Also according to Sharma et al, a careful history is important and the electroretinogram (ERG) is the single most important diagnostic test.
The purpose of this study was to investigate and integrate anatomic and physiologic findings from a group of patients that present pigmentary retinopathy resembling RP in just one eye and to use this information to propose mechanisms of disease pathogenesis. A mutation associated with RP has been identified among the subjects studied. We propose that either of 2 different genetic mechanisms may cause the unilateral presentation of the disease: mosaicism or somatic mutation.
Methods
A prospective cross-sectional study was performed.
Subjects
Clinical records of 5 women who ranged in age from 8 to 60 years were examined. They were from unrelated families and presented unilateral retinal pigmentary abnormalities. Exclusion criteria were the presence of other ocular diseases affecting the retina and optic nerve (e.g., glaucoma, diabetes). Patients were enrolled with the approval of the Institutional Review Board at each institution. All research procedures were performed in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all study subjects before their enrollment and Health Insurance Portability and Accountability Act compliance was maintained.
The 5 women were studied with full medical history, best-corrected visual acuity, and dilated fundus examination performed by at least one retina consultant. In addition, all patients received fundus autofluorescence (FAF), spectral domain optical coherence tomography (SD-OCT), infrared imaging (IR), and standardized full-field scotopic and photopic electroretinogram (ERG). Blood samples were obtained for genetic screening. Fluorescein angiography and B-scan were performed in selected cases.
Fundus autofluorescence and infrared imaging
Fundus autofluorescence and IR imaging were performed with a confocal scanning laser ophthalmoscope (SLO, Heidelberg Retina Angiograph 2; Heidelberg Engineering, Heidelberg, Germany) after pupil dilation. Both imaging modalities were performed using a 30° ′ 30° field of view at a resolution of 1536 ′ 1536 pixels. Fundus autofluorescence images were obtained with an optically pumped solid-state laser with an excitation wavelength of 488 nm and a barrier filter of 495 nm used to modulate the blue argon excitation light. Standard procedure was followed for the acquisition of FAF images, including focus of the retinal image in the infrared reflection mode with an excitation wavelength of 820 nm, further focus and sensitivity adjustment at 488 nm, and acquisition of at least 9 single 30° ′ 30° FAF images encompassing the entire macular area with at least a portion of the optic disc. The 9 single images were computationally averaged to produce a single frame with improved signal-to-noise ratio.
Spectral domain optical coherence tomography
Spectral domain OCT was performed with the OCT-SLO Spectralis (Heidelberg Engineering) in all the studied patients, except in case 3. This equipment allows for simultaneous OCT scans and FAF imaging and subsequent image superimposition. The simultaneous acquisition of OCT and FAF images facilitates point-to-point correlation between the en face and cross-sectional images. The OCT imaging was acquired by a broadband 870 nm superluminescent diode that scanned the retina at 40,000 A-scans per second with an optical axial depth resolution of 7 μm. The standard protocol included at least 25 OCT scans averaged to reduce signal-to-noise ratio by a factor of 5. The scans included at least one 9-mm horizontal line scan through the fovea.
In patient 3, SD-OCT images were obtained using the Cirrus Spectral Domain OCT (Carl Zeiss Meditec Inc., Dublin, California, USA). The acquisition protocol consisted of a 5-line raster scan and a macular cube 512 ′ 128 scan pattern in which a 6 ′ 6 mm region of the retina was scanned (a total of 65,536 sampled points) within a scan time of 2.4 seconds. After image acquisition, those with a signal strength ≤8 were excluded. Horizontal line scans through the center of the foveal region were repeated 3 times and images were registered.
Fluorescein angiography
Fluorescein angiography was performed following the usual procedure in ophthalmology.
B-scan
B-scan was performed with the Innovative Imaging B-scan (Innovative Imaging, Sacramento, California, USA).
Electroretinogram
Ganzfeld full-field ERGs (Diagnosys LLC, Lowell, Massachusetts, USA) were recorded from both eyes with DTL electrodes according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standard in both scotopic and photopic states to assess retinal function. The amplitudes and implicit times obtained from both eyes of each patient were compared to age-matched normal values, in which lower limits were calculated as 2 standard deviations below the mean.
Genetic analysis
All patients were screened for genetic mutations. DNA was extracted from whole blood with the QIAamp DNA Blood Maxi Kit 51194 (Qiagen Inc., Valencia, California, USA). RPar genotyping microarray (Asper Ophthalmics, Tartu, Estonia) was used to screen for 585 mutations in CERKL, CNGA1, CNGB1, MERTK, PDE6A, PDE6B, PNR, RDH12, RGR, RLBP1, SAG, TULP1, CRB, RPE65, USH2A, USH3A, LRAT, and PROML1 genes; RPad genotyping microarray (Asper Ophthalmics) was used to screen 370 mutations in CA4, FSCN2, IMPDH1, NRL, PRPF3, PRPF31, PRPF8, RDS, RHO, ROM1, RP1, RP9, CRX, TOPORS, and PNR genes; early onset retinal dystrophy array was used to screen for 495 disease-associated sequences in AIPL1, CRB1, CRX, GUCY2D, LRAT, TULP1, MERTK, CEP290, RDH12, RP-GRIP1, LCA5, and RPE65 genes. Usher array and Bardet Biedl array (Asper Ophthalmics) were also used.
Results
The 5 patients showed signs of asymmetric retinal degeneration consistent with RP. None had family history of poor vision or night blindness and no other systems or tissues were affected. X-linked disorder was further discussed, but no men affected by RP were known in the studied families and this diagnosis could be ruled out. Clinical history excluded traumatic etiology. Complementary testing excluded inflammatory and infectious etiologies.
Medical history
Patient 1 is a 9-year-old girl who presented difficulties seeing with her right eye, which was previously diagnosed with amblyopia. Her visual acuity was 20/80 OD and 20/20 OS. Patient 2 is an 8-year-old girl with decreased vision and difficulties seeing at night with her right eye. Visual acuity was 20/20 in both eyes.
Patient 3 is a 25-year-old woman who presented with decreased vision in her left eye. She had medical history of papillitis. Visual acuity was 20/20 OD and 20/80 OS. Patient 4 is a 53-year-old woman who presented nyctalopia of the right eye and developed cataract and cystoid macular edema (CME). She had been prescribed dorzol-amide drops for her macular edema. Visual acuity was 20/50 OD and 20/20 OS.
Patient 5 is a 60-year-old woman with visual impairment in the left eye first noted at age 7 when glasses were prescribed. Her best-corrected visual acuity at the time of the study was 20/20 OD and 20/125 OS.
Each case presented unilateral abnormalities with normal fellow eye.
Fundus examination
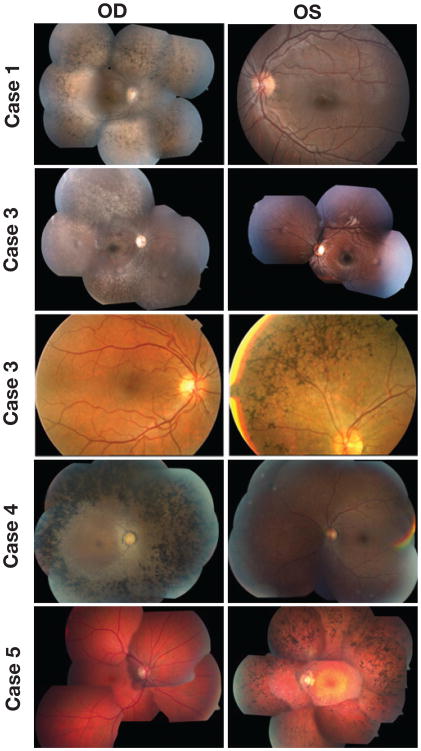
Fundus examination revealed intraretinal bone spicule pigmentation, attenuated retinal vessels, and loss of retinal pigment epithelium (Fig. 1), as well as swelling at the optic disc area of patient 1 (Fig. 1, case 1).
Fig. 1.
Color fundus photographs of both eyes of each patient showing typical retinitis pigmentosa (RP) abnormalities, including attenuated retinal vessels, intraretinal clumps of black pigment, and loss of retinal pigment epithelium in the affected eye of each patient; the fellow eye of each case presents a normal aspect. Color fundus images show typical RP changes and swelling at the optic disc area of the right eye of patient 1; affected right eye of patients 2 and 4 and typical RP abnormalities in the left eye of patients 3 and 5.
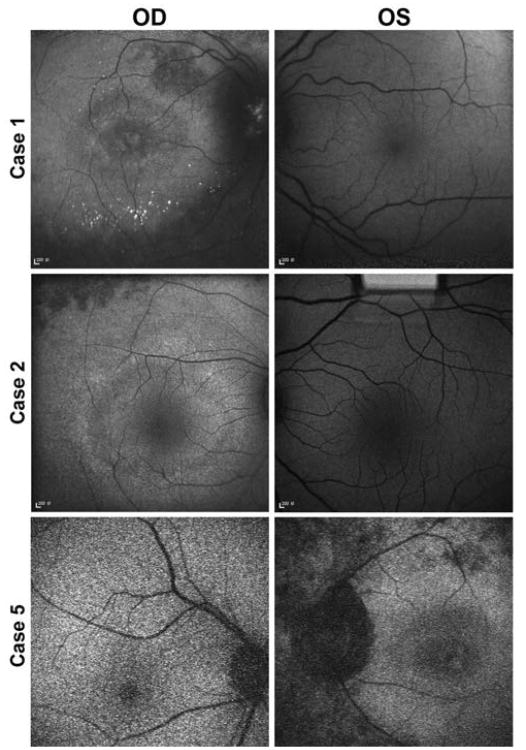
Autofluorescence
Autofluorescence showed areas of decreased intensity corresponding to cell death, a hyperautofluorescent ring in the macular region (Fig. 2), and calcifications at the posterior pole and at the optic disc in case 1 (Fig. 2A).
Fig. 2.
Fundus autofluorescence showing bull's eye maculopathy and hypoautofluorescent areas corresponding to loss of retinal pigment epithelium due to cell death as a consequence of photoreceptor degeneration in the affected eye of each patient (right eye of cases 1 and 2 and left eye of case 5). Calcifications at the posterior pole and at the optic disc of case 1 are observed. The fellow eye has a normal autofluorescent aspect in all the cases.
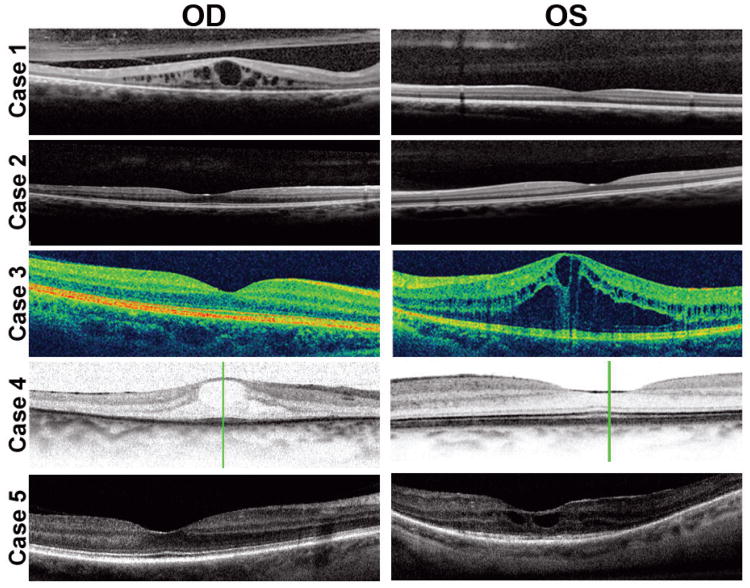
High-resolution OCT
Spectral domain OCT showed CME in all the cases, except patient 2 (Fig. 3).
Fig. 3.
Optical coherence tomography (OCT) showing cystoid macular edema in the affected eye of cases 1, 3, 4, and 5. The fellow eye of these patients presents normal OCT aspect, as do both eyes of case 2.
Fluorescein angiography
Fluorescein angiography confirmed CME in cases 1 (Fig. 4A) and 3 (Fig. 4C) and showed leakage at the optic disc in case 1 (Fig. 4A).
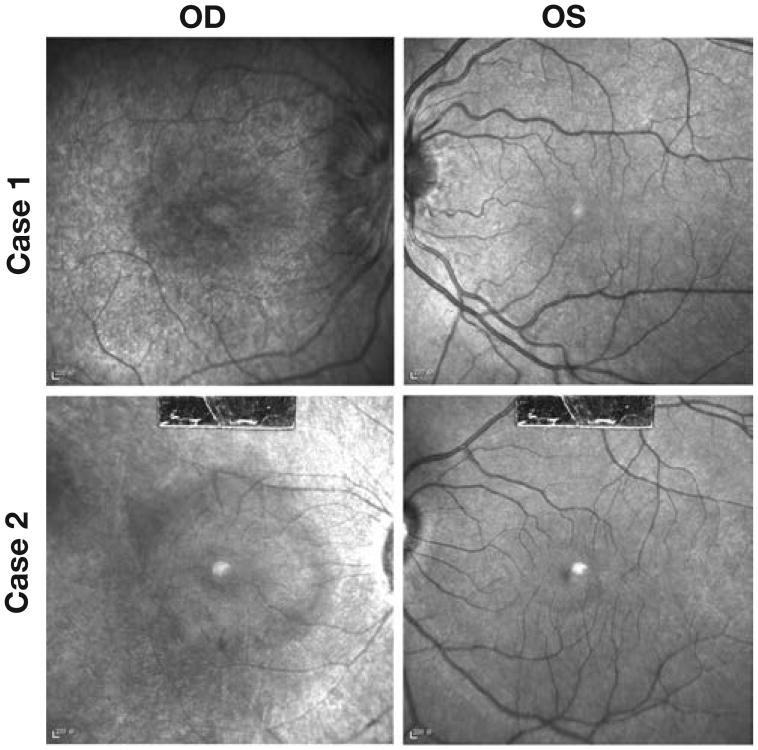
Fig. 4.
Infrared imaging shows bull's eye maculopathy in the affected eye of cases 1 and 2 (OD). The fellow eye presents a normal aspect in each patient (OS).
Infrared imaging
Infrared imaging revealed bull's eye maculopathy aspect in case 1 (Fig. 4D) and case 2 (Fig. 4F).
B-scan
B-scan was performed in patient 1 and revealed optic nerve drusen.
Standardized full-field electroretinogram
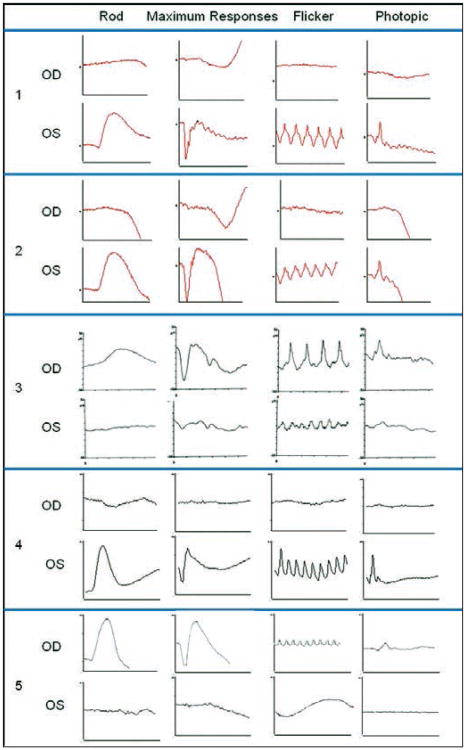
Electroretinogram showed decreased amplitude and increased implicit time in each case (Fig. 5).
Fig. 5.
International Society for Clinical Electrophysiology of Vision standardized full-field electroretinogram (ERG) was performed in both eyes of each case. Rod-specific, maximum scotopic, photopic 30-Hz flicker, and transient photopic responses were determined in each patient. A decrease in the amplitude and an increase in the implicit time are observed in the affected eye of each case: right eye (OD) of patients 1, 2, and 4, and left eye (OS) of patients 3 and 5. A normal ERG tracing was found in the fellow eye of each patient. Scale used for cases 1 and 2 is 50 milliseconds and 50 microvolts. In case 3, scale is 25 milliseconds and 25 microvolts for rod-specific response and 50 microvolts for the other determinations. In case 4, scale is 100 milliseconds and 100 microvolts for maximum response and 50 microvolts for the other determinations. In case 5, scale is 20 milliseconds and 100 microvolts for rod scotopic ERG and maximum response, 25 milliseconds and 250 microvolts for 30-Hz flicker, and 20 milliseconds and 250 microvolts for photopic ERG.
Genetic testing
Genetic testing was performed in all patients, except in case 3; patient 2 presented the USH2AW4149R mutation and we are awaiting the results in other patients tested.
Serology
Serology was performed in all cases for every possible infection affecting the retina.
Patient 1 was prescribed acetazolamide. Four weeks later, OD presented visual acuity of 20/60, no optic nerve head swelling, and decreased macular edema compared to the previous OCT.
Discussion
Before establishing the RP diagnosis, it is essential to rule out other possible etiologies such as trauma, infection, inflammation, and cancer in every patient with pigmentary retinopathy that demonstrates disparity between both eyes. None of our cases presented traumatic history of the head or eye. Workup ruled out infectious diseases that can affect the retina, such as Lyme disease, bartonellosis, toxocariasis, toxoplasmosis, and viral infections. Inflammatory diseases were ruled out by clinical testing and epidemiologic data (2). Cystoid macular edema is not unusual in RP and improves with carbonic anhydrase inhibitors, as in our cases (3-7); CME due to intraocular inflammation does not typically improve with this treatment. Acute zonal occult outer retinopathy has not been reported in children and presents autofluorescence abnormalities at the peripapillary region which were not found in our cases (8). The reduced ERG in these cases ruled out mitochondrial retinal diseases and viral papillitis. Disc swelling can be seen in RP (3, 4, 9). Cancer-associated retinopathy does not present a similar fundus appearance to that of RP.
Although family history and genetic confirmation are not required for RP diagnosis and high-throughput screening capable of detecting mutations in RP is still in development (10-12), the USH2AW4149R mutation has been confirmed in one case. Mutations in USH2A are the most common cause of simplex RP (13-16), but typically present bilaterally.
These cases highlight the importance of combining imaging and functional testing to elucidate the unilateral pattern of disease and to monitor photoreceptor degeneration (17-22). Genetic diseases can affect just one eye, as happens in some cases of retinoblastoma. Since the 1940s, many unilateral RP cases have been reported (23-26), but mutation confirmation was only recently reported (27). We have combined imaging and functional testing to confirm the dysfunctions in the affected eye and the normality in the fellow eye in all the patients and in one case we obtained the USH2AW4149R mutation confirmation. Even with clinically and genetically clear indications of RP affecting just one eye, there remains controversy in the scientific community regarding this diagnosis. As such, we would like to propose 2 genetic mechanisms that could explain the unusual unilateral presentation of RP: mosaicism and somatic mutations. Both mechanisms may cause asymmetric and partial involvement of a genetic disease throughout the body.
In conclusion, imaging and functional testing are important in elucidating the unilateral pattern of the disease and in monitoring these individuals, and mosaicism or somatic mutations may cause unusual, unilateral genetic disease presentation.
Acknowledgments
The authors thank their fellow ophthalmologists for sharing their opinion on these cases and the members of the retinal research unit at the Edward S. Harkness Eye Institute for support.
Supported by NIH grant R01EY018213, DOD-W81XWH-09-1-0575, and the Foundation Fighting Blindness. S.H.T. is a Fellow of the Burroughs-Wellcome Program in Biomedical Sciences, and has been supported by the Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness Award and Foundation Fighting Blindness, Dennis W. Jahnigen Award of the American Geriatrics Society, Crowley Family Fund, Joel Hoffman Fund, Gale and Richard Siegel Stem Cell Fund, Charles Culpeper Scholarship, Schneeweiss Stem Cell Fund, Irma T. Hirschl Charitable Trust, Bernard and Anne Spitzer Stem Cell Fund, Barbara & Donald Jonas Family Fund, Eye Surgery Fund, and Professor Gertrude Rothschild Stem Cell Foundation.
Footnotes
Proprietary interest: None.
Copyright of European Journal of Ophthalmology is the property of Wichtig Editore and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
References
- 1.Landauer W. Phenocopies and genotype, with special reference to sporadically-occurring developmental variants. Am Nat. 1957;91:79–90. [Google Scholar]
- 2.Charbel Issa P, Scholl HP, Helb HM, Fleckenstein M, Inhetvin-Hutter C, Holz FG. Unilateral pigmented paravenous retinochoroidal atrophy. Klin Monatsbl Augenheilkd. 2007;224:791–3. doi: 10.1055/s-2007-963600. [DOI] [PubMed] [Google Scholar]
- 3.Ozdek S, Ozdogan S, Sezgin T, Gurelik G. Bilateral disc edema and unilateral macular hole in a patient with retinitis pigmentosa. Eur J Ophthalmol. 2006;16:487–90. doi: 10.1177/112067210601600324. [DOI] [PubMed] [Google Scholar]
- 4.Vinores SA, Küchle M, Derevjanik NL, et al. Blood-retinal barrier breakdown in retinitis pigmentosa: light and electron microscopic immunolocalization. Histol Histopathol. 1995;10:913–23. [PubMed] [Google Scholar]
- 5.Küchle M, Nguyen NX, Martus P, Freissler K, Schalnus R. Aqueous flare in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 1998;236:426–33. doi: 10.1007/s004170050101. [DOI] [PubMed] [Google Scholar]
- 6.Thobani A, Fishman GA. The use of carbonic anhydrase inhibitors in the retreatment of cystic macular lesions in retinitis pigmentosa and X-linked retinoschisis. Retina. 2011;31:312–5. doi: 10.1097/IAE.0b013e3181e587f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genead MA, Fishman GA. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with retinitis pigmentosa and usher syndrome. Arch Ophthalmol. 2010;128:1146–50. doi: 10.1001/archophthalmol.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30:1206–16. doi: 10.1097/IAE.0b013e3181e097f0. [DOI] [PubMed] [Google Scholar]
- 9.Villa AM, Anderson SF, Abundo RE. Bilateral disc edema in retinitis pigmentosa. Optom Vis Sci. 1997;74:132–7. doi: 10.1097/00006324-199703000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Clark GR, Crowe P, Muszynska D, et al. Development of a diagnostic genetic test for simplex and autosomal recessive retinitis pigmentosa. Ophthalmology. 2010;117:2169–77. doi: 10.1016/j.ophtha.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Simpson DA, Clark GR, Alexander S, Silvestri G, Willoughby CE. Molecular diagnosis for heterogeneous genetic diseases with targeted high-throughput DNA sequencing applied to retinitis pigmentosa. J Med Genet. 2011;48:145–51. doi: 10.1136/jmg.2010.083568. [DOI] [PubMed] [Google Scholar]
- 12.Bowne SJ, Sullivan LS, Koboldt DC, et al. Identification of disease-causing mutations in autosomal dominant retinitis pigmentosa (adRP) using next-generation DNA sequencing. Invest Ophthalmol Vis Sci. 2011;52:494–503. doi: 10.1167/iovs.10-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 14.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125:151–8. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ávila-Fernández A, Cantalapiedra D, Aller E, et al. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol Vis. 2010;16:2550–8. [PMC free article] [PubMed] [Google Scholar]
- 16.McGee TL, Seyedahmadi BJ, Sweeney MO, Dryja TP, Berson EL. Novel mutations in the long isoform of the USH2A gene in patients with Usher syndrome type II or non-syndromic retinitis pigmentosa. J Med Genet. 2010;47:499–506. doi: 10.1136/jmg.2009.075143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakabayashi T, Sawa M, Gomi F, Tsujikawa M. Correlation of fundus autofluorescence with photoreceptor morphology and functional changes in eyes with retinitis pigmentosa. Acta Ophthalmol. 2010;88:e177–83. doi: 10.1111/j.1755-3768.2010.01926.x. [DOI] [PubMed] [Google Scholar]
- 18.Robson AG, Michaelides M, Saihan Z, et al. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence: a review and update. Doc Ophthalmol. 2008;116:79–89. doi: 10.1007/s10633-007-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robson AG, Saihan Z, Jenkins SA, et al. Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br J Ophthalmol. 2006;90:472–9. doi: 10.1136/bjo.2005.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robson AG, Tufail A, Fitzke F, et al. Serial imaging and structure-function correlates of high-density rings of fundus autofluorescence in retinitis pigmentosa. Retina. 2011;31:1670–9. doi: 10.1097/IAE.0b013e318206d155. [DOI] [PubMed] [Google Scholar]
- 21.Popović P, Jarc-Vidmar M, Hawlina M. Abnormal fundus autofluorescence in relation to retinal function in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2005;243:1018–27. doi: 10.1007/s00417-005-1186-x. [DOI] [PubMed] [Google Scholar]
- 22.Lupo S, Grenga PL, Vingolo EM. Fourier-domain optical coherence tomography and microperimetry findings in retinitis pigmentosa. Am J Ophthalmol. 2011;151:106–11. doi: 10.1016/j.ajo.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Dreisler KK. Unilateral retinitis pigmentosa: two cases. Acta Ophthalmol. 1948;26:385–93. doi: 10.1111/j.1755-3768.1948.tb07596.x. [DOI] [PubMed] [Google Scholar]
- 24.Gordon DM. Unilateral retinitis pigmentosa: report of a case. Am J Ophthalmol. 1949;32:1350–3. [PubMed] [Google Scholar]
- 25.Thakur A, Puri L. Unilateral retinitis pigmentosa. Clin Exp Optom. 2010;93:102–4. doi: 10.1111/j.1444-0938.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 26.Farrell DF. Unilateral retinitis pigmentosa and cone-rod dystrophy. Clin Ophthalmol. 2009;3:263–70. doi: 10.2147/opth.s5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay R, Holder GE, Moore AT, Webster AR. Unilateral retinitis pigmentosa occurring in an individual with a germline mutation in the RP1 gene. Arch Ophthalmol. 2011;129:954–6. doi: 10.1001/archophthalmol.2011.171. [DOI] [PubMed] [Google Scholar]