Abstract
Objective
α4 Integrins are major players in lymphoid cell trafficking and immune responses. However, their importance in lymphoid reconstitution and function, studied by antibody blockade or in genetic models of chimeric animals with α4KO embryonic stem (ES) cells, competitive repopulation experiments with fetal liverKO cells, or in β1/β7 doubly-deficient mice has yielded disparate conclusions.
Materials and Methods
To study the role of α4 integrin (α4β1, α4β7) during adult life, we transplanted lethally irradiated Rag2−/− mice with α4Δ/Δ or α4f/f adult bone marrow (BM) cells and evaluated recipients at several points after transplantation.
Results
Lymphomyeloid repopulation (8 months later) was entirely donor-derived in all recipients, and novel insights regarding lymphoid reconstitution and function were revealed. Thymic repopulation was impaired in all α4Δ/Δ recipients, likely because of homing defects of BM-derived progenitors, although a role of α4 integrin in intrathymic expansion/maturation of T cells cannot be excluded; reconstitution of gut lymphoid tissue was also greatly diminished because of homing defects of α4Δ/Δ cells; impaired immunoglobulin (Ig) M and IgE, but normal IgG responses were seen, suggesting compromised initial B-/T-cell interactions, whereas interferon-γ production from ovalbumin-stimulated cells was increased, possibly reflecting a bias against Th2 stimulation.
Conclusion
These data complement previous observations by defending the role of α4 integrin in thymic and gut lymphoid tissue homing, and by strengthening evidence of attenuated B-cell responses in α4-deficient mice.
Integrins are heterodimeric (α/β) cell-adhesion receptors with important roles in many physiologic or pathologic cell processes, including cell migration and lymphocyte trafficking during homeostasis, recruitment of leukocytes in inflammatory sites, or metastatic spread of leukemic or tumor cells [1,2]. Their effects are achieved not only through adhesion-dependent processes, but also bidirectional signaling (outside-in, inside-out) and cross-talk with other cell receptors (receptor tyrosine kinases or chemokine receptors) or signaling molecules, contributing to generation of a wide diversity of signals [3]. Among integrins, α4β1 (very–late antigen 4 [VLA4], CD49d/CD29), expressed in both hematopoietic and nonhematopoietic cells, and β2 (CD18) integrins, expressed only in hematopoietic cells, are major players in cell-migration events and lymphocyte trafficking. Circulating leukocytes are recruited to inflammatory tissues through interactions with tissue selective adhesion/migration cascades. α4β1 participates in all the classic three steps of the trafficking cascade (rolling/adhesion/migration), in contrast to many molecules participating only at specific steps in this cascade, and it can further augment cell migration by other integrins in a transdominant fashion [4,5].
Lymphocyte migration to secondary lymphoid tissues is necessary for maintaining immune defense and is regulated by multiple adhesion cascades controlled by shared participation of β1 and β2 integrins. Stromal cell networks in lymphoid tissues serve as guides directing or limiting the migration of T and B cells in and out of these tissues. Recently, there have been significant advances in defining the trafficking signals that control the movement of distinct subsets of immune cells in and out of specific tissue sites [6,7]. Thymus function and maintenance of its population relies on the continuous supply of bone marrow (BM)–derived lymphoid progenitors. Significant knowledge in dissecting the molecular cascades that dictate the thymic tropism of BM-derived progenitors, the characterization of progenitors endowed with thymic tropism, and the intra-thymic trafficking molecules responsible for intrathymic differentiation has recently been gained. Common lymphoid progenitors (CLP; Lin−/Sca1lo/IL-7Ra+]) can give rise to B, T, natural killer (NK), and dendritic cells, but these are not present in thymus and need to be differentiated further within BM before their emigration to thymus. In fact, it is the CLP-2 population (c-kit−/B220+/CD19−) that possesses thymic tropism and the latter is supported by both the lymphocyte function-associated antigen-1 (CD11a/CD18) and α4 integrin with the participation of GTP-binding protein coupled receptor engagement [7]. Within the thymus, in addition to lymphocyte function– associated antigen-1 and α4β1, α5β1, CD44, and the CCL9/CCR25 chemokine pathway are working in concert for migration of developing thymocytes [7,8], whereas other molecules (i.e., S1P/S1PR) control egress from thymus [9]. Thus, maintenance of thymic population in the adult is dependent on the coordination of several events, i.e., generation of BM-derived progenitors endowed with thymic tropism; their proper emigration and homing to thymus; and their appropriate intrathymic development. Defects along any of these steps significantly impair thymic population.
Genetic studies with α4 null chimeric mice have uncovered an important role of α4β1 integrin in lymphopoiesis and myelopoiesis with a severe defect in B-cell differentiation [10]. In addition, emigration of T-cell precursors from BM and their homing to thymus was inhibited in these mice, corroborating a large body of antibody blockade studies against α4 integrins or vascular cell-adhesion molecule (VCAM)-1 [11]. However, recent competitive transplantation experiments using fetal α4 null or α4+/+ cells with adult BM α+/+ competitor cells, concluded that thymic repopulation was normal, but impairment in Peyer's Patches (PPs) re-constitution was seen like in the chimeras [12]. Further, in contrast to the evidence from α4 null chimeric mice, β1 integrin–induced deletion in adult hematopoietic cells proved not to be essential for hematopoiesis/lymphopoiesis and for lymphocyte trafficking with normal homing to lymph nodes and PPs [13]. Only a transient defect in thymic colonization and/or intrathymic differentiation of T cells was seen in these mice, but an impairment in immunoglobulin M (IgM) response was present [13]. Thus, there are great discrepancies in data obtained in different genetic models of α4 integrin deficiency, or in different experimental settings used, and data with antibody blockade have not been predictive of results obtained in models of genetic deficiencies.
We have previously described adult mice with conditional ablation of α4 integrin and studied its effects on hematopoiesis [14]. No gross hematopoietic defects were seen in our model, but a sustained alteration in biodistribution of progenitor and stem cells at homeostasis was seen and α4-ablated cells had a competitive disadvantage in long-term hematopoietic repopulation [15]. However, no detailed studies on lymphopoiesis and lymphoid cell function were previously done in these mice. In the present studies, we transplanted α4-ablated BM cells from adult mice into lethally irradiated Rag2 null mice and made detailed observations in lymphoid organ repopulation and lymphocyte function in fully donor-reconstituted recipients. We have uncovered distinct defects in thymus reconstitution, in homing to gut lymphoid tissue, and in IgM-mediated responses. Similarities and differences with previously used models are discussed, with an attempt to reconcile divergence in outcomes and further our understanding of the role of α4 integrin in adult lymphopoiesis.
Materials and methods
Mice
Generation of α4f/f and MxCre+α4f/f mice [14], or Tie2Cre+α4Δ/Δ mice were previously described [16], Rag 2−/− (CD45.1) mice were from Taconic (Germantown, NY, USA). For transplantation experiments using these mice, single cell suspensions of 5 × 106 donor BM cells in prewarmed Hanks Balanced Salt Solution were injected via tail veins into each of lethally irradiated (800 cGy) Rag2−/− recipients (n = 10/group). Recipient animals were studied 8 to 10 weeks and up to 8 months posttransplantation.
Antibodies and fluorescein-activated cell-sorting (FACS) evaluation
Nucleated cells from donor or recipient mice were analyzed using CellQuest software on a FACSCalibur (BD Immunocytometry Systems, San Jose, CA, USA). Antibodies and their clone numbers included CD3 (145-2C11), CD8 (53-6.7), CD25 (7D4), CD44 (KM114), CD28 (37.51), B220 (RA3-6B2), Gr-1 (RB6-8C5), and α4β7 (DATK32) purchased from BD Biosciences (San Diego, CA, USA); anti-α4 integrin (PS/2 from Southern Biotech, Birmingham, AL, USA), CD19 (ID3) from Serotec Ltd. (Raleigh, NC, USA), CD34 (RAM34) from Caltag/Invitrogen (Carlsbad, CA, USA) and CD62L (MEL-14) in addition to Mouse Regulatory T Cell Staining Kit (w/PE Foxp3 FJK-16s, fluorescein isothiocyanate CD4, allophycocyanin CD25) from eBioscience, San Diego, CA, USA). Irrelevant isotype-matched antibodies (BD Biosciences) were used as controls.
Preparation of tissues for cellularity and FACS evaluation
BM, spleen, thymus, PPs, mesenteric lymph nodes (MLNs), cervical lymph nodes, axillary lymph nodes and inguinal lymph nodes were used for studies. The lymph nodes (LN) from the anatomical locations mentioned here were surgically removed and the sacs were gently teased using two bent syringe needles in Dulbecco's phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) on ice. Thymus glands were gently rubbed between the rough surfaces of two histological slides until only a cell suspension remained. Single cell suspensions were made by gently pushing through narrow gauge needles once or twice and then debris or large-membrane particles from the sac removed by passing through 40-μm Nitex filter (Sefar America, Depew, NY, USA), centrifuged, then resuspended in fresh PBS plus 0.1% BSA. For PP cellularity assessment, the junction between the small and large intestines (segment of ∼2.5 cm) was surgically removed, gut contents removed, washed repeatedly, cut into small pieces, and digested with 0.1% collagenase Type I (Sigma Chemical Co., St. Louis, MO, USA) for 1 hour in a 37°C water bath with periodic vortexing. The resulting cell suspension was washed in PBS to remove the enzyme, filtered through a nylon mesh, and then resuspended in PBS plus 0.1% BSA for staining and evaluation by FACS. The subset distribution among CD45+ cells was determined. Cellularity was determined using a Particle Z cell counter from Beckman Coulter (Miami, FL, USA).
Immunohistochemistry
Tissues for immunohistochemistry were processed as described previously [17].
Immunization with trinitrophenyl ovalbumin (TNP-OVA)
TNP-OVA (Biosearch Technologies, Novato, CA, USA) and incomplete Freud's adjuvant (Sigma Chemical Co.) were emulsified and injected (1:1 ratio) 100/μg in 100/μL subcutaneously in the back of each mouse. Mice were bled 7 and 14 days later and TNP-antibody titers in serum were measured by enzyme-linked immunosorbent assay (Sigma Chemical Co.). Goat anti-mouse IgM-alkaline phosphatase (AP), IgG1-AP, IgG2a-AP, IgG3-AP and rat anti-mouse IgE-AP were used for detection of various antibody isotypes. Calculated endpoint titers represent the greatest dilution of plasma with a signal (optical density) of 10% of maximum [18].
Proliferative responses and cytokine secretions by lymphoid cells
Splenocytes were prepared from spleens and cell suspensions were treated with red cell lysis buffer (Tris-NH4Cl). CD4+ or CD8+ T cells were purified from splenocytes by positive selection magnetic-activated cell sorting (Miltenyi Biotec, Auburn, CA, USA) (>95% purity) and stimulated with anti-CD28 (4 μg/mL) in the presence of various concentrations of anti-CD3 for 3 days. Antigen-presenting cells (APCs) were purified by Thy1.1 (Miltenyi Biotec) depletion using magnetic-activated cell sorting columns and were activated with various concentrations of lipopolysaccharide (Sigma) or anti-IgM (BD Biosciences). All cell cultures were performed in RPMI-1640 with proper supplements. To assay proliferation, cultures were pulsed with 1 μCi/well of tritiated thymidine (Perkin Elmer, formerly New England Nuclear, Waltham, MA, USA) for the last 6 hours of the 72-hour incubation period. To measure cytokines, aliquots of supernatants were harvested at 72 hours after initiation of cultures. Interleukin-4 (IL-4) and interferon-γ (IFN-γ) were analyzed by enzyme-linked immunosorbent assay using monoclonal antibodies and recombinant cytokine standards from eBioscience. Detection units were interleukin (IL)-4, 40 pg/mL and IFN-γ, 100 pg/mL. Antigen-specific responses were detected using OVA-specific trangenic T-cells (OT-11) mice. CD4+ OT-11 T cells and APCs were isolated as above. The 1 × 105 OT-11 T cells were cultured with 3 × 105 γ-irradiated (3000 cGy) APCs and various concentrations of OVA323-339 peptide (Invitrogen). Proliferation and cytokine production was measured as mentioned previously.
Results
Hemopoietic reconstitution by donor cells in Rag 2−/− recipients
Because transplantation of nonirradiated Rag 2−/− mice leads to very low hematopoietic reconstitution and to a largely ineffective thymic repopulation by donor cells [19], we used lethally irradiated Rag 2−/− recipients in two independent experiments using 20 mice given either control (α4f/f, 10 mice) or α4-deficient (α4Δ/Δ, 10 mice) donor BM cells. Expression of α4 in the donor population was >95% in the controls and <3% in the α4Δ/Δ BM cells. A total of 5 × 106 donor cells were transplanted by tail-vein injection within 3 to 4 hours after irradiation in the recipient mice. To assess reconstitution by donor cells, we tested recipient mice from 8 to 34 weeks posttransplantation. Of the 10 recipients given α4Δ/Δ cells, 1 died at 5 days and 1 at 119 days posttransplantation (with BM hypoplasia). When mice were tested at 8 to 10 weeks posttransplantation the cohort of Rag 2−/− recipients given α4+/+ cells was completely reconstituted by donor cells (CD45.2+ cells), whereas among recipients of α4Δ/Δ cells there were >7% residual host cells α4+ (CD45.1+) compared to 2% in control recipients (Fig. 1 and Table 1). These data of somewhat delayed reconstitution by α4-deficient cells are consistent with homing and reconstitution defects seen previously in transplantation experiments with α4-deficient donor cells [14]. At 6 months, half of the recipient mice of either donor cells were sacrificed to study cellularity and differentiation parameters in all hemopoietic organs. Data from BM, peripheral blood (PB), and spleen are presented in Figure 2A. In all these tissues, there was similar to increased cellularity in the recipients of α4Δ/Δ cells compared to the control group. Comparable data were obtained when the rest of recipient mice were sacrificed at 8 months posttransplantation, suggesting that this pattern is stably maintained long-term (Fig. 2B). The complete donor cell reconstitution for each cohort was confirmed not only by replacement posttransplantation by CD45.2 (donor) cells, but also by the level of α4 positivity in Gr-1+ cells in recipients of α4f/f vs α4Δ/Δ donor cells (BM α4+, 86.8% vs 0.88%; PB, 89% vs 3.3%; spleen, 78.8% vs 15.35% cells, respectively). Presence of differentiated erythro/myeloid cells (Gr-1+, TER119+, Mac-1) in all these tissues was not significantly different between the two groups; however, differences were seen in the proportion of lymphoid cells. For example, there were decreased proportions of B220+ cells in the BM of α4Δ/Δ compared to α4f/f repopulated mice (19.5% vs 25.0%, respectively). Among B-cell subsets, pro B (B220+/CD34−) and especially mature B cells (B220+/CD19+ or IgM+) were significantly decreased in BM of α4Δ/Δ recipients (Table 2A). In PB the opposite was seen, with all B220+ cells being increased, especially the early types (B220+/CD19−, or B220+/CD34+) in the recipients of α4Δ/Δ cells. Total T cells in BM were increased, but the proportion of activated T cells (CD4+ CD25+ regulatory cells, CD3+CD44+-activated T cells) was diminished (the former from 50.5% in α4f/f vs 10.4% in α4Δ/Δ and the latter from 85.6% in α4f/f to 20.5% in α4Δ/Δ).
Figure 1.

Rag 2−/− recipient reconstitution by α4f/f or α4Δ/Δ donor cells at 8 weeks after transplantation. Note the donor (CD45.2) reconstitution in (A) and that residual GR-1+/CD45.1+ host cells are present in recipients of α4Δ/Δ cells (B).
Table 1. Peripheral blood 8 weeks after transplantation.
| Donor cell type | CD3+ (% α4+) | B220 (% α4+) | Gr-1 (% α4+) |
|---|---|---|---|
| α4f/f | 28.3 ± 3.4 (99.7) | 45.5 ± 32.5 (96.3) | 27.2 ± 3.1 (97.8) |
| α4Δ/Δ | 33.2 ± 4.8 (1.3) | 45.0 ± 2.8 (1.6) | 18.5 ± 2.5 (2.11) |
Values are percentages. Lymphoid and myeloid reconstitution at 8 weeks after transplantation.
Figure 2.

Cell numbers recovered from different tissues at 6 and 8 months posttransplantation. BM = bone marrow CLN = cervical lymph nodes; LNI = inguinal lymph nodes; LNX = axillary lymph nodes; MLN = mesenteric lymph nodes; PB = peripheral blood; PP = Peyer's Patches; Thy = thymus. Baseline: refers to nucleated cell counts in nontransplanted Rag2−/− mice. *p < 0.05.
Table 2. 2A Total cellularity (× 10E6) in hematopoietic tissues of Rag2−/− recipients (pooled data from 6 and 8 months posttransplantation).
| B220+ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Donor cells | CD3+ | CD4+ | CD8+ | CD4+ CD25+ | CD3+ CD44+ | Ratio CD4:CD8 | CD34+ | CD34− | CD 19+ | CD19+ | lgM+ | |
| BM | α4f/f | 9.05±1.45 | 6.03± 1.054 | 3.02±0.08 | 4.57±1.18 | 7.7±2.09 | 1.99:1 | 0.7±0.15 | 2.07±0.84 | 1.94±0.05 | 3.3 ±1.07 | 0.58±0.02 |
| α4Δ/Δ | 13.2±3.05 | 9.32± 1.054 | 3.84±0.65 | 1.37±1.07 | 3.9±1.13 | 2.42:1 | 0.9±0.17 | 0.15±0.04 | 0.29±0.08 | 1.56±0.43 | 0.67±0.01 | |
| PB | α4f/f | 3.96±0.68 | 2.64±0.08 | 1.32±0.08 | 1.59±0.78 | 2.9±0.13 | 2.4:1 | 0.03 ±0.001 | 0.39±0.67 | 0.05±0.001 | 0.34±0.11 | 2.05±0.22 |
| α4Δ/Δ | 5.2±0.11 | 2.6±0.31 | 2.52±0.06 | 1.7±0.5 | 4.69 ±1.05 | 1.08:1 | 0.14±0.07 | 1.34±0.01 | 0.46±0.001 | 1.34±0.62 | 1.77±0.03 | |
| Spleen | α4f/f | 13.8±3.97 | 9.8±1.97 | 3.9±1.06 | 6.2±0.17 | 12.3 ±2.06 | 2.5:1 | 10.91±3.38 | 0.62±0.04 | 1.09±0.06 | 10.4±3.31 | 28.8±4.82 |
| α4Δ/Δ | 36.5±6.14 | 28.4±3.14 | 8.1 ±1.033 | 13.5±3.91 | 35.1 ±4.47 | 3.5:1 | 39.23±1.19 | 3.83±1.83 | 4.96±0.67 | 71.2± 14.21 | 51.62±2.33 | |
| PP | α4f/f | 1.04±0.09 | 0.8±0.01 | 0.17±0.01 | 0.85±0.27 | 0.82±0.25 | 4.7:1 | 11.07±3.91 | 0.096 ±0.003 | 0.005±0.001 | 0.8±0.01 | 1.32±0.43 |
| α4Δ/Δ | 0.06±0.02 | 0.5±0.02 | 0.1 ±0.003 | 0.29 ±0.11 | 0.56±0.13 | 5:1 | 2.98±0.93 | 3.35±1.39 | 0.03±0.01 | 0.2±0.01 | 0.826±0.13 | |
| MLN | α4f/f | 28.59±3.06 | 19.96±3.07 | 9.53±1.08 | 18.0±2.47 | 24.4±2.21 | 2.09:1 | 7.87 ±1.09 | 0.71±0.21 | 0.12±0.04 | 4.86±2.91 | 21.84±3.96 |
| α4Δ/Δ | 8.7±1.02 | 6.8±1.02 | 1.86±0.07 | 2.9±1.07 | 6.15±1.93 | 3.4:1 | 6.59±1.17 | 0.5±0.03 | 0.08±0.002 | 1.5 ±0.3 | 10.92±2.49 | |
| PLN | α4f/f | 9.8±1.07 | 5.1 ±0.07 | 4.6 ±0.74 | 3.9±0.03 | 5.1±1.84 | 1.1:1 | 1.8±0.75 | 0.71±0.05 | 0.65±0.02 | 3.0±0.75 | 6.1 ±2.92 |
| α4Δ/Δ | 9.4±1.95 | 6.8±0.95 | 2.5 ±0.04 | 1.66±0.75 | 3.1±1.16 | 2.72:1 | 1.47±0.28 | 1.06±0.03 | 0.61 ±0.03 | 2.7±0.35 | 7.55±1.12 | |
|
| ||||||||||||
| BM=bone marrow; MLN mesenteric lymph nodes; PB =peripheral blood; PLN=peripheral lymph nodes; PP=Peyer's Patches. Numbers in bold letters: p<0.05 | ||||||||||||
|
| |||||
| Table 2B. Thymus | |||||
|
| |||||
| %DN | %DP | %CD4 SP | %CD8 SP | Ratio CD4:CD8 | |
| α4f/f (n=56) | 7.06±2.72 | 48.67±5.37 | 39.28±5.05 | 5.0±0.51 | 7.8:1 |
| α4Δ/Δ (n=53) | 5.89±2.06 | 45.61±4.10 | 43.46±1.55 | 5.04±1.20 | 8.7:1 |
In addition to cell numbers we tested progenitor cells colony forming unit-culture (CFU-C) in all these tissues in the same recipients. Like the increased cellularity, a significant increase in progenitor content was found, especially in spleen and in peripheral blood (Fig. 3). The increase in progenitors concerned all subtypes (burst-forming unit erythroid, CFU granulocyte-macrophage, and CFU granulocyte-erythrocyte-megakaryocyte-monocyte, data not shown). It is of interest that the above quantitative changes in Rag 2−/− recipients of cells were similar to those we previously described for donor α4Δ/Δ mice [14], including increased numbers of B cells in circulation, although mature B cells (IgM+) were much less represented. These data do suggest, that for this phenotype exemplified by changes in progenitor biodistribution and early release of B cells in circulation, the absence of α4 only in hematopoietic cells is necessary and sufficient [16].
Figure 3.

Number of progenitor cells in bone marrow (BM), peripheral blood (PB), and spleen in Rag 2−/− recipients of α4f/f or α4Δ/Δ cells at 6 months posttransplantation. Burst-forming erythroid, colony-forming unit (CFU) granulocyte-macrophage, CFU-Mix present are pooled and shown as CFU-C. No significant differences in proportions of different types of progenitors are seen. *p < 0.05.
Repopulation of lymphoid organs with α4Δ/Δ donor cells
Having documented that the Rag 2−/− recipients were fully reconstituted by donor (CD45.2) cells, we next assessed the repopulation status of several lymphoid tissues. We tested thymus, peripheral lymph nodes (inguinal, axillary, and cervical) and gut lymphoid tissues, i.e., PPs and MLNs.
Thymus
Repopulation of thymus was significantly impaired in Rag 2−/− recipients of α4Δ/Δ cells, compared to those that received α4f/f donor cells (Figs. 2A and B). A decrease in total cellularity (by 43% at 6 months) was again demonstrable at 8 months (Fig. 2B) posttransplantation, indicating no restorative evidence with time posttransplantation. To test whether the decrease in cellularity concerned only certain subsets of lymphoid cells vs all cell types, we carried out FACS analyses using several lymphoid-specific markers with differential expression at different stages of activation or differentiation. These data are presented in Figure 4 and Table 2B. Double-positive (DP, CD4+/CD8+) population was the predominant one in Rag2−/− recipients of α4Δ/Δ or α4f/f donor cells. The CD4:CD8 ratio greatly favored the CD4 population (∼8:1). Thus, the data in Figures 2 and 4 and Table 2B suggest that the total repopulation of thymus was impaired, likely because of impaired migration of BM-derived progenitors to thymus, although their subsequent maturation (to DP) was not grossly impaired in the absence of α4 integrins. However, it is notable that CD8+ cells were at very low levels in thymus and lower than controls, in contrast to levels in PB (∼ 1.9:1, Table 2).
Figure 4.
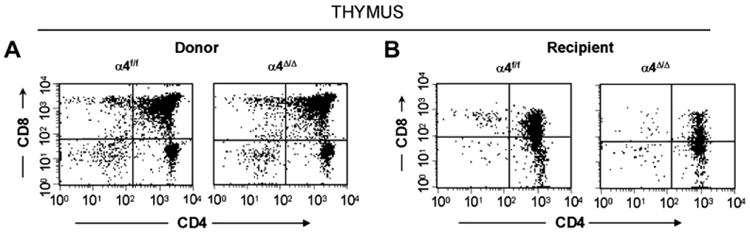
Fluorescein-activated cell-sorting analyses of cells from thymi of donor (α4Δ/Δ, α4f/f) compared to recipient mice (of α4Δ/Δ cells, α4f/f cells) at 6 months posttransplantation. Note the low abundance of CD4+, CD8+, or double-negative populations and high numbers of double-positive cells in all recipient (Rag2−/−) mice, indicating suboptimal reconstitution of thymus in transplanted Rag2−/− mice.
Peripheral lymph nodes
Cellularity in cervical, axillary, and inguinal lymph nodes was similar to controls (i.e., recipients of α4f/f donor cells). Detailed evaluation of subset distribution showed that there were modestly decreased proportions of mature B cells (B220+IgM+) in all LNs tested, or decreased proportions of activated T cells (CD3+/CD25+, CD3+/CD44+), but their absolute numbers were not significantly different from control groups (Table 2A). There was a tendency for preferential migration of CD45RC−/CD4+ (memory) cells to lymph nodes, whereas CD45RC+/CD4+ (naive) cells instead preferentially migrated to spleen and thymus in α4Δ/Δ recipients. In spleen, as noted above, the cellularity, especially of red pulp, was significantly increased (Fig. 2) and concerned all developmental stages of B cells and of total T cells (Table 2A). The picture of T- and B-cell distribution in spleen is more in line with what is present in PB, and contrasts that of BM (Table 2A) described above most likely because of longer retention and maturation of these cells in the splenic environment compared to BM. CD40+ (dendritic cells) were lower in all organs except the spleen, where the proportion, but not the total number, was low (data not shown).
PPs and MLNs
In Rag−/− recipients of α4Δ/Δ cells both at 6 and 8 months posttransplantation, there was a significant reduction in cell numbers recovered from these tissues compared to controls (about 17-fold in PPs, 67% less in MLNs) (Fig. 2). All subsets of B and T cells (Table 2A) were severely reduced in PPs and MLNs repopulated by α4Δ/Δ cells. The CD4:CD8 ratio in PPs favored a CD4+ profile, as seen in thymus. These data, like the ones in thymus, suggest significant homing impairment of all α4Δ/Δ cells (Table 2A) to these tissues.
Functional status of α4-deficient lymphoid cells B cells
To test the ability of α4-deficient B cells for antibody production, recipient mice were injected with TNP-OVA (intraperitoneally) and antibody titers were tested 7 and 14 days later. Determination of TNP-OVA-specific endpoint titers showed a significant reduction of titers for IgM (endpoint titers 1/2269.167 vs 1/858.199 in α4Δ/Δ) and IgE (titers: 1/8248.8 vs 1/1187.127 in α4Δ/Δ) in α4Δ/Δ recipients' sera, but no significant differences in IgG1 and IgG3 from α4f/f recipient's sera (Fig. 5A). This impairment in IgM response suggests that α4-integrin–dependent signals are necessary for the initial B-cell activation and IgM secretion. Because IgG1 and IgG3, or total IgG levels were not impaired, the data suggest no global defect in immune response or in Ig class switch. Further in vitro testing of B-cell proliferation supports this notion. Indeed, there was no defect in the in vitro activation of α4-deficient APCs (CD19+/CD3−) in the presence of increasing concentrations of lipopolysaccharide or anti-IgM (data not shown). In addition, there was no defect in activation and cytokine production by OT-II T cells (α4+) when OVA peptide was presented by α4Δ/Δ APCs as compared to α4f/f APCs (data not shown).
Figure 5.

Functional responses of B and T cells in recipients of α4f/f or α4Δ/Δ donor cells. (A) Trinitrophenyl ovalbumin (TNP-OVA)–specific immunoglobulins in response to in vivo stimulation by TNP-OVA in recipients of α4f/f or α4Δ/Δ donor cells. (B) Left panel: Interferon-γ (IFN-γ) production by freshly isolated CD4+ T cells from recipients of α4f/f or α4Δ/Δ donor cells stimulated with anti-CD3 and anti-CD28. Middle panel: α4f/f or α4Δ/Δ mice were immunized with OVA and 7 days later, CD4+ T cells were isolated from spleen, restimulated with anti-CD3 and anti-CD28, then IFN-γ production measured. Right panel: Mice were stimulated with OVA and 7 days later, splenocytes were restimulated with OVA, then IFN-γ production measured. (C) CD4+CD25− naïve cells were sorted and tested under Th1 conditions: anti-CD3/anti-CD28/anti-IL-4/IFN-γ or under Th2 conditions: anti-CD3/anti-CD28/anti-IFN-γ/IL-4, cultured for 7 days with IL-2 and restimulated with phorbol myristate acetate/ionomycin. Cytokines were detected by intracellular staining. Numbers above the upper right quadrant indicate the % of CD4+ T cells producing each cytokine. *p < 0.05.
T cells
The proliferative response of purified CD4+ or CD8+ or total splenocytes from α4Δ/Δ recipients was assessed by measuring proliferation in the presence of anti-CD3 and anti-CD28, or irradiated syngeneic APCs (for costimulation). Using both modalities we identified no differences in proliferation of purified CD4+, CD8+, or total splenocytes between the α4-deficient or control test population. Also, no differences were seen after phorbol myristate acetate or Ionomycin stimulation (data not shown). To test whether secretion of cytokines by stimulated cells was normal, we measured under both costimulatory conditions (i.e., CD28 or irradiated APCs) the secretion of IFN-γ, IL-2, IL-4, and IL-10. We found significantly decreased levels of IFN-γ by freshly isolated α4-deficient CD4+ T cells (Fig. 5B, left panel). There were low to undetectable levels of IL-2, IL-4, and IL-10 in these cultures stimulated with anti-CD3 and anti-CD28. To investigate whether the reduced IFN-γ production was due to an intrinsic defect of α4Δ/Δ CD4+ T cells, CD4+CD25− (naïve) cells were sorted from α4Δ/Δ spleens and activated with anti-CD3 and anti-CD28 under Th1 (+IFN-γ) and Th2 (+IL-4) polarizing conditions. The percentage of α4Δ/Δ naïve CD4+ T cells producing IFN-γ under Th1-polarizing conditions was similar to control CD4+ T cells (Fig. 5C). In addition, the percentage of IL-4–producing cells under Th2 conditions was comparable. Similar results were seen when CD4+CD62L+ or negative cells were sorted and activated with anti-CD3 and anti-CD28 (data not shown). This indicates there is no inherent defect by naïve α4Δ/Δ CD4 cells to produce IFN-γ. To test whether α4Δ/Δ can also mount a good response after in vivo stimulation, we immunized mice with OVA (7 days previously) and restimulated with anti-CD3 + anti-CD28 or OVA. As seen in Figure 5B, in contrast to the response of naïve α4-deficient T cells, the OVA-activated α4-deficient T cells produced higher than control levels of IFN-γ in two independent experiments when restimulated with anti-CD3 + anti-CD28 (Fig. 5B, middle panel). Similar results were also seen when splenocytes from mice immunized with OVA were restimulated with OVA (Fig. 5B, right panel). These data may indicate that more antigen-specific cells were maintained in the spleens of α4Δ/Δ mice, as the proliferative response to OVA was also increased (data not shown and [20]). However, further studies are needed to secure this point. Although not tested in Rag 2−/− recipients, we demonstrated no differences in the proportion of T regulatory (Tregs, CD4+CD25+, FoxP3+) cells in donor α4-ablated mice, both at young (4 months old), and old (16 months) age (Suppl. Fig. 2).
Discussion
In the present studies, in contrast to previous observations using anti-VLA4 monoclonal antibodies [21] or transplant experiments of mixed chimeras with embryonic stem (ES) cells or fetal cells [10,12,22], we assessed lymphomyeloid hematopoietic reconstitution in lethally irradiated Rag 2−/− recipients transplanted with adult BM α4-deficient (α4Δ/Δ) donor cells. As the phenotype of transplanted animals may be different in the presence of normal host cells [22] or with normal competitor cells [12] because of paracrine or other undefined effects of normal companion cells, meaningful comparisons to the phenotype of donor animals were not feasible in previous studies.
The complete reconstitution of hematopoiesis by α4-deficient BM donor in our Rag2−/− recipients had similar general features to that achieved in other recipients of adult α4 deficient cells [14]. These data reinforce our previous conclusions, that the cellular composition and the premature, ongoing release of progenitors from BM to blood are dictated by the absence of α4 integrin in hematopoietic cells, with no demonstrable contribution by α4-deficient microenvironmental cells [16]. Reconstitution of lymphoid organs in the recipients of α4Δ/Δ cells and comparison to data in α4-ablated donor mice several months postablation were not studied previously. Such an evaluation in the present study revealed a constellation of novel, insightful findings with both similarities and differences from other relevant models, i.e., chimeras with α4KO ES cells, or competitive repopulation experiments using fetal liver α4KO cells [12,22].
Repopulation of adult thymus was thought to be dependent on ongoing colonization by BM-derived progenitors [6,7]. Consistent with this view was the finding of atrophic thymi postnatally in the chimeras with α4KO cells [22]. Because general hematopoiesis was not contributed by α4KO ES cells beyond the first month of postnatal life in these chimeric studies [23], the thymic homing competency of the BM-derived progenitors could not be tested in this model. Subsequent transplantation studies of feta liver α4KO cells suggested no significant defects in thymic repopulation of the recipient mice [12]. However, only partial hematopoietic reconstitution was present in these mixed chimeras and absolute thymic cellularity was not presented. Our data showed decreased cellularity of thymus up to 8 months posttransplantation in the Rag 2−/− recipients of α4-deficient cells. Subset analysis suggested that the thymic hypocellularity was likely due to impaired homing of thymic progenitors and less so to their subsequent intrathymic development, as the profiles of double-negative, double-positive, and, single positive were not significantly different between α4f/f vs α4Δ/Δ recipients (Fig. 4). Recent studies indicate that homing to thymus is accomplished through preferential migration of the CLP-2 population, which coexpresses P-selectin glycoprotein ligand-1, α4β1, and αLβ2 integrins and interacts with their respective ligands on thymic endothelial cells [7,24]. This interaction, as well as the correct localization of homed cells in thymic cortex, is enforced by chemoattractant CCL25 expressed mainly in cortex and its receptor (CCR9) on CLP-2 cells. CCR9 is coexpressed with α4β1 in the latter cells and is required for homing, but had no effect on their subsequent T-cell development [25]. The latter appeared to be dependent on another chemokine, CXCL12, which did not affect thymic homing, but had a critical role in expansion and differentiation of thymocytes after their homing to thymus [26,27]. However, in other studies, pertussis toxin treatment of cells reduced the homing of CLP-2 to thymus partially, but had no effect on CLP-2 homing to BM [7]. Because thymic homing can be subserved by P-selectin glycoprotein ligand-1 or αLβ2 as indicated by these previous studies, it may not be surprising that the homing defects of thymic progenitors were partial in the absence of α4β1 alone, or after ablation of β1 postengraftment [2], although in the latter case only transient impairment in thymic repopulation was seen and some homing to thymus might have preceded their β1 ablation, which was initiated post–full engraftment. As the total numbers of DP and single positive cells were decreased in thymus of α4Δ/Δ recipients, additional effects on their development cannot be excluded. In line with this view are antibody data implicating VLA4 in trafficking between cortex and medulla [28], thereby suggesting a non-redundant role of α4β1 integrin for intrathymic expansion/maturation of thymic progenitors [8]. Such an outcome also advocated involvement of the CXCL12/CXCR4 pathway for the intrathymic traffic and correct localization of progenitor cells for their further maturation [27]. It is of note that expansion of T cells in both fetal and adult thymi was reduced in CXCL12/CXCR4KOS[26], like in our studies. Thus, on the strength of these data, one could conclude that cooperation between the two pathways likely through inside-out signaling affecting α4β1, may be operative in this process. It is important to emphasize that the effect of α4β1, CXCL12, or αLβ2 by antibody blockade [7], like the present data, was partial, suggesting additional alternative pathways. The presence of only a very small population of CD8+ cells in the reconstituted thymus, in contrast to the normal CD4:CD8 ratio in blood is of interest. It is unlikely that it is due to the inability of CD8+ α4-deficient cells to reenter thymus [29], as this was also observed in thymi reconstituted with control cells. It was also seen previously in ES α4 null chimeras with Rag2−/− ES cells [22]. Whether some alterations in Rag2−/− micro-environments (stroma and endothelial elements [19,30,31] play a role is only speculative at present. Furthermore, the detailed molecular signaling of chemokine/integrin interactions in the thymus has not been fully explored, but efforts to elucidate such molecular pathways are underway [7].
Like the reduced repopulation of thymus, severely reduced cellularity was also seen in gut associated lymphoid cells, i.e., PPs and MLNs. As all lymphocyte subsets were similarly reduced in these tissues, the data implied a severe homing problem. A similar outcome was seen in the recipients of FL α4KO cells [12] and in all prior data with α4β1 antibody blockade [32,33]. Of interest, anti–VCAM-1 [34] did not block homing to PP, unless αLβ2 was also blocked [34]. Because homing to PPs is mainly dependent on α4β7/ MadCAM-1 pathway (and most of the adult CD3+ cells in these tissues are negative for β1 integrin), this was not an unexpected finding. However, PPs were reported to be normal in β7 KOS and were normal in number (but of smaller size) in β1/β7 doubly-deficient mice [2]. Because the total cellularity was not assessed in the previous study with FL α4 KO cells [12] or in β1/β7 double KOS, differences may be explained on methodological or technical grounds. The presence of VCAM-1+ and ICAM-1+ stroma was deemed important for PP organogenesis [35] and for colonization by CD4+/CD3− cells, a unique subset of cells expressing both α4β1 and α4β7 integrins [36]. Also of interest, MLNs provide a supportive function for DP cells, as the only such site outside thymus [37]. The presence of CCL25 in the stromal cells of both tissues may be mediating this effect. Thus, our data suggest that similar α4 integrin-dependent interactions with stromal tissue cells seem to play major roles both for homing and intrathymic development of T lymphocytes and for colonization of mucosal tissues by T-lymphoid cells.
Cellularity of all tested peripheral lymph nodes, as well as that of spleen, was similar to one found in animals repopulated with control cells (Fig. 2). Likewise, no abnormalities have been reported in these tissues in any of the other models [2,12]. Nevertheless, certain abnormalities in the functional properties of mature lymphoid cells were found here and are of interest. There was a decrease in initial IgM response after immunization and antibody-specific IgE responses α4Δ/Δ cells compared to ones with α4f/f (Fig. 5A). A similar impairment in initial B-cell responses was noted in VCAM-1–deficient mice and in mice with ablated β1-integrin post-engraftment [2,38,39]. Furthermore, OVA-specific IgE response after induction of allergen (OVA)-dependent acute asthma was significantly impaired when α4-deficient mice were used [20]. All these concordant data suggest weakened interactions between T helper and B cells. Whether the reduced response reflects inadequate cell contact of B cells, or their subsequent expansion and stimulation is unclear, but is consistent with recent observations suggesting involvement and incorporation of α4β1 in the activation complex (pSMAC) during immune synapse formation between APCs and T cells [40]. An important mechanistic insight into the requirement of VLA4/VCAM-1 pathway for B-cell activation was recently presented. The interaction is mediated either by B-cell tethering to the target membrane and thereby facilitating BCR/antigen engagement, or by enhancing the level of B-cell signaling [41]. Thus, VCAM-1, expressed on the surface of target cells (follicular dendritic cells, vascular endothelium) may capture B cells through interaction with VLA4 and enhance a mature immune synapse formation and B-cell activation [41]. Nevertheless, we found no generalized attenuation of immune responses by α4-deficient cells. B-cell proliferation to lipopolysaccharide or anti-IgM in vitro was similar to controls, but different microenvironment-dependent responses in vivo cannot be excluded. Interferon responses of stimulated T cells were also of interest. Purified naïve CD4+CD62L+ α4-deficient lymphocytes had similar IFN-γ secretion, although freshly isolated splenic CD4+ α4-deficient T cells produced less IFN-γ (Fig. 5B). This conflicting result may be due to contamination of CD4+ expressing cells by other cells, such as NK T cells. If NK T cells are reduced in α4-deficient mice, then we might expect to observe a reduction in IFN-γ production from freshly isolated splenocytes. This reduction would not be seen when CD4+CD62L+ cells were isolated, as NK T cells do not express CD62L. However, for definitive conclusions, further investigation is needed. Once stimulated, α4-deficient T cells turned into IFN-γ hyper-producing cells (Fig. 5B), due to an increase in the number and proliferation of antigen-specific cells retained in the spleen. In addition to lack of migration out of the spleen, this result could also reflect a bias of α4-deficient lymphocytes toward Th1 responses with secondary suppression of Th2 responses. Failure to induce allergen-dependent acute asthma in our α4-deficient mice was primarily due to the inability of α4Δ/Δ lymphocyte migration to lung as well as to airways, but Th2-dependent responses in these mice were also reduced [20]. In this context, it is of interest that miR-155 was found to be critically involved in the in vivo immune response by exerting its function at the level of cytokine production, i.e., less IFN-γ but more IL-4, favoring Th2 differentiation [42]. Future studies may shed more light on this issue. In summary, the data presented herein amplify and fine tune previous observations on the role of α4 integrins in the repopulation of lymphoid organs, by securing its role in homing to thymus and gut lymphoid tissue and by strengthening previous evidence of attenuated B-cell responses by α4-deficient cells.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grant HL46557) to T.P.
Footnotes
Online version of this article contains a data supplement.
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.exphem.2008.03.008.
References
- 1.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 2.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 4.Berlin C, Berg EL, Briskin MJ, et al. Alpha4beta7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 5.Rose DM. The role of the alpha4 integrin-paxillin interaction in regulating leukocyte trafficking. Exp Mol Med. 2006;38:191–195. doi: 10.1038/emm.2006.23. [DOI] [PubMed] [Google Scholar]
- 6.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. PNAS. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmeissner PJ, Xie H, Smilenov LB, Shu F, Marcantonio EE. Integrin functions play a key role in the differentiation of thymocytes in vivo. J Immunol. 2001;67:3715–3724. doi: 10.4049/jimmunol.167.7.3715. [DOI] [PubMed] [Google Scholar]
- 9.Halin C, Scimone ML, Bonasio R, et al. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood. 2005;106:1314–1322. doi: 10.1182/blood-2004-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1108. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 11.Bell E, Sparshott S, Ager A. Migration pathways of CD4 T cell subsets in vivo: the CD45RC- subset enters the thymus via alpha 4 in-tegrin-VCAM-1 interaction. Int Immunol. 1995;7:1861–1871. doi: 10.1093/intimm/7.11.1861. [DOI] [PubMed] [Google Scholar]
- 12.Gribi R, Hook L, Ure J, Medvinsky A. The differentiation program of embryonic definitive hematopoietic stem cells is largely alpha4 integrin independent. Blood. 2006;108:501–509. doi: 10.1182/blood-2005-10-4209. [DOI] [PubMed] [Google Scholar]
- 13.Bungartz G, Stiller S, Bauer M, et al. Adult murine hematopoiesis can proceed without beta1 and beta7 integrins. Blood. 2006;108:1857–1864. doi: 10.1182/blood-2005-10-007658. [DOI] [PubMed] [Google Scholar]
- 14.Scott L, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priestley GV, Scott LM, Ulyanova T, Papayannopoulou T. Lack of alpha4 integrin expression in stem cells restricts competitive function and self-renewal activity. Blood. 2006;107:2959–2967. doi: 10.1182/blood-2005-07-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priestley GV, Ulyanova T, Papayannopoulou T. Sustained alterations in biodistribution of stem/progenitor cells in Tie2cre+ alpha4f/f mice are hematopoietic cell autonomous. Blood. 2007;109:109–111. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulyanova T, Scott L, Priestley G, et al. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106:86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdam AJ, Greenwald RJ, Levin MA, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 19.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early t cell progenitors. J Immunol. 2004;173:1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee ER, Jiang Y, Henderson JWR, Scott LM, Papayannopoulou T. alpha4 and beta2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only alpha4 is critical. Exp Hematol. 2007;35:605–617. doi: 10.1016/j.exphem.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Issekutz T. Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo. J Immunol. 1991;147:4178–4184. [PubMed] [Google Scholar]
- 22.Arroyo AG, Taverna D, Whittaker CA, et al. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for alpha5, alphav, and alpha4 integrins. J Immunol. 2000;165:4667–4675. doi: 10.4049/jimmunol.165.8.4667. [DOI] [PubMed] [Google Scholar]
- 23.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11:555. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 24.Rossi FMV, Corbel SY, Merzaban JS, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 25.Parmo-Cabanas M, Garcia-Bernal D, Garcia-Verdugo R, Kremer L, Marquez G, Teixido J. Intracellular signaling required for CCL25-stimulated T cell adhesion mediated by the integrin alpha4 beta1. J Leukoc Biol. 2007;82:380–391. doi: 10.1189/jlb.1206726. [DOI] [PubMed] [Google Scholar]
- 26.Ara T, Itoi M, Kawabata K, et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170:4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 27.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and Tcell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 28.Crisa L, Cirulli V, Ellisman MH, et al. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: the roles of fibronectin, VLA4, and VLA5. J Exp Med. 1996;184:215–228. doi: 10.1084/jem.184.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sitnicka E, Buza-Vidas N, Ahlenius H, et al. Critical role of FLT3 ligand in IL-7 receptor-independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 30.Boyd RL, Tucek CL, Godfrey DI, et al. The thymic microenvironment. Immunol Today. 1993;14:445–459. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 31.van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunol Today. 1994;15:214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 32.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 33.Xu B, Wagner N, Pham LN, et al. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197:1255–1267. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berlin-Rufenach C, Otto F, Mathies M, et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finke D, Kraehenbuhl JP. Formation of Peyer's Patches. Curr Opin Genet Dev. 2001;11:561–567. doi: 10.1016/s0959-437x(00)00233-1. [DOI] [PubMed] [Google Scholar]
- 36.Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl JP. CD4+CD3- cells induce Peyer's Patch development: role of alpha4-beta1 integrin activation by CXCR5. Immunity. 2002;17:363–373. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 37.Maillard I, Schwarz BA, Sambandam A, et al. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–3519. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med. 2001;193:755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barreiro O, de la Fuente H, Mittelbrunn M, Sanchez-Madrid F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol Rev. 2007;218:147–164. doi: 10.1111/j.1600-065X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 41.Carrasco YR, Batista FD. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 2006;25:889–899. doi: 10.1038/sj.emboj.7600944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thai TH, Calado DP, Casola S, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


