Normal hematopoiesis is sustained by a relatively small pool of self-renewing primitive hematopoietic stem cells (HSCs). In murine hematopoietic repopulation studies, human cells with bright expression of CD34, but no expression of CD38, are highly enriched in HSC activity.1 Cells with similar immunophenotype are implicated as leukemia stem cells (LSCs),2 the initiating and repopulating reservoir for acute myeloid leukemia (AML). CD34+CD38neg cell frequency at diagnosis correlates with the incidence of minimal residual disease following achievement of complete remission (CR) in adult AML3 and with worse event-free survival in pediatric AML.4 A similar, but not identical, population of cells known as ‘side population’ (SP) has been defined based on ability to efflux fluorescent dye Hoechst 33342 via the ABCG2 multidrug resistance-mediating transporter that can be inhibited by fungal toxin Fumitremorgin C (FumC).5 The SP was originally characterized in mice6 and was demonstrated to contain primitive HSCs. The stem cell reservoir in nearly all normal tissues resides in the SP,7 and it also contains the primitive stem cells for solid tumors, including breast, prostate and lung cancer.8–10
The relationship between SP and CD34+CD38dim cells has not been previously explored in AML. Three studies demonstrated that SP could be isolated from AML patients. The first study of 16 patients showed that presence of the SP was not related to expression of the multidrug resistance gene (MDR-1), expressed variable CD38, was present with different types of cytogenetics abnormalities, and variably engrafted immunodeficient mice.11 The second study demonstrated that the SP was present in 80% of 61 patients, that 3 out of 28 cases could engraft NODscid mice and that 11/11 exhibited abnormal cytogenetics.12 The third study highlighted the heterogeneity of the SP derived from 48 patients, although there was uniform expression of the stem cell marker CLL-1.13
LSCs appear to provide a reservoir for relapse of AML following chemotherapy.14 Chemoresistance may reflect the relative quiescent state of the LSC and/or increased expression of drug efflux mediating transporters that extrude chemotherapy drugs.15 The latter possibility motivated us to determine whether SP correlated with outcome in AML. In particular, we investigated the relationship between the frequencies of CD34+ CD38neg, CD34+ CD38low and SP blasts and clinical prognostic variables, such as age, cytogenetics, achievement and duration of first CR, in patients with newly diagnosed AML.
All studies were conducted with approval of the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board. Normal control samples were purchased from the FHCRC Stem Cell Core. Blood and bone marrow samples were obtained from consecutive newly diagnosed AML patients with informed consent. Clinical characteristics are provided in Supplementary Table 1. Mononuclear cells were suspended ± FumC at 10 μg/ml for 30 min prior to Hoechst stain, incubated with 5 μm Hoechst 33342 at 37°C for 90 min in the dark and then labeled with conjugated antihuman CD45, antihuman CD34, antihuman CD38 and 7-amino-actinomycin D. The CD38neg population was determined using fluorescence minus one controls. CD38low population was defined by CD38 above negativity threshold and below that of mature precursors. Flow cytometry analysis was performed using in-house software Woodlist (Dr Brent Wood). Mean fluorescence intensity ratio (MFIR) was calculated by dividing mean fluorescence of CD38 in bulk blasts by that of SP blasts.
G-banding karyotype analysis was performed at the University of Washington (UW) Cytogenetics Laboratory. Fluorescence in situ hybridization (FISH) was performed by the Cytogenetics Laboratory of the Seattle Cancer Care Alliance.
NODscid IL2R γc−/− (NOD.Cg-Prkdcscid ll2rgtm1Wjl/SzJ) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Animal care protocols were approved by the UW Institutional Animal Care and Use Committee. Fluorescence-activated cell-sorted SP or other cell fractions were infused by tail vein injection in NODscid IL2Rγc−/− mice after sublethal irradiation. Analysis of engraftment was performed by flow cytometry.
Statistical analysis was performed by Wilcoxon rank-sum test, Kruskal–Wallis test and Spearman's correlation. Two patients (unique patient number 63 and 69) had < 1 year of CR1 duration and were excluded from the CR duration analyses.
We first confirmed that ABCG2 was the primary transporter responsible for the SP phenotype in AML as in normal HSCs. All normal (20/20) and nearly all AML (21/22) samples demonstrated dramatic reduction of SP following FumC treatment (median SP inhibition 98%) (Figure 1A). We then addressed whether SP and CD34+ CD38neg populations in normal marrow identify similar primitive hematopoietic cells. In each normal sample, the SP blast population contained >80% CD38neg blasts and >60% of CD38neg blasts were present in the SP (Supplementary Figure 1).
Figure 1.
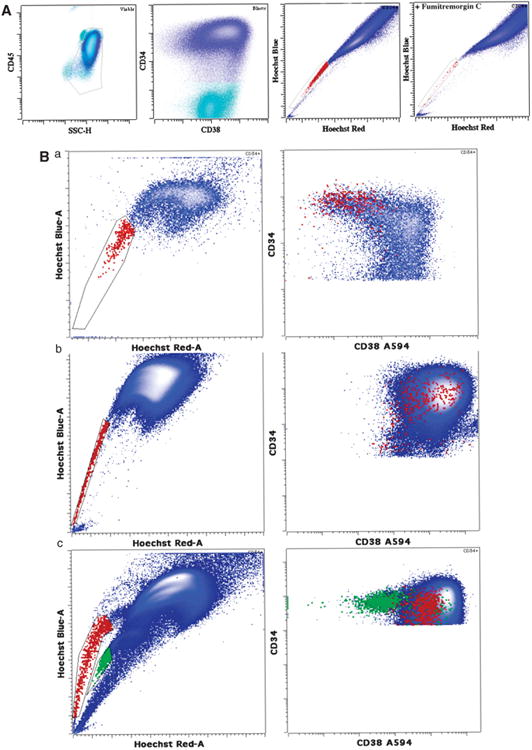
Flow cytometry analysis of SP phenotype in relation to CD38 expression. (A) Typical example of FumC-mediated inhibition of SP phenotype in AML. CD34+ blasts first gated based on CD45 and side scatter and then on CD34 expression (shown in blue) demonstrated a SP of 0.55% (shown in red). When FumC was added to the sample, the proportion was reduced to 0.03% with identical gating. Dead cells were excluded using 7-amino-actinomycin D uptake prior to gating. (B) (a) Some AML cases demonstrate mild decrease of CD38 expression on SP blasts. SP blasts are depicted in red and bulk blasts are shown in blue. (b) The majority of AML cases exhibit bright CD38 expression. SP blasts are depicted in red and bulk blasts are shown in blue. (c) A single case demonstrated two distinct blast populations of different ploidy, one derived from the leukemic clone and one from normal population. The population with brighter Hoechst blue and red staining (higher ploidy) demonstrated SP (red) primarily with brighter CD38. Both the SP and the higher ploidy population demonstrated numerous AML-related genomic abnormalities. In contrast, the population with lower ploidy showed a SP (green) that had dim CD38 and did not contain any detectable cytogenetic abnormalities suggestive of residual normal blasts.
In contrast to normal samples, most AML lacked correspondence between the SP and CD34 + CD38neg or low populations. Only rare cases showed a significant overlap between CD38 neg or low blasts and SP (Figure 1Bb). Rather, most AML samples showed SP populations with a higher level of CD38 more typical of lineage committed progenitors (Figure 1Bc). Combining data from all cases showed no significant difference in MFIR of CD38 between AML SP and bulk CD34+ blasts (MFIR 0.94 (P>0.1, two-tailed paired t-test)) (Supplementary Figure 2). SP blasts contained the same cytogenetic abnormalities at similar frequency as bulk blasts in the four consecutive cases tested (Supplementary Table 2). In one case, there were two distinct blast populations that were distinguishable by DNA content, normal and abnormal. The SP with higher DNA content (tetraploid) showed the multiple FISH abnormalities of the bulk blasts and also had increased CD38 expression. A second population (diploid) showed a lower DNA content, dim expression of CD38 and no FISH-defined abnormalities, suggesting a residual normal blast population (Figure 1Bc). CD38 expression thus clearly distinguishes the majority of AML SP cells from the residual normal precursors.
Individual patient fractions of stem cell populations and clinical features are provided in Table 1. The SP percent did not correlate with age, cytogenetic risk category, response, CR1 duration or Flt3 (all P>0.05). Higher percents of CD38low and CD38neg were each associated with lack of CR (P=0.01 and P<0.01, respectively), longer CR1 duration (P<0.01 and P=0.01, respectively) and increased age (P=0.01). Higher percent of CD38neg also correlated with worse cytogenetic risk category (P= 0.01).
Table 1. Individual patient data.
| UPN | % SP | % CD38low blasts | % CD38neg blasts | Response | Cytogenetics | Age | Flt3 | NPM1 | CR1 duration |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 0.021 | 1.64 | 0.096 | NR | Complex-unfavorable | 52.5 | ND | ND | 0 |
| 12 | 0.021 | 5.45 | 0.005 | CR | Diploid-intermediate | 53.3 | ND | ND | 293 days |
| 13 | 0.77 | 7.82 | 0.057 | NR | Diploid-intermediate | 80.6 | 0 | 0 | 0 |
| 14 | 0.038 | 4.91 | 0.17 | NR | Complex-unfavorable | 72.4 | 0 | 0 | 0 |
| 17 | 0.33 | 0.51 | 0 | CR | Inv16-favorable | 63.4 | 0 | 0 | 269 days |
| 26 | 0.026 | 0.96 | 0.00093 | CR | Trisomy8-intermediate | 59.8 | 0 | 0 | 464 days |
| 28 | 13.28 | 4 | 0.027 | NR | Complex-unfavorable | 67.6 | 0 | 0 | 0 |
| 29 | 0.036 | 0.081 | 0 | CR | Inv16-favorable | 26.2 | ND | ND | 469 days |
| 32 | 0.42 | 0.035 | 0 | CR | t(8;21); − 9q favorable | 30.3 | 1 | 0 | >422.5 m |
| 38 | 0.034 | 0.02 | 0.001 | CR | Diploid-intermediate | 51.5 | 0 | 0 | >421.1 m |
| 39 | 0.078 | 0.27 | 0.014 | CR | Diploid-intermediate | 51.4 | 0 | 0 | 133 days |
| 44 | 0.36 | 0.0053 | 0.00068 | CR | Diploid-intermediate | 58.5 | 0 | 0 | >418.6 m |
| 51 | 0.0029 | 17.41 | 0.73 | NR | Complex-unfavourable | 83.5 | ND | ND | 0 |
| 52 | 0.32 | 1.88 | 0.098 | NR | Diploid-intermediate | 67.8 | 0 | 0 | 0 |
| 55 | 0.0012 | 8.93 | 0.52 | NR | Diploid-intermediate | 69.4 | 0 | 0 | 0 |
| 56 | 0 | 0.65 | 0.0016 | NR | Complex-unfavorable | 69.1 | 1 | 0 | 0 |
| 59 | 0.041 | 0.062 | 0.00077 | NR | Diploid-intermediate | 53 | 1 | 0 | 0 |
| 60 | 7.6 | 2.57 | 0.094 | NR | Complex-unfavorable | 61.7 | 0 | 0 | 0 |
| 61 | 0.013 | 0.2 | 0.0071 | CR | Complex-unfavorable | 75.8 | 0 | 0 | 71 days |
| 63 | 6.2 | 0.26 | 0.0076 | CR | Inv16, + 8-favorable | 19.4 | 0 | 0 | >11.7 m |
| 67 | 0.029 | 5.08 | 0.24 | NR | Complex-unfavorable | 72.1 | 0 | 0 | 0 |
| 69 | 0.18 | 8.14 | 0.021 | CR | Inv16-favorable | 30.5 | 0 | 0 | >9.6 m |
| Mean | 1.48 | 2.88 | 0.092 | ||||||
| Median | 0.039 | 1.3 | 0.011 | ||||||
| Range | 0–13.2 | 0.02–17.4 | 0–0.92 |
Abbreviations: CR, complete remission; NR, no response; UPN, unique patient number. The clinical variables including age, cytogenetic risk, mutational status response and duration of first complete remission (CR1) are provided by UPN.
The Wilcoxon rank-sum analysis demonstrated that there was a marked difference between the percents of CD38neg or CD38low between responders (CR) and nonresponders (no CR): the median for CD38neg % for responders was 0.001 and for nonresponders was 0.096, and the median for CD38low % for responders was 0.260 and for nonresponders was 3.996 (Supplementary Figure 3). Among the top quartile (highest % CD38neg) of six patients, there were no CRs, whereas for the lowest quartile, there were five out of six CRs, with four of them of duration >1 year. For both CR and CR duration, P-value was 0.015. Frequencies of both CD38low and CD38neg blasts demonstrated a high degree of correlation between frozen and fresh samples (0.71 and 0.76, respectively).
To assess the frequency of LSC in the SP by the conventional definition of ‘scid repopulating cells’, we performed engraftment experiments in NODscid IL2Rγc−/− mice with 1700–120000 SP cells per mouse from patients 32, 34, 37, 56, 67 and 69. At 12–24 weeks, the bone marrow samples exhibited 1–35.6% (mean 7.8%), the spleen 0.4–38.3% (mean 10.2%) and the blood 0.3–27.4% (mean 10.1%) human CD45 + cells. The cells from one patient demonstrated significant engraftment of 70000 human SP cells per mouse resulting in 24.1% human CD45+ cells in the blood and 35.6% human CD45+ cells in the bone marrow, comparable to the level obtained from 1 000 000 CD34+ cells per mouse from the same patient.
In summary, SP cells can be derived from the majority of patient AML blast populations and can exhibit robust dye exclusion and variable expression of CD38. They usually have the same cytogenetic abnormality as the parent leukemia and are capable of variable engraftment in the NODscid IL2Rγc−/− mouse. However, the frequency of this population does not correlate with any known prognostic features in AML, in contrast to the frequency of CD34 + CD38low or neg cells. We therefore favor the phenotype of putative LSCs as CD34 + CD38low or neg rather than SP, as an increased proportion of CD34 + CD38low or neg cells is correlated with increased age, unfavorable cytogenetics, chemotherapy resistance and short duration of CR1. Targeting this population will likely be critical to eradication of leukemia.
Supplementary Material
Acknowledgments
This project was supported by a grant from the Leukemia and Lymphoma Society Translational Research Program (to PSB). We thank Ms Caroline Stamato for her assistance with the manuscript.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 3.van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- 4.Witte KE, Ahlers J, Schafer I, Andre M, Kerst G, Scheel-Walter HG, et al. High proportion of leukemic stem cells at diagnosis is correlated with unfavorable prognosis in childhood acute myeloid leukemia. Pediatr Hematol Oncol. 2011;28:91–99. doi: 10.3109/08880018.2010.528171. [DOI] [PubMed] [Google Scholar]
- 5.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 6.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 8.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 9.Christgen M, Geffers R, Ballmaier M, Christgen H, Poczkaj J, Krech T, et al. Down-regulation of the fetal stem cell factor SOX17 by H33342: a mechanism responsible for differential gene expression in breast cancer side population cells. J Biol Chem. 2010;285:6412–6418. doi: 10.1074/jbc.M109.082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oates JE, Grey BR, Addla SK, Samuel JD, Hart CA, Ramani VA, et al. Hoechst 33342 side population identification is a conserved and unified mechanism in urological cancers. Stem Cells Dev. 2009;18:1515–1521. doi: 10.1089/scd.2008.0302. [DOI] [PubMed] [Google Scholar]
- 11.Feuring-Buske M, Hogge DE. Hoechst 33342 efflux identifies a subpopulation of cytogenetically normal CD34 + CD38 − progenitor cells from patients with acute myeloid leukemia. Blood. 2001;97:3882–3889. doi: 10.1182/blood.v97.12.3882. [DOI] [PubMed] [Google Scholar]
- 12.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 13.Moshaver B, van Rhenen A, Kelder A, van der Pol M, Terwijn M, Bachas C, et al. Identification of a small subpopulation of candidate leukemia-initiating cells in the side population of patients with acute myeloid leukemia. Stem Cells. 2008;26:3059–3067. doi: 10.1634/stemcells.2007-0861. [DOI] [PubMed] [Google Scholar]
- 14.Essers MA, Trumpp A. Targeting leukemic stem cells by breaking their dormancy. Mol Oncol. 2010;4:443–450. doi: 10.1016/j.molonc.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentles AJ, Plevritis SK, Majeti R, Alizadeh AA. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA. 2010;304:2706–2715. doi: 10.1001/jama.2010.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


