Abstract
Research over the last decade has uncovered roles for bile acids (BAs) that extend beyond their traditional functions in regulating lipid digestion and cholesterol metabolism. BAs are now recognized as signaling molecules that interact with both plasma membrane and nuclear receptors. Emerging evidence indicates that by interacting with these receptors, BAs regulate their own synthesis, glucose and energy homeostasis, and other important physiological events. Herein, we provide a comprehensive review of the actions of BAs on cardiovascular function. In the heart and the systemic circulation, BAs interact with plasma membrane G‐protein‐coupled receptors, for example, TGR5 and muscarinic receptors, and nuclear receptors, for example, the farnesoid (FXR) and pregnane (PXR) xenobiotic receptors. BA receptors are expressed in cardiovascular tissue, however, the mechanisms underlying BA‐mediated regulation of cardiovascular function remain poorly understood. BAs reduce heart rate by regulating channel conductance and calcium dynamics in sino‐atrial and ventricular cardiomyocytes and regulate vascular tone via both endothelium‐dependent and ‐independent mechanisms. End‐stage liver disease, obstructive jaundice, and intrahepatic cholestasis of pregnancy are prominent conditions in which elevated serum BAs alter vascular dynamics. This review focuses on BAs as newly recognized signaling molecules that modulate cardiovascular function. Clin Trans Sci 2011; Volume 4: 210–218
Keywords: bile acids, arteries, vascular tone, endothelium, cardiomyocyte, cirrhosis, receptors, potassium channels
Introduction
Bile acids (BAs) are synthesized in the liver as by‐products of cholesterol metabolism and secreted into the duodenum. They journey through the small intestine, undergo metabolism by intestinal flora, are reabsorbed efficiently by specific transporters in the ileum and circulate back to the liver via the mesenteric and portal veins. 1 In the liver, BAs are transported into hepatocytes and conjugated with amino acids before excretion into bile ducts. BAs are stored in the gallbladder and released into the duodenum with meals. This cycle of BA metabolism in the liver and recovery from the intestines, designated as enterohepatic circulation, is a highly efficient system whereby more than 95% of the BA pool is recycled. In normal circumstances, the remaining small fraction of the BA pool either enters the colon to be excreted in feces or spills into the systemic circulation. This small percentage of fecal BAs is important for maintaining normal defecatory function.
In recent years, the recognized role of BAs has expanded beyond fat absorption and cholesterol metabolism. Interaction of BAs with plasma membrane G‐protein‐coupled (GPCRs) and nuclear receptors in various tissues triggered investigation into the role of BAs in regulating glucose homeostasis, obesity, thyroid function, and other important physiological processes; this is reviewed elsewhere. 2 , 3 Herein, we focus on the role of BAs and their receptors in regulating cardiovascular function in health and disease.
The role of BAs in regulating cardiovascular function was investigated sporadically in the past and more actively in recent years. In large part, this renewed interest stems from identification of BA receptors in cardiovascular tissue and recognition of BAs as vasoactive ligands that regulate vascular tone and myocardial contractility in disease. Recent advances highlight the importance of this line of investigation. As liver disease progresses toward cirrhosis, the BA pool shifts from the enterohepatic to the systemic circulation. 4 Cirrhosis is associated with increased cardiac output, reduced arterial pressure, and reduced systemic vascular resistance. 5 In cirrhosis, several mechanisms and ligands including BAs are proposed to induce the systemic and splanchnic vasodilation that yields a hyperdynamic circulation. Intrahepatic cholestasis of pregnancy (ICP) is another disorder that is accompanied by elevated serum BA levels. 6 In ICP, BA‐induced cardiac arrhythmias are proposed to cause fetal morbidity and mortality. 6 , 7 In addition, activation of vascular farnesoid (FXR) may play a role in atherosclerosis. Hence, elucidating the interaction of BAs with cardiovascular tissues is likely to provide novel mechanistic insights into their regulatory role.
BA Metabolism
BAs have complex structural and biochemical properties that vary amongst vertebrates. 8 In humans, they are synthesized exclusively in hepatocytes as by‐products of cholesterol metabolism. While preserving the cholesterol backbone (C‐27 and four fused rings: A‐D) ( Figure 1 ), modifications consisting primarily of key hydroxylations and shortening of the C‐8 to a C‐5 side chain yield a predominantly C‐24 BA pool. 9 BAs are divided into two groups based on the orientation of C‐5 proton with respect to the C‐19 methyl group. The C‐5 proton in trans and cis configurations renders 5α‐ or 5β‐BAs, respectively. The 5α‐BAs have all four rings in same plane, while 5β‐BAs have ring A at an approximate right angle to the other rings. The majority of human BAs are in the 5β‐configuration. Conversion of cholesterol into BAs via neutral and acidic pathways involves multiple steps that are catalyzed by enzymes expressed in different hepatocyte compartments. 10 The neutral pathway is regulated by the rate‐limiting 7α‐hydroxylase‐CYP7A1, a microsomal cytochrome P450 enzyme. In the “acidic pathway,” the initial step is catalyzed by CYP27A1, and chenodeoxycholic acid (CDCA) is the main product. This pathway may contribute up to 50% of the BA pool. Cholesterol 25‐hydroxylase is a minor BA synthesis pathway.
Figure 1.
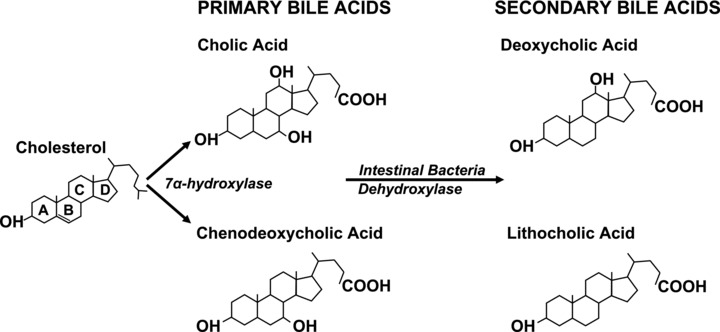
This scheme shows the structure of major human BAs derived from cholesterol metabolism. The steroid nucleus and ring lettering for both primary and secondary human BAs are shown. Dehydroxylases derived from intestinal bacteria regulate formation of secondary BAs. The degree of hydroxylation of the steroid nucleus determines the biochemical and functional characteristics of both primary and secondary BAs. Whereas cholic acid has three hydroxyl groups, chenodeoxycholic acid and deoxycholic acid have two, and lithocholic acid has one.
Cholic acid (CA) and CDCA are the primary human BAs ( Figure 1 ). In the small intestine, bacterial 7α‐dehydroxylase converts a fraction of these molecules into secondary BAs—deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. Further hepatic and bacterial modifications lead to formation of tertiary BAs, for example, ursodeoxycholic acid (UDCA). In human adults, 95% of the 3‐ to 5‐g pool consists of primary and secondary BAs. Normally, less than 5% of the BA pool escapes reabsorption to be lost in feces, whereupon it is replaced by hepatic synthesis. Before secretion into bile, BA solubility is increased through amidation of the carboxyl group with either glycine or taurine. In adults, the ratio of glycine to taurine conjugates is normally 3:1. Dietary intake of taurine, primarily in meat, dictates the size of taurine‐conjugated BA pool as hepatocytes do not synthesize that amino acid. Adhering to a proposed nomenclature, taurine conjugates of CA, CDCA, DCA, LCA, and UDCA are designated cholyltaurine (CT), chenodeoxycholyltaurine (CDCT), deoxycholyltaurine (DCT), lithocholyltaurine (LCT), and ursodeoxycholyltaurine (UDCT). 11 Glycine conjugates are named likewise (e.g., cholylglycine). A summary of BA nomenclature and abbreviations is provided in Table 1 .
Table 1.
Recommended designation and abbreviations for major human bile acids.*
| Bile acid (BA) | Abbreviation for unconjugated BA | Abbreviation for taurine‐conjugated BA | Abbreviation for glycine‐conjugated BA |
|---|---|---|---|
| Cholic acid | CA | CT | CG |
| Deoxycholic acid | DCA | DCT | DCG |
| Chenodeoxycholic acid | CDCA | CDCT | CDCG |
| Lithocholic acid | LCA | LCT | LCG |
| Ursodeoxycholic acid | UDCA | UDCT | UDCG |
*From Hofmann et al. 9
In the enterohepatic circulation, BAs are transported across the cell membranes via various transport proteins. 12 , 13 In the liver, BAs are transported into hepatocytes by Na+‐taurocholate cotransporting polypeptide and organic anion transport polypeptide (OATP). BAs are secreted from hepatocytes by the bile salt export pump (BSEP) and multidrug resistance proteins. In the small intestine, BAs are absorbed by enterocytes via ASBT in apical membranes, transported across the cells by ileal BA binding protein, and released into portal circulation via organ solute transporters (OSTα‐OSTβ). The small fraction of BAs spilled into the systemic circulation is reabsorbed by renal proximal tubule cells via ASBT and OSTα‐OSTβ for hepatic reuptake. Bile duct epithelium also expresses ASBT and OSTα‐OSTβ on the apical and basolateral membranes, respectively, which mediate transcellular transport of BAs.
BA Receptors in the Circulation
BA interaction with nuclear receptors
In 1999, FXR, previously an orphan nuclear receptor, was identified as a BA receptor. 14 Since then, three additional nuclear BA receptors were identified; rodent xenobiotic receptor, that is, pregnane (PXR), its human analog, steroid and xenobiotic receptor, and the vitamin D receptor (VDR). 15 , 16 , 17 VDR is classified as an endocrine nuclear receptor; FXR and PXR are classified as “adopted nuclear receptors.” 18 While these receptors are expressed primarily in tissues exposed to high‐BA concentrations such as liver, kidneys, and gastrointestinal tract, FXR expression was also detected in cardiovascular organs such as the coronary arteries, aorta, heart, and atherosclerotic arteries. 19 , 20 Amongst all nuclear receptors, FXR is the most studied. Whereas CDCA is the most potent endogenous FXR agonist, FXR is also activated by DCA and LCA. Interaction of conjugated BAs with FXR requires the presence of plasma membrane BA transporters. 14 CDCA binding to FXR triggers a conformational change that facilitates formation of a heterodimer with the retinoic acid receptor (RXR) in the cytoplasm, which then translocates into the nucleus and recognizes DNA‐sequence motif in the promoter region of FXR target genes. 21
VDR is also expressed in endothelial and vascular smooth cells and may play a role in atherosclerosis. 22 Structure‐function analysis revealed that 1,25(OH)2D3 (vitamin D) and LCA interact with different sets of amino acids in the ligand‐binding pocket of VDR resulting in distinct conformational responses to 1,25(OH)2D3 and LCA binding. 23 , 24 Docking models suggest that compared to 1,25(OH)2D3, LCA and 3‐keto‐LCA are weakly accommodated in the VDR ligand‐binding pocket; 23 , 24 such ligand and receptor‐site specific interactions may explain different biological actions of 1,25(OH)2D3 and LCA. Nonetheless, a direct impact of LCA‐mediated regulation of cardiovascular function has yet to be demonstrated.
PXR plays a major role in xenobiotic metabolism and was shown to act as an LCA sensor. 25 Similar to FXR, PXR is expressed predominantly in the gastrointestinal tract and liver and forms a heterodimer with RXR to regulate gene transcription. 26 A recent report suggested that PXR is expressed in mesenteric arteries and may contribute to vascular adaptation during pregnancy. 27 In view of this single report, the role of PXR in vascular tone remains preliminary. Studies elucidating the interaction of PXR with BAs in regulating cardiac function are lacking.
BA Interaction with Plasma Membrane GPCRs
BAs interact with two GPCRs—muscarinic receptors and TGR5. Muscarinic receptors are widely expressed in the body, including all regions of the gastrointestinal tract, intestinal smooth muscle, and the central nervous system. In 1998, Raufman and colleagues reported that conjugated secondary BAs interact functionally with muscarinic receptors. Five muscarinic receptors (M1 to M5) couple to different G proteins (M1,3,5 are coupled to Gαq/G11 and M2,4 to Gαi/G0). 28 In CHO cells expressing rat M3 muscarinic receptors (M3R), DCA, LCA, and their taurine and glycine conjugates inhibited binding of a muscarinic radioligand, 3 [H]N‐methylscopolamine. Similarly, DCT and DCG inhibited acetylcholine (ACh)‐induced postreceptor signaling (increase in inositol phosphate formation and MAPK phosphorylation). 29 , 30 Computer modeling revealed that BA and ACh molecular surface structures show striking similarities; 29 the charge distribution on the LCT amide side chain closely resembles the charge distribution on ACh. Hence, LCT structure favors interaction with M3R. Khurana and colleagues reported that conjugated LCA and DCA interact with rat and human (M3R). 29 , 30 , 31 , 32 A recent study indicates that CT regulates cardiac contractility by interacting with M2 muscarinic receptors; 33 M2 and M3 muscarinic receptors are the major subtypes that regulate cardiac function. The role of M3R in BA‐mediated modulation of cardiac function was not investigated.
In 2002, TGR5, a Gαs‐protein‐coupled receptor that is activated by conjugated and free BAs, was discovered. 34 Gαs‐coupled receptors stimulate cAMP synthesis and, via PKA, activate a cAMP response element binding protein that induces expression of many genes. In cell culture, LCA and DCA were the most efficacious activators of TGR5. 34 TGR5 is widely expressed, with the greatest expression in gallbladder, spleen, intestine, adipose tissue, and immune cells. In thermogenic tissues (brown adipose tissue and muscle), BA–TGR5 interaction increases energy expenditure. 35 Recently, TGR5 expression was identified in hepatic sinusoidal endothelial cells and cardiomyocytes. 36 , 37
Nonreceptor‐Mediated BA Actions
In addition to GPCRs and nuclear receptors, BAs interact with large conductance Ca2+‐activated K+ (BKCa) channels that regulate arterial tone. Vascular smooth muscle stretch activates BKCa to favor hyperpolarization, vessel relaxation, and preservation of tissue perfusion. That mechanism has been proposed to function as a brake on myogenic constriction and local blood flow. 38 It appears that BAs activate these channels directly to a degree that is inversely related to the number of hydroxyl groups in the BA molecule. 39 Various aspects of this interaction such as the effect of BA conjugation and conformational changes in channel structure remain to be elucidated. A summary of BA receptors, their expression in cardiovascular tissue, and their role in BA‐mediated regulation of cardiovascular function are provided in Table 2 .
Table 2.
Expression of BA receptors that regulate cardiovascular function.
| BA receptors | Tissue type | Direct BA action demonstrated | References |
|---|---|---|---|
| Nuclear receptors | |||
| FXR | Endothelial cells | Yes | 17, 72 |
| Vascular smooth muscle cells | Yes | 74, 75, 76 | |
| Cardiomyocytes | No | 17 | |
| PXR | Mesenteric arteries | No | 24 |
| VDR | Cardiomyocytes | No | 49 |
| G‐Protein‐coupled Receptors | |||
| TGR5 | Hepatic sinusoidal endothelial cells | Yes | 33 |
| Cardiomyocytes | No | 34 | |
| M2R | Cardiomyocytes | Yes | 30 |
| M3R | Endothelial cells | Yes | 66 |
| Potassium channels | |||
| BKCa | Vascular smooth muscle cells | Yes | 36, 63 |
BAs and Cardiac Function
Effects of BAs on cardiac function may be categorized as indirect and direct. Indirect effects involve BA and cholesterol metabolic pathways that regulate blood cholesterol levels, atherosclerotic plaque formation, and myocardial function. Direct effects require interaction of BAs with myocytes, thereby influencing myocardial conduction and contraction; these actions may be receptor dependent or independent. A summary of the effects of specific BAs on cardiovascular tissue is outlined in Table 3 .
Table 3.
Summary of the effects of bile acids on cardiovascular tissue
| BA/ligand | Tissue/model | Effect | Signaling/mediator | References |
|---|---|---|---|---|
| DCT | Rat cardiomyocyte | ↓ Contraction | Not known | 43 |
| CA | Rat heart | Bradycardia | ↓ Cholinergic stimulation | 44, 45 |
| CT | Rat cardiomyocyte; rabbit sinoatrialnode | ↓ action potential duration | ↓ inward Na+ and Ca2+ currents, and ↑ outward K+ currents | 46, 47 |
| CT,CG | Neonatal rat cardiomyocytes | ↓ contraction rate, amplitude and synchronization | Altered intracellular calcium dynamics; M2R‐mediated ↓ cAMP | 33, 49, 50 |
| UDCA | Mouse heart | ↓ allograft rejection | Immune‐mediated | 58, 59 |
| UDCA | Rat cardiac ischemia‐reperfusion model | ↓ myocyte apoptosis | Activation of PI3K‐AKT | 60 |
| DCT, CDCT | Rat aorta, portal vein, mesenteric and carotid arteries | Vasodilation | M3R‐mediated NO release;? VDCC activation | 32, 63, 64 |
| DCA, DCT, CDCA | Endothelial cells | NO and K+ current generation | ↑ cytoplasmic [Ca2+] | 68 |
| LCT, CT, CDCT | Hepatic sinusoidal endothelial cells | NOS up‐regulation and NO generation | TGR5‐mediated cAMP generation | 36 |
| LCA | Cerebral arteries | Vasodilation | BKCa activation | 39, 66 |
| CDCA | Endothelial cells | NOS up‐regulation | FXR activation | 75 |
| GW4064 | Rabbit mesenteric arteries | ↓ NO sensitivity | ↓ generation of cGMP | 76 |
| CDCA | Rat pulmonary artery endothelial cells | ↓ ET‐1 expression | FXR activation | 77 |
| CDCA GW4064 | Rat aortic smooth muscle cells | ↑ AT2R expression | FXR activation | 78 |
| 6ECDCA GW4064 | Rat and human aortic smooth muscle cells | ↓ inflammation and migration | ↓ IL‐1β ‐mediated iNOS and COX2 expression | 79 |
| INT‐747 | ApoE–/– mice | ↓ aortic plaque area and calcification | ↓ expression of IL‐1β, IL‐6 and CD11b | 80, 81 |
| CDCA | Endothelial cells; human esophageal cancer xenograft | ↑ angiogenesis | FXR activation; COX‐2‐dependent VEGF production | 88, 89 |
| UDCA | Chick embryo CAM; laser‐induced CNV | ↓ angiogenesis | Not known | 90, 91 |
↓: decreased; ↑: increased; INT‐747 is a synthetic CDCA derivative. GW4064 and 6α‐ethyl‐chenodeoxycholic acid (6ECDCA) are synthetic FXR agonists; CAM: chorioallantoic membrane assay; CNV: choroidalneovascularization ; VDCC: Voltage‐dependent calcium channels
Human diseases associated with elevated serum BAs (>200–400 μM) and altered cardiac function include ICP, obstructive jaundice, chronic viral hepatitis, and cirrhosis. 4 , 40 ICP is complicated by fetal distress, preterm labor, and intrauterine death. In the fetus, both bradycardia and tachycardia ≤100 or ≥180 beats/minute) are observed. 40 Experimental and clinical studies indicate that cirrhosis is associated with impaired myocardial contractility and electrophysiological abnormalities. The syndrome of “cirrhotic cardiomyopathy” is discussed elsewhere. 41
Clinical observations regarding the effect of bile components on the heart are not new. In 1863, Röhrig proposed that BAs might be responsible for jaundice‐associated bradycardia. 42 In 1909, John King reported that in dogs, bile pigments such as biliverdin, rather than BAs, induce an atropine sensitive bradycardia and hypotension. 42 Such earlier reports suggested that bile constituents have cardiovascular effects and implicated cholinergic mechanisms. In the early 1980s, the effect of individual BAs on cardiac function was demonstrated, both in vitro and in vivo. DCT and serum from bile duct ligated rats reduced spontaneous contraction of cultured rat cardiac myocytes in a concentration‐ and time‐dependent manner. 43 Compared to controls, 20 μM DCT reduced the rate of myocyte contraction within 2 hours (86 ± 6 and 67 ± 7 beats/minute, respectively) and by 24 hours, the rate was reduced by 60%. Higher DCT concentrations, that is, 40 and 60 μM, abolished cardiac myocyte contraction. Further, in rats, bradycardia was observed 7 days after bile duct ligation, and infusion of CA induced dose‐dependent bradycardia that was inhibited by vagotomy and atropine. 44 , 45 These early studies provided initial evidence that BAs can exert a direct effect on cardiac function.
Binah et al. demonstrated that BAs reduce the duration of the action potential in ventricular myocytes. 46 In rat, voltage‐clamp experiments demonstrated that 1 μM CT reduced the slow inward Na+ and Ca2+ currents and increased the outward K+ current. Similarly, experiments with the rabbit sino‐atrial node demonstrated that CT (30–300 μM) slowed the sinus rate by reducing diastolic depolarization accompanied by similar depression of ion fluxes. 47 Mechanistic insights into BA‐induced effects on cardiac function in ICP were provided in neonatal rat cardiomyocytes by Julia Gorelik’s laboratory. 48 CT and CG (both 1 mM) reduced cardiomyocyte contraction rates by 46% and 11%, respectively. CT reduced the contraction amplitude and prevented cardiomyocyte synchronization. By comparison, CG did not affect those characteristics but, at 300 μM, altered calcium dynamics, characterized by a biphasic change in calcium wave frequency or cellular calcium overload. 49 , 50 These data demonstrated that BAs alter the “pacemaker” function of cardiac myocytes.
Cardiac myocytes express FXR and VDR. Since FXR and VDR are transcription factors, it is unlikely that they account for immediate changes in myocyte function that occur with BA stimulation. In contrast, BA–GPCR interaction provides a more likely mechanism. In a variety of cell systems, BAs interact with muscarinic receptors to activate intracellular signaling. 51 CT interacts with muscarinic receptor subtype 2 (M2R) on neonatal rat cardiac myocytes to reduce intracellular cAMP and exert a negative chronotropic effect. 33 In those cells, pharmacological inhibition and siRNA‐knockdown of M2R completely abolished the effects of CT on contraction, calcium transient amplitude, and synchronization, without implicating participation of FXR and TGR5.
Like M2R, the GPCR TGR5, is expressed on cardiac myocytes. In vitro, in both neonatal mouse cardiomyocytes and cardiomyocytes isolated in biliary fibrosis, LCA and CDCT stimulated phosphorylation of AKT and GSK‐β suggesting TGR5‐mediated activation. 37 However, these observations only provided a temporal association between TGR5 and modulation of cardiac function. More definitive experiments that establish a direct effect are lacking.
VDR may play a role in regulating cardiac function. Cardiac myocytes isolated from VDR knockout mice demonstrated accelerated contraction and relaxation rates compared to wild type controls. 52 1,25(OH)2D3 altered the contractility and rate of relaxation of cardiac myocytes. 52 Although VDR can interact with LCA and keto‐LCA, it is not clear that this plays a role in BA‐mediated effects. 53 Further, to postulate access to nuclear receptors, such an interaction requires transport of BAs into cardiomyocytes. Cardiac myocytes express anion transporters such as BSEP, multidrug resistance gene 3 (MDR3), and OATP; however, their role in BA‐mediated regulation of cardiac function is unknown. 54
Indirect evidence suggests that additional mechanisms may mediate BA‐induced changes in cardiac function. In rabbits, using a complicated surgical model whereby biliovenous catheterization, cholecystoduodenal fistula, or exteriorization of biliary drainage was achieved after bile duct ligation; Martinez‐Rodenas et al. demonstrated that bile constituents increase serum levels of atrial natriuretic peptide (ANP). 55 In patients with obstructive jaundice, investigators from the same group demonstrated that elevated ANP levels were associated with myocardial dysfunction. Internal biliary drainage not only reduced ANP levels but also improved cardiac function. 56 Despite these studies, evidence implicating specific BAs in ANP release from cardiomyocytes has yet to be demonstrated.
In nonhepatobiliary tissue, UDCA and its taurine‐conjugate are the most extensively studied BAs. UDCA and UDCT inhibit apoptosis and promote cell survival, although the mechanisms remain to be determined. 57 A few studies have evaluated their role on cardiomyocyte survival. After heart transplantation, treatment with UDCA may reduce the risk of acute cardiac rejection. 58 , 59 In animal models, transfer of splenocytes or CD4+ cells from UDCA‐treated allograft recipients resulted in indefinite survival of allografts in naive secondary recipients. This effect is most likely due to UDCA‐mediated regulation of immune cell function rather than to a direct effect on cardiomyocytes. In another experimental system, pretreatment of rats with UDCA (40 mg/kg over 30 minutes) protected the heart against ischemia‐reperfusion injury by activating the PI3K‐AKT survival pathway and reducing cardiomyocytes loss and infarct size. 60 Hence, UDCA may have cardioprotective effects but the relative roles of VDR, TGR5, or muscarinic receptors are unclear.
In summary, these observations suggest that BAs regulate cardiac function by interacting with more than one receptor and more than one cell type, though mechanistic insights are limited. The availability of knockout mice (e.g., M2R−/−, M3R−/−, FXR−/−, VDR−/−, and TGR5−/− mice) may help elucidate receptor specific effects of BAs on cardiac function. However, since nuclear receptors such as FXR and PXR also influence BA metabolism, cardiomyocyte‐selective gene deletion may be required to determine their cell‐specific roles.
BAs and Vascular Function
The effect of BAs on vascular tone has been evaluated in a variety of vascular beds. While earlier studies demonstrated the impact of BA infusion and bile duct ligation on blood pressure, later studies demonstrated the role of BAs in isolated vascular preparations and cell culture models.
In 1983, Lautt and colleagues demonstrated in cats that intravenous administration of CT‐induced vasodilation of mesenteric and hepatic arteries. 61 Studies using rat portal veins, isolated 3–5 days after bile duct ligation, demonstrated an attenuated contractile response to noradrenalin. 62 Similarly, isolated rat hind limb preparations incubated with CT had blunted noradrenalin‐mediated contraction. Pak et al. evaluated the effects of dihydroxylated BAs (i.e., UDCT, CDCT, and DCT) on vascular tone. 63 In rats, intravenous infusion of increasing doses of CDCT and DCT increased mesenteric arterial blood flow and reduced arterial pressure; UDCT infusion had no effect. In isolated preparations of mesenteric and carotid arteries and portal vein, CDCT and DCT induced dose‐dependent (1 μM to 10 mM) vasodilation, mimicking in vivo experiments. Again, UDCT had no effect. Further investigation suggested that receptor‐operated and voltage‐gated calcium channel modulation may play a role in BA‐induced vasodilation. 64
Role of Endothelium
The role of endothelium in BA‐mediated vasodilation has been investigated with varying results. Pak et al. reported that endothelium denudation did not alter DCT‐mediated vasodilation of rat mesenteric arteries. 64 Ljubuncic et al. made similar observations; endothelial denudation did not alter DCA‐mediated vasodilation of rat aorta. 65 Both studies observed that incubation of endothelium‐intact vessels with L‐NAME, a nitric oxide synthase (NOS) inhibitor, did not alter DCT‐ and DCA‐mediated vasodilation of rat mesenteric arteries and abdominal aorta, respectively. 64 , 65 Bukiya et al. found that LCA mediates nonendothelial‐dependent vasodilation of cerebral arteries by directly activating BKCa channels on vascular smooth muscle. 66 In contrast, noradrenalin‐ and 5‐hydroxytryptamine‐mediated contraction was accentuated in endothelium‐denuded renal arteries from mongrel dogs with bile duct ligation. 67 In the same preparation, ACh‐mediated vasodilation was abolished by endothelial removal. Nakajima et al. employed fluorescence microscopy to determine the effect of BAs on bovine aortic and human umbilical vein endothelial cells. 68 DCA, CDCA, and DCT induced concentration‐dependent increase in cytoplasmic Ca2+ ([Ca2+]i) and NO production.
Investigations in our laboratory favor a role for the endothelium in BA‐mediated vasodilation. In rat and mouse aorta, we demonstrated that DCT mediates concentration‐dependent vasodilation that is attenuated by endothelium denudation or incubation with L‐NAME. 32 Further, DCT‐induced vasodilation (0.1 μM–1 mM) was reduced by a synthetic ACh: BA hybrid that acts as an M3R antagonist. 69 Similarly, ACh‐ and DCT‐mediated relaxation of mouse aortic rings was reduced by M3R gene ablation. 32 These data support the interpretation that systemic vasodilatory actions of DCT are mediated by an M3R‐dependent mechanism. The role of TGR5 in BA‐mediated vasodilation is less clear. One report suggests that TGR5 is expressed in hepatic sinusoidal endothelial cells. In these cells, LCT, CT, and CDCT increased cAMP‐induced endothelial NO synthase (eNOS) mRNA expression, eNOS Ser1177 phosphorylation, and NO production. 36 However, the study did not directly link TGR5 stimulation to the BA‐mediated eNOS activation. Absence of specific TGR5 inhibitors is a major limitation and studies using TGR5 knockdown are lacking. The expression and role of TGR5 in other vascular beds is not known.
Role of Potassium Channels
BA‐mediated activation of Ca2+‐dependent K+ currents in cultured endothelial cells has been demonstrated. 68 BAs also activate Ca2+‐dependent K+ channels in vascular smooth muscle cells. In rabbit, mesenteric artery smooth muscle cells, using patch‐clamp techniques, Dopico et al. demonstrated that BAs reversibly activate BKCa channels. 39 In pressurized cerebral resistance arteries, endothelium‐independent vasodilation by LCA was blocked by the BKCa channel blocker, iberiotoxin. LCA failed to stimulate vasodilation in arteries from BK β‐1 subunit knockout mice implicating an important role for the BK β‐1 subunit in LCA activation. 66 It appears that LCA effects are mediated by interaction with the second transmembrane domain of the BK β‐1 subunit. 70 , 71 Collectively, these data demonstrated that BAs stimulate receptor‐independent vasodilation by activating smooth muscle K+ channels.
Role of Nuclear Receptors
Studies of the role of FXR in vascular function were sparked by identification of its expression in the vasculature. 20 Generation of FXR and PXR null mice broadened the scope of these investigations, but study of Fxr−/− mice, in particular, must be interpreted with caution. fxr (and pxr) directly impact BA metabolism, and Fxr−/− mice have eight‐fold elevations of BA concentrations. 72 , 73 , 74
Since nuclear receptors are transcription factors, it is expected that they regulate vascular function by altering the expression of vasoactive molecules and other receptors. In cultured endothelial cells, CDCA and GW4064 (a chemical FXR agonist) increased eNOS expression. 75 In contrast, chronic stimulation of FXR impaired NO‐dependent vasodilation due to blunted increase of cGMP in smooth muscle cells. 76 These observations suggest that short‐ and long‐term FXR stimulation have differential effects on NO generation and sensitivity. Further, in endothelial cells, FXR ligands increased FXR expression and reduced ET‐1 expression; 77 in vascular smooth muscle cells, FXR ligands increased angiotensin type 2 receptor expression. 78 These data provide evidence that in vascular tissue, FXR, in addition to regulating its own expression, alters the generation of vasodilatory and vasoconstrictor molecules.
FXR also appears to regulate vascular inflammation and, thereby, may affect generation of atherosclerosis. In vascular smooth muscle cells, FXR ligands inhibited IL‐1β‐mediated induction of iNOS and COX‐2. 79 Apolipoprotein E‐deficient (ApoE−/−) mice fed INT‐747, a CDCA derivative, for 12 weeks had 95% reduction in aortic plaque formation; reduced aortic expression of IL‐1β, IL‐6, and CD11b mRNA; and increased expression of FXR. 80 FXR was also induced during osteogenic transformation of bovine calcifying vascular cells (CVCs) and in the aorta of partially nephrectomized ApoE−/− mice. 81 In CVCs, INT‐747 inhibited phosphate‐induced mineralization and triglyceride accumulation, a feature of atherosclerotic calcification. FXR inhibition augmented mineralization of CVCs and blocked the anticalcific effect of INT‐747. It is not clear if TGR5 plays a role in some effects of INT‐747 in these models; the role of FXR in atherosclerosis appears to be complex and needs to be further elucidated.
Interestingly, Fxr−/− mice have a proatherogenic lipid profile but do not display enhanced atherosclerosis, even when fed a high‐fat/high‐cholesterol diet. 72 , 82 , 83 Fxr−/–male mice generated on an apoe−/− background and fed an atherogenic diet had more severe atherosclerosis and reduced survival compared to controls. 82 In contrast, Guo et al. reported fewer atherosclerotic lesions in female Fxr−/−/apoe−/− mice. 84 These conflicting observations suggest that studies are needed to adequately assess the functional impact of long‐term FXR stimulation and inhibition, and further in vivo studies, using organ‐specific gene ablation, are required to determine the impact of BA–FXR interactions on atherosclerosis. In addition, the role of vascular FXR in regulating vascular tone in cirrhosis or ICP has not been evaluated. Specifically, mice with selective knockout of Fxr in endothelial and vascular smooth muscle may be needed to delineate the role of vascular FXR in BA‐mediated changes in vascular tone.
Compared to FXR, the roles of PXR and VDR in vascular function have received little attention. PXR mRNA is expressed in mouse mesenteric arteries, but it is not clear if PXR is expressed in endothelial cells, vascular smooth muscle cells, or both. 27 Compared to nonpregnant mice, arteries from pregnant mice have reduced phenylephrine‐induced constriction and enhanced bradykinin‐induced vasodilation, an effect that is eliminated by PXR knockout. 27 Further experiments suggested that PXR‐dependent increases in vasorelaxation may be caused by activation of cytochrome P450 epoxygenases. In contrast, no studies evaluated the role of VDR in vascular tone. Although VDR activators reduce vascular calcification, a role for BAs has not been shown. 22
Finally, evidence regarding BA‐mediated neural regulation of vascular tone is limited. Patients with obstructive jaundice have decreased sympathetic and vagal components of the baroreflex that correlates inversely with plasma ANP levels. 85 Preserved baroreflex is associated with improved survival in sepsis. 86 However, these observations are preliminary and studies in isolated arterial preparations are lacking.
BAs and Neovascularization
Neovascularization in response to injury results in organ repair or dysfunction. Cirrhosis is characterized by intrahepatic vascular remodeling with capillarization of sinusoids, formation of intrahepatic shunts, and extrahepatic portal‐systemic collaterals. 87 Due to the shift of the BA pool to the systemic circulation, the role of BAs in neovascularization associated with cirrhosis is plausible. In vitro, CDCA increased endothelial cell motility, matrigel tube formation, and focal adhesion plaques; all were inhibited by FXR siRNA. 88 In a xenograft mouse model of human esophageal cancer, intraperitoneal administration of CDCA (50 mg/kg twice weekly for 5 weeks) increased tumor volume, neovascularization, and COX‐2‐dependent production of vascular endothelial growth factor. 89 In contrast, in chick embryo chorioallantoic membrane assay, UDCA‐inhibited angiogenesis. 90 Further, in a rat model of laser‐induced choroidal neovascularization (CNV), intraperitoneal administration of UDCA (500 mg/kg) and UDCT (100 mg/kg) for 14 days reduced fluorescein leakage and the size of CNV lesions. 91 Contrasting effects of CDCA and UDCA on promotion and inhibition of angiogenesis, respectively, suggest that different BAs may have opposing effect on neovascularization. However, studies evaluating the role of BAs in neovascularization in animal models of cirrhosis are lacking.
Conclusions and Perspective
BAs regulate cardiovascular function by receptor‐dependent and ‐independent mechanisms and can modify vascular tone byinteracting with muscarinic receptors and transcription factors such as FXR and PXR ( Figure 2 ). Although some studies failed to demonstrate direct interaction of BAs with FXR, the bulk of evidence indicates that short‐term effects of BAs induce vasodilation and long‐term effects modulate transcription of vasoactive molecules. These studies defined hormone‐like actions of BAs and their pharmacological potential to regulate cardiovascular function. Many questions remain regarding the interaction of BAs with cardiovascular tissue. For example, while FXR activation modifies expression of vasoactive and proatherogenic molecules, the mechanistic role of BAs other than CDCA remains to be defined. FXR is activated by unconjugated CDCA and DCA, whereas conjugated forms of these BAs activate FXR only in cells that coexpress BA transporters. While expression of BA transporters such as MDR3 and OATP in cardiac myocytes is established and a few reports suggest that similar anion transporters are expressed in choroid plexus endothelium, 92 , 93 their expression and role in the systemic vasculature remains to be elucidated. Similarly, the role of PXR, VDR, and TGR5 in regulating vascular and cardiac function has received minimal attention. Despite expression of VDR and TGR5 in cardiovascular tissue, evidence regarding BAs interaction with these receptors in regulation of cardiovascular function is at best circumstantial.
Figure 2.
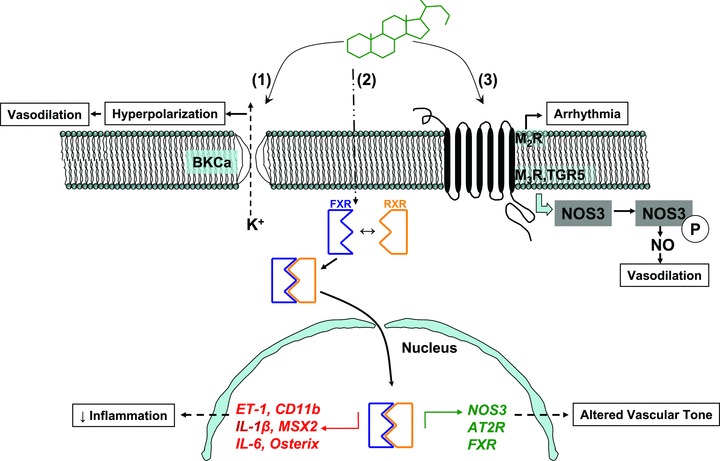
This cartoon outlines the interaction of BAs with various cellular molecules. (A) Nonreceptor‐mediated interaction of BAs with BKCa that leads to K+ efflux and hyperpolarization and relaxation of vascular smooth muscle. (B) BA interaction with nuclear receptors. In the cytoplasm, BA binding to FXR triggers dimerization with RXR that leads to translocation of FXR into the nucleus, where FXR binds to target gene regulatory elements. Downward (red) and upward (green) arrows indicate down‐regulation and up‐regulation of molecules, respectively. MSX2 and osterix are osteogenic transcription factors. (C) BA interaction with GPCRs that can lead to negative chronotropic response (for M2R) in cardiac myocytes and NO generation (for M3R and TGR5) in endothelial cells. AT2R: angiotensin type 2 receptor ; BKCa: big potassium, calcium‐activated channels; CD 11b: cluster of differentiation 11b; ET‐1: endothelin‐1; IL‐1,6: interleukin‐1 and ‐6); M2R: muscarinic receptor subtype 2; M3R: muscarinic receptor subtype 3); MSX2: muscle segment homebox 2; NOS3: nitric oxide synthase 3.
The ability of BAs to interact with transcription factors, a variety of GPCRs (TGR5, M3R, and M2R) and potassium channels indicates that BAs are promiscuous signaling molecules. While such interactions may mediate acute events such as vasodilation, long‐term events have not been adequately investigated. Studies are needed to provide mechanistic insight into long‐term effects of GPCR–BA interactions and their role in resetting cardiovascular function. These insights may be particularly relevant for disorders such as cirrhosis and ICP, where serum BAs concentrations can be very elevated. Amidation and hydroxylation play major roles in BA solubility and interaction. The effect of BA hydroxylation on BK channel activity was elegantly demonstrated by Dopico et al. 39 Similarly, the effect of hydroxylation on BA interaction with muscarinic receptors has been explored. 29 However, the impact of amidation on BA interaction with vascular transcription factors is unknown.
Vascular beds differ in the mechanisms that regulate vascular tone. While TGR5 appears to play a role in regulating the hepatic microcirculation, the impact of BAs in regulating vascular tone in the renal or cerebral circulation is not clear. Renal failure is a major cause of mortality in end‐stage liver disease and FXR is expressed in renal tissue; however, few studies have identified a role for BAs in regulation of the renal circulation. 94 It is clear that BAs regulate cardiovascular function by multiple mechanisms. In view of the findings, such as, cardiovascular expression of FXR, PXR and TGR5, and BA‐mediated activation of K+ channels, the use of tissue‐selective knockout mice is likely to provide additional mechanistic insight into the actions of BAs in cardiovascular tissue.
Acknowledgements
Sandeep Khurana is supported by NIH grant K08 DK081479. Thomas L. Pallone is supported by NIH grants R01DK067621, R37DK042495, and P01HL078870. Jean‐Pierre Raufman is supported by NIH grants R01CA107345 and R01CA120407.
References
- 1. Muller M, Jansen, PLM . Mechanisms of bile secretion In: Zakim D, Boyer T, editors. Hepatology: A Textbook of Liver Disease, Vol 1, 4th ed Philadelphia , PA : Saunders Publishing; 2003: 271–290. [Google Scholar]
- 2. Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009; 32(Suppl2): S237–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vallim TQ, Edwards PA. Bile acids have the gall to function as hormones. Cell Metab. 2009; 10(3): 162–164. [DOI] [PubMed] [Google Scholar]
- 4. Ohkubo H, Okuda K, Iida S, Ohnishi K, Ikawa S, Makino I. Role of portal and splenic vein shunts and impaired hepatic extraction in the elevated serum bile acids in liver cirrhosis. Gastroenterology. 1984; 86(3): 514–520. [PubMed] [Google Scholar]
- 5. Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008; 57(9): 1300–1314. [DOI] [PubMed] [Google Scholar]
- 6. Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004; 40(2): 467–474. [DOI] [PubMed] [Google Scholar]
- 7. Zecca E, De Luca D, Marras M, Caruso A, Bernardini T, Romagnoli C. Intrahepatic cholestasis of pregnancy and neonatal respiratory distress syndrome. Pediatrics. 2006; 117(5): 1669–1672. [DOI] [PubMed] [Google Scholar]
- 8. Haslewood GA. Bile salt evolution. J Lipid Res. 1967; 8(6): 535–550. [PubMed] [Google Scholar]
- 9. Hofmann AF, Sjovall J, Kurz G, Radominska A, Schteingart CD, Tint GS, Vlahcevic ZR, Setchell KD. A proposed nomenclature for bile acids. J Lipid Res. 1992; 33(4): 599–604. [PubMed] [Google Scholar]
- 10. Fuchs M. Bile acid regulation of hepatic physiology: III. Regulation of bile acid synthesis: past progress and future challenges. Am J Physiol Gastrointest Liver Physiol. 2003; 284(4): G551–G557. [DOI] [PubMed] [Google Scholar]
- 11. Colpaert CG, Vandenbroucke MP, Andries LJ, Van Marck EA, Brutsaert DL. Role of endocardial endothelium in the positive inotropic effect of cholic acid in isolated myocardium. J Cardiovasc Pharmacol. 1992; 20(Suppl12): S179–S182. [DOI] [PubMed] [Google Scholar]
- 12. Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009; 50(12): 2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007; 24(10): 1803–1823. [DOI] [PubMed] [Google Scholar]
- 14. Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999; 284(5418): 1365–1368. [DOI] [PubMed] [Google Scholar]
- 15. Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998; 92(1): 73–82. [DOI] [PubMed] [Google Scholar]
- 16. Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow‐Backman M, Ohlsson R, Postlind H, Blomquist P, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998; 95(21): 12208–12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kliewer SA, Willson TM. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 2002; 43(3): 359–364. [PubMed] [Google Scholar]
- 18. Mukherjee S, Mani S. Orphan nuclear receptors as targets for drug development. Pharm Res. 2010; 27(8): 1439–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res.c2008; 18(11): 1087–1095. [DOI] [PubMed] [Google Scholar]
- 20. Bishop‐Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci U S A. 2004; 101(10): 3668–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X‐activated receptor. J Biol Chem. 2000; 275(14): 10638–10647. [DOI] [PubMed] [Google Scholar]
- 22. Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008; 19(8): 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi M, Yamamoto K, Itoh T, Makishima M, Mangelsdorf DJ, Moras D, DeLuca HF, Yamada S. Interaction between vitamin D receptor and vitamin D ligands: two‐dimensional alanine scanning mutational analysis. Chem Biol. 2003; 10(3): 261–270. [DOI] [PubMed] [Google Scholar]
- 24. Adachi R, Shulman AI, Yamamoto K, Shimomura I, Yamada S, Mangelsdorf DJ, Makishima M. Structural determinants for vitamin D receptor response to endocrine and xenobiotic signals. Mol Endocrinol. 2004; 18(1): 43–52. [DOI] [PubMed] [Google Scholar]
- 25. Staudinger JL, Goodwin B, Jones SA, Hawkins‐Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001; 98(6): 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frank C, Makkonen H, Dunlop TW, Matilainen M, Vaisanen S, Carlberg C. Identification of pregnane X receptor binding sites in the regulatory regions of genes involved in bile acid homeostasis. J Mol Biol. 2005; 346(2): 505–519. [DOI] [PubMed] [Google Scholar]
- 27. Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension. 2007; 49(2): 328–333. [DOI] [PubMed] [Google Scholar]
- 28. Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007; 6(9): 721–733. [DOI] [PubMed] [Google Scholar]
- 29. Raufman JP, Chen Y, Cheng K, Compadre C, Compadre L, Zimniak P. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur J Pharmacol. 2002; 457(2–3): 77–84. [DOI] [PubMed] [Google Scholar]
- 30. Raufman JP, Chen Y, Zimniak P, Cheng K. Deoxycholic acid conjugates are muscarinic cholinergic receptor antagonists. Pharmacology. 2002; 65(4): 215–221. [DOI] [PubMed] [Google Scholar]
- 31. Raufman JP, Zimniak P, Bartoszko‐Malik A. Lithocholyltaurine interacts with cholinergic receptors on dispersed chief cells from guinea pig stomach. Am J Physiol. 1998; 274(6 Pt 1): G997–G1004. [DOI] [PubMed] [Google Scholar]
- 32. Khurana S, Yamada M, Wess J, Kennedy RH, Raufman JP. Deoxycholyltaurine‐induced vasodilation of rodent aorta is nitric oxide‐ and muscarinic M(3) receptor‐dependent. Eur J Pharmacol. 2005; 517(1–2): 103–110. [DOI] [PubMed] [Google Scholar]
- 33. Sheikh Abdul Kadir SH, Miragoli M, Abu‐Hayyeh S, Moshkov AV, Xie Q, Keitel V, Nikolaev VO, Williamson C, Gorelik J. Bile acid‐induced arrhythmia is mediated by muscarinic M2 receptors in neonatal rat cardiomyocytes. PLoS One. 5(3): e9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al A G protein‐coupled receptor responsive to bile acids. J Biol Chem. 2003; 278(11): 9435–9440. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006; 439(7075): 484–489. [DOI] [PubMed] [Google Scholar]
- 36. Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, Haussinger D, Kubitz R. The G‐protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007; 45(3): 695–704. [DOI] [PubMed] [Google Scholar]
- 37. Desai MS, Shabier Z, Taylor M, Lam F, Thevananther S, Kosters A, Karpen SJ. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology. 2010; 51(6):2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, et al Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1‐subunit. J Physiol. 2009; 587(Pt 12): 3025–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dopico AM, Walsh JV, Jr ., Singer JJ. Natural bile acids and synthetic analogues modulate large conductance Ca2+‐activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol. 2002; 119(3): 251–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mays JK. The active management of intrahepatic cholestasis of pregnancy. Curr Opin Obstet Gynecol. 2010; 22(2): 100–103. [DOI] [PubMed] [Google Scholar]
- 41. Moller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010; 53(1): 179–190. [DOI] [PubMed] [Google Scholar]
- 42. King JH, Stewart HA. Effect of the injection of bile on the circulation. J Exp Med. 1909; 11(5): 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bogin E, Better O, Harari I. The effect of jaundiced sera and bile salts on cultured beating rat heart cells. Experientia. 1983; 39(11): 1307–1308. [DOI] [PubMed] [Google Scholar]
- 44. Joubert P. Cholic acid and the heart: in vitro studies of the effect on heart rate and myocardial contractility in the rat. Clin Exp Pharmacol Physiol. 1978; 5(1): 9–16. [DOI] [PubMed] [Google Scholar]
- 45. Joubert P. An in vivo investigation of the negative chronotropic effect of cholic acid in the rat. Clin Exp Pharmacol Physiol. 1978; 5(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 46. Binah O, Rubinstein I, Bomzon A, Better OS. Effects of bile acids on ventricular muscle contraction and electrophysiological properties: studies in rat papillary muscle and isolated ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1987; 335(2): 160–165. [DOI] [PubMed] [Google Scholar]
- 47. Kotake H, Itoh T, Watanabe M, Hisatome I, Hasegawa J, Mashiba H. Effect of bile acid on electrophysiological properties of rabbit sino‐atrial node in vitro. Br J Pharmacol. 1989; 98(2): 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gorelik J, Shevchuk A, de Swiet M, Lab M, Korchev Y, Williamson C. Comparison of the arrhythmogenic effects of tauro‐ and glycoconjugates of cholic acid in an in vitro study of rat cardiomyocytes. Bjog. 2004; 111(8): 867–870. [DOI] [PubMed] [Google Scholar]
- 49. Williamson C, Gorelik J, Eaton BM, Lab M, de Swiet M, Korchev Y. The bile acid taurocholate impairs rat cardiomyocyte function: a proposed mechanism for intra‐uterine fetal death in obstetric cholestasis. Clin Sci (Lond). 2001; 100(4): 363–369. [PubMed] [Google Scholar]
- 50. Gorelik J, Harding SE, Shevchuk AI, Koralage D, Lab M, de Swiet M, Korchev Y, Williamson C. Taurocholate induces changes in rat cardiomyocyte contraction and calcium dynamics. Clin Sci (Lond). 2002; 103(2): 191–200. [DOI] [PubMed] [Google Scholar]
- 51. Shah N, Khurana S, Cheng K, Raufman JP. Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol. 2009; 296(2): C221–C232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t‐tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149(2): 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002; 296(5571): 1313–1316. [DOI] [PubMed] [Google Scholar]
- 54. Gorelik J, Patel P, Ng’andwe C, Vodyanoy I, Diakonov I, Lab M, Korchev Y, Williamson C. Genes encoding bile acid, phospholipid and anion transporters are expressed in a human fetal cardiomyocyte culture. Bjog. 2006; 113(5): 552–558. [DOI] [PubMed] [Google Scholar]
- 55. Martinez‐Rodenas F, Pereira JA, Jimenez W, Gubern JM, Sitges‐Serra A. Circulating bile is the main factor responsible for atrial natriuretic peptide release in experimental obstructive jaundice. Br J Surg. 1998; 85(4): 480–484. [DOI] [PubMed] [Google Scholar]
- 56. Padillo J, Puente J, Gomez M, Dios F, Naranjo A, Vallejo JA, Mino G, Pera C, Sitges‐Serra A. Improved cardiac function in patients with obstructive jaundice after internal biliary drainage: hemodynamic and hormonal assessment. Ann Surg. 2001; 234(5): 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009; 50(9): 1721–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bahrle S, Szabo G, Stiehl A, Theilmann L, Dengler TJ, Zimmermann R, Kubler W. Adjuvant treatment with ursodeoxycholic acid may reduce the incidence of acute cardiac allograft rejection. J Heart Lung Transplant. 1998; 17(6): 592–598. [PubMed] [Google Scholar]
- 59. Zhang Q, Nakaki T, Iwami D, Niimi M, Shirasugi N. Induction of regulatory T cells and indefinite survival of fully allogeneic cardiac grafts by ursodeoxycholic acid in mice. Transplantation. 2009; 88(12): 1360–1370. [DOI] [PubMed] [Google Scholar]
- 60. Rajesh KG, Suzuki R, Maeda H, Yamamoto M, Yutong X, Sasaguri S. Hydrophilic bile salt ursodeoxycholic acid protects myocardium against reperfusion injury in a PI3K/Akt dependent pathway. J Mol Cell Cardiol. 2005; 39(5): 766–776. [DOI] [PubMed] [Google Scholar]
- 61. Lautt WW, Daniels TR. Differential effect of taurocholic acid on hepatic arterial resistance vessels and bile flow. Am J Physiol. 1983; 244(4): G366–G369. [DOI] [PubMed] [Google Scholar]
- 62. Bomzon A, Finberg JP, Tovbin D, Naidu SG, Better OS. Bile salts, hypotension and obstructive jaundice. Clin Sci (Lond). Aug 1984; 67(2): 177–183. [DOI] [PubMed] [Google Scholar]
- 63. Pak JM, Lee SS. Vasoactive effects of bile salts in cirrhotic rats: in vivo and in vitro studies. Hepatology. 1993; 18(5): 1175–1181. [PubMed] [Google Scholar]
- 64. Pak JM, Adeagbo AS, Triggle CR, Shaffer EA, Lee SS. Mechanism of bile salt vasoactivity: dependence on calcium channels in vascular smooth muscle. Br J Pharmacol. 1994; 112(4): 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ljubuncic P, Said O, Ehrlich Y, Meddings JB, Shaffer EA, Bomzon A. On the in vitro vasoactivity of bile acids. Br J Pharmacol. 2000; 131(3): 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large‐conductance Ca2+‐activated K+ channels and dilation in small, resistance‐size arteries. Mol Pharmacol. 2007; 72(2): 359–369. [DOI] [PubMed] [Google Scholar]
- 67. Utkan ZN, Utkan T, Sarioglu Y, Gonullu NN. Effects of experimental obstructive jaundice on contractile responses of dog isolated blood vessels: role of endothelium and duration of bile duct ligation. Clin Exp Pharmacol Physiol. 2000; 27(5–6): 339–344. [DOI] [PubMed] [Google Scholar]
- 68. Nakajima T, Okuda Y, Chisaki K, Shin WS, Iwasawa K, Morita T, Matsumoto A, Suzuki JI, Suzuki S, Yamada N, et al Bile acids increase intracellular Ca(2+) concentration and nitric oxide production in vascular endothelial cells. Br J Pharmacol. 2000; 130(7): 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cheng K, Khurana S, Chen Y, Kennedy RH, Zimniak P, Raufman JP. Lithocholylcholine, a bile acid/acetylcholine hybrid, is a muscarinic receptor antagonist. J Pharmacol Exp Ther. 2002; 303(1): 29–35. [DOI] [PubMed] [Google Scholar]
- 70. Bukiya AN, Vaithianathan T, Toro L, Dopico AM. The second transmembrane domain of the large conductance, voltage‐ and calcium‐gated potassium channel beta(1) subunit is a lithocholate sensor. FEBS Lett. 2008; 582(5): 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bukiya AN, Vaithianathan T, Toro L, Dopico AM. Channel beta2–4 subunits fail to substitute for beta1 in sensitizing BK channels to lithocholate. Biochem Biophys Res Commun. 2009; 390(3): 995–1000. [DOI] [PubMed] [Google Scholar]
- 72. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000; 102(6): 731–744. [DOI] [PubMed] [Google Scholar]
- 73. Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009; 50(8): 1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009; 89(1): 147–191. [DOI] [PubMed] [Google Scholar]
- 75. Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, Zhang LM, Pitt BR, Xie W, Li S. FXR‐mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc Res. 2008; 77(1): 169–177. [DOI] [PubMed] [Google Scholar]
- 76. Kida T, Murata T, Hori M, Ozaki H. Chronic stimulation of farnesoid X receptor impairs nitric oxide sensitivity of vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009; 296(1): H195–H201. [DOI] [PubMed] [Google Scholar]
- 77. He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, et al Downregulation of endothelin‐1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006; 98(2): 192–199. [DOI] [PubMed] [Google Scholar]
- 78. Zhang Q, He F, Kuruba R, Gao X, Wilson A, Li J, Billiar TR, Pitt BR, Xie W, Li S. FXR‐mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc Res. 2008; 77(3): 560–569. [DOI] [PubMed] [Google Scholar]
- 79. Li YT, Swales KE, Thomas GJ, Warner TD, Bishop‐Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007; 27(12): 2606–2611. [DOI] [PubMed] [Google Scholar]
- 80. Mencarelli A, Renga B, Distrutti E, Fiorucci S. Antiatherosclerotic effect of farnesoid X receptor. Am J Physiol Heart Circ Physiol. 2009; 296(2): H272–H281. [DOI] [PubMed] [Google Scholar]
- 81. Miyazaki‐Anzai S, Levi M, Kratzer A, Ting TC, Lewis LB, Miyazaki M. Farnesoid X receptor activation prevents the development of vascular calcification in ApoE‐/‐ mice with chronic kidney disease. Circ Res. 2010; 106(12): 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E‐deficient mice. J Lipid Res. 2005; 46(12): 2595–2604. [DOI] [PubMed] [Google Scholar]
- 83. Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, Edwards PA. FXR deficiency causes reduced atherosclerosis in Ldlr‐/‐ mice. Arterioscler Thromb Vasc Biol. 2006; 26(10): 2316–2321. [DOI] [PubMed] [Google Scholar]
- 84. Guo GL, Santamarina‐Fojo S, Akiyama TE, Amar MJ, Paigen BJ, Brewer B Jr, Gonzalez FJ. Effects of FXR in foam‐cell formation and atherosclerosis development. Biochim Biophys Acta. 2006; 1761(12): 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Song JG, Cao YF, Sun YM, Ge YH, Xu XW, Yang LQ, Liu ZQ, Song SL, Yu WF. Baroreflex sensitivity is impaired in patients with obstructive jaundice. Anesthesiology. 2009; 111(3): 561–565. [DOI] [PubMed] [Google Scholar]
- 86. Shen FM, Guan YF, Xie HH, Su DF. Arterial baroreflex function determines the survival time in lipopolysaccharide‐induced shock in rats. Shock. 2004; 21(6): 556–560. [DOI] [PubMed] [Google Scholar]
- 87. Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009; 50(3): 604–620. [DOI] [PubMed] [Google Scholar]
- 88. Das A, Yaqoob U, Mehta D, Shah VH. FXR promotes endothelial cell motility through coordinated regulation of FAK and MMP‐9. Arterioscler Thromb Vasc Biol. 2009; 29(4): 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Soma T, Kaganoi J, Kawabe A, Kondo K, Tsunoda S, Imamura M, Shimada Y. Chenodeoxycholic acid stimulates the progression of human esophageal cancer cells: a possible mechanism of angiogenesis in patients with esophageal cancer. Int J Cancer. 2006;119(4): 771–782. [DOI] [PubMed] [Google Scholar]
- 90. Suh H, Jung EJ, Kim TH, Lee HY, Park YH, Kim KW. Anti‐angiogenic activity of ursodeoxycholic acid and its derivatives. Cancer Lett. 1997; 113(1–2): 117–122. [DOI] [PubMed] [Google Scholar]
- 91. Woo SJ, Kim JH, Yu HG. Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser‐treated rat model. J Ocul Pharmacol Ther. 2010; 26(3): 223–229. [DOI] [PubMed] [Google Scholar]
- 92. Gao B, Stieger B, Noe B, Fritschy JM, Meier PJ. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem. 1999; 47(10): 1255–1264. [DOI] [PubMed] [Google Scholar]
- 93. Kusuhara H, Suzuki H, Naito M, Tsuruo T, Sugiyama Y. Characterization of efflux transport of organic anions in a mouse brain capillary endothelial cell line. J Pharmacol Exp Ther. 1998; 285(3): 1260–1265. [PubMed] [Google Scholar]
- 94. Ackerman Z, Karmeli F, Amir G, Rachmilewitz D. Renal vasoactive mediator generation in portal hypertensive and bile duct ligated rats. J Hepatol. 1996; 24(4): 478–486. [DOI] [PubMed] [Google Scholar]


