Abstract
The effects of dietary cholesterol on plasma lipoproteins and cholesterol homeostasis in blood mononuclear cells have been examined in healthy adults. Addition of 1,500 mg of cholesterol to the daily diet of 37 subjects for 14 d was associated with a wide range of response of plasma total cholesterol concentration (from −6 to +75 mg/dl; mean change, +29 mg/dl; P < 0.001). Increases in plasma cholesterol reflected increased cholesterol concentrations in intermediate density lipoprotein (IDL; 1.006-1.019 g/ml), low density lipoprotein (LDL; 1.019-1.063 g/ml), and the HDL2 subclass (1.063-1.125 g/ml) of high density lipoprotein, which on average accounted for 20, 58, and 22%, respectively, of the total increment. Similar responses occurred in 14 other subjects given 750 mg cholesterol per day for 28 d. Plasma apolipoprotein B concentrations in IDL and LDL also increased.
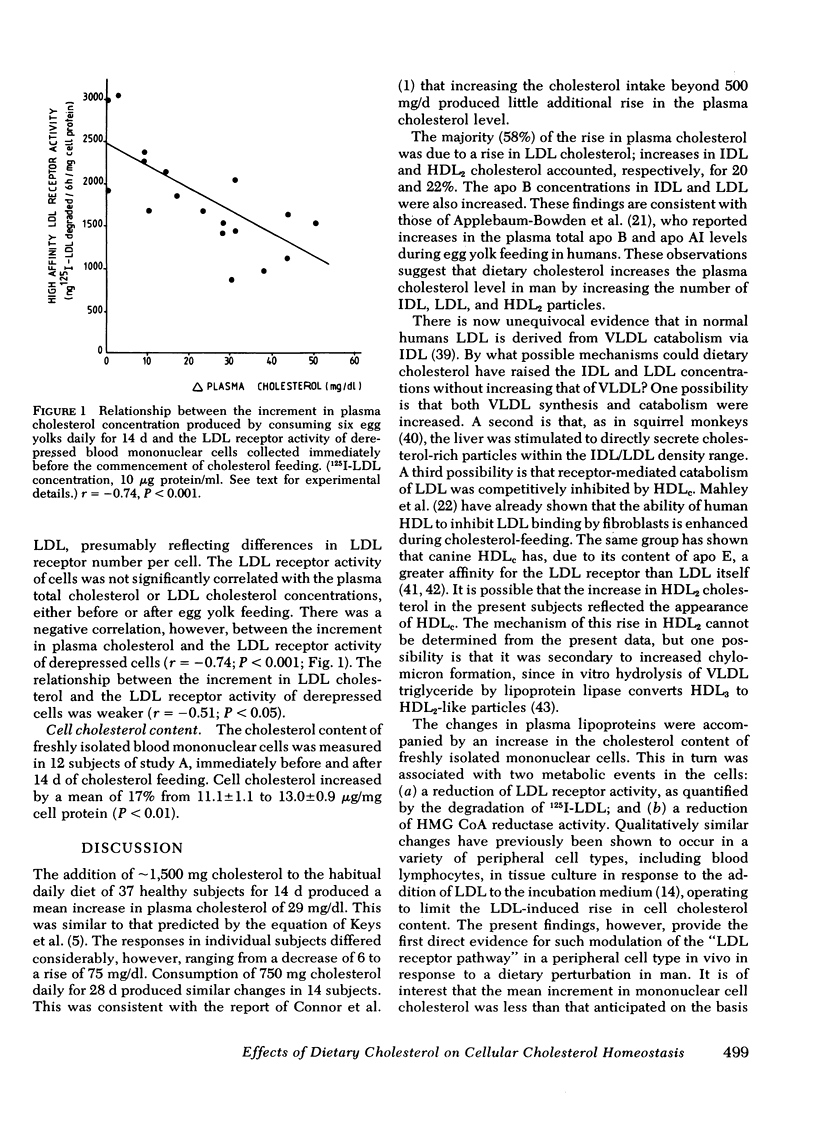
These effects on plasma lipoproteins were accompanied by three changes in freshly isolated blood mononuclear cells: (a) an increase in cell cholesterol content (mean change, +17%; P < 0.01); (b) suppression of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase activity (−32%; P < 0.001); and (c) reduction of LDL receptor activity (−74%; P < 0.01), quantified as the rate of degradation of 125I-LDL to noniodide trichloroacetic acid-soluble material. These results provide the first direct evidence for the modulation of LDL receptor activity and HMG CoA reductase activity in a peripheral cell type in response to a dietary perturbation of human lipoprotein metabolism.
The percentage increase in LDL cholesterol was negatively correlated with the percentage decrease in HMG CoA reductase activity (r = −0.49, P < 0.01). An additional negative correlation existed between the increment in plasma cholesterol concentration and the capacity of cells to degrade 125I-LDL after derepression by preincubation for 72 h in lipoprotein-deficient medium (r = −0.74, P < 0.001). Thus, differences between individuals in the responses of the plasma lipoproteins to dietary cholesterol appear to be related in part to differences in the capacity of peripheral cells to catabolize LDL and to down-regulate cholesterol synthesis.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in 15 tissues of rat. II. Role of rat and human high and low density plasma lipoproteins and of rat chylomicron remnants. J Biol Chem. 1977 Jun 10;252(11):3652–3659. [PubMed] [Google Scholar]
- Applebaum-Bowden D., Hazzard W. R., Cain J., Cheung M. C., Kushwaha R. S., Albers J. J. Short-term egg yolk feeding in humans. Increase in apolipoprotein B and low density lipoprotein cholesterol. Atherosclerosis. 1979 Aug;33(4):385–396. doi: 10.1016/0021-9150(79)90031-5. [DOI] [PubMed] [Google Scholar]
- BEVERIDGE J. M., CONNELL W. F., MAYER G. A., HAUST H. L. The response of man to dietary cholesterol. J Nutr. 1960 May;71:61–65. doi: 10.1093/jn/71.1.61. [DOI] [PubMed] [Google Scholar]
- BHATTATHIRY E. P., SHIPERSTEIN M. D. FEEDBACK CONTROL OF CHOLESTEROL SYNTHESIS IN MAN. J Clin Invest. 1963 Oct;42:1613–1618. doi: 10.1172/JCI104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brown M. S. Evidence for regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in nonhepatic tissues of rat. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2564–2568. doi: 10.1073/pnas.73.8.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Ho Y. K., Brown M. S., Anderson R. G., Goldstein J. L. Genetics of the low density lipoprotein receptor. Diminished receptor activity in lymphocytes from heterozygotes with familial hypercholesterolemia. J Clin Invest. 1978 Mar;61(3):678–696. doi: 10.1172/JCI108980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR W. E., HODGES R. E., BLEILER R. E. The serum lipids in men receiving high cholesterol and cholesterol-free diets. J Clin Invest. 1961 May;40:894–901. doi: 10.1172/JCI104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COURTICE G. C., MORRIS B. The exchange of lipids between plasma and lymph of animals. Q J Exp Physiol Cogn Med Sci. 1955 Apr;40(2):138–148. doi: 10.1113/expphysiol.1955.sp001105. [DOI] [PubMed] [Google Scholar]
- Camejo G., Bosch V., Arreaza C., Mendez H. C. Early changes in plasma lipoprotein structure and biosynthesis in cholesterol-fed rabbits. J Lipid Res. 1973 Jan;14(1):61–68. [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. I. N Engl J Med. 1970 May 14;282(20):1128–1138. doi: 10.1056/NEJM197005142822005. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Hirono H., Arakawa T. Idiopathic hypercholesterolemia: demonstration of an impaired feedback control of cholesterol synthesis in vivo. Tohoku J Exp Med. 1965 Nov 25;87(2):155–167. doi: 10.1620/tjem.87.155. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth D. R. Metabolism of lipoproteins in nonhuman primates. Studies on the origin of low density lipoprotein apoprotein in the plasma of the squirrel monkey. Biochim Biophys Acta. 1975 Apr 18;388(1):38–51. doi: 10.1016/0005-2760(75)90060-0. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979 May 25;254(10):4186–4190. [PubMed] [Google Scholar]
- KEYS A., ANDERSON J. T., MICKELSEN O., ADELSON S. F., FIDANZA F. Diet and serum cholesterol in man; lack of effect of dietary cholesterol. J Nutr. 1956 May 10;59(1):39–56. doi: 10.1093/jn/59.1.39. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Sata T., Hamilton R. L., Havel R. J. Apoprotein composition of very low density lipoproteins of human serum. J Clin Invest. 1975 Dec;56(6):1622–1634. doi: 10.1172/JCI108245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser N. L., Roheim P. S., Edelstein D., Eder H. A. Serum lipoproteins of normal and cholesterol-fed rats. J Lipid Res. 1973 Jan;14(1):1–8. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lewis B., Chait A., Wootton I. D., Oakley C. M., Krikler D. M., Sigurdsson G., February A., Maurer B., Birkhead J. Frequency of risk factors for ischaemic heart-disease in a healthy British population. With particular reference to serum-lipoprotein levels. Lancet. 1974 Feb 2;1(7849):141–146. doi: 10.1016/s0140-6736(74)92438-6. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Bersot T. P., Lipson A., Margolis S. Alterations in human high-density lipoproteins, with or without increased plasma-cholesterol, induced by diets high in cholesterol. Lancet. 1978 Oct 14;2(8094):807–809. doi: 10.1016/s0140-6736(78)92588-6. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T., Brewer H. B., Jr, Assmann G. Swine lipoproteins and atherosclerosis. Changes in the plasma lipoproteins and apoproteins induced by cholesterol feeding. Biochemistry. 1975 Jul;14(13):2817–2823. doi: 10.1021/bi00684a005. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Nestel P. Changes in cholesterol metabolism with dietary cholesterol in children with familial hypercholesterolaemia. Clin Sci (Lond) 1979 Apr;56(4):377–380. doi: 10.1042/cs0560377. [DOI] [PubMed] [Google Scholar]
- Mattson F. H., Erickson B. A., Kligman A. M. Effect of dietary cholesterol on serum cholesterol in man. Am J Clin Nutr. 1972 Jun;25(6):589–594. doi: 10.1093/ajcn/25.6.589. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Miller N. E., Weinstein D. B., Carew T. E., Koschinsky T., Steinberg D. Interaction between high density and low density lipoproteins uptake and degradation by cultured human fibroblasts. J Clin Invest. 1977 Jul;60(1):78–88. doi: 10.1172/JCI108772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Poyser A. Changes in cholesterol synthesis and excretion when cholesterol intake is increased. Metabolism. 1976 Dec;25(12):1591–1599. doi: 10.1016/0026-0495(76)90112-8. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Patsch J. R., Gotto A. M., Jr, Olivercrona T., Eisenberg S. Formation of high density lipoprotein2-like particles during lipolysis of very low density lipoproteins in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4519–4523. doi: 10.1073/pnas.75.9.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Arnold K. S., Mahley R. W. Rate and equilibrium constants for binding of apo-E HDLc (a cholesterol-induced lipoprotein) and low density lipoproteins to human fibroblasts: evidence for multiple receptor binding of apo-E HDLc. Proc Natl Acad Sci U S A. 1979 May;76(5):2311–2315. doi: 10.1073/pnas.76.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr Effects of dietary cholesterol on the regulation of total body cholesterol in man. J Lipid Res. 1971 Mar;12(2):233–247. [PubMed] [Google Scholar]
- Reichl D., Simons L. A., Myant N. B., Pflug J. J., Mills G. L. The lipids and lipoproteins of human peripheral lymph, with observations on the transport of cholesterol from plasma and tissues into lymph. Clin Sci Mol Med. 1973 Sep;45(3):313–329. doi: 10.1042/cs0450313. [DOI] [PubMed] [Google Scholar]
- Shore V. G., Shore B., Hart R. G. Changes in apolipoproteins and properties of rabbit very low density lipoproteins on induction of cholesteremia. Biochemistry. 1974 Apr 9;13(8):1579–1585. doi: 10.1021/bi00705a004. [DOI] [PubMed] [Google Scholar]
- Sigurdsson G., Nicoll A., Lewis B. Conversion of very low density lipoprotein to low density lipoprotein. A metabolic study of apolipoprotein B kinetics in human subjects. J Clin Invest. 1975 Dec;56(6):1481–1490. doi: 10.1172/JCI108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. D., Avigan J. In vitro effects of serum proteins and lipids on lipid synthesis in human skin fibroblasts and leukocytes grown in culture. Biochim Biophys Acta. 1972 Mar 23;260(3):413–423. doi: 10.1016/0005-2760(72)90056-2. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Cowell T. K., Grimes A. J. The automatic defibrination of normal human blood. Scand J Haematol. 1969;6(3):197–204. doi: 10.1111/j.1600-0609.1969.tb01826.x. [DOI] [PubMed] [Google Scholar]



