Short abstract
The facet joint is a crucial anatomic region of the spine owing to its biomechanical role in facilitating articulation of the vertebrae of the spinal column. It is a diarthrodial joint with opposing articular cartilage surfaces that provide a low friction environment and a ligamentous capsule that encloses the joint space. Together with the disc, the bilateral facet joints transfer loads and guide and constrain motions in the spine due to their geometry and mechanical function. Although a great deal of research has focused on defining the biomechanics of the spine and the form and function of the disc, the facet joint has only recently become the focus of experimental, computational and clinical studies. This mechanical behavior ensures the normal health and function of the spine during physiologic loading but can also lead to its dysfunction when the tissues of the facet joint are altered either by injury, degeneration or as a result of surgical modification of the spine. The anatomical, biomechanical and physiological characteristics of the facet joints in the cervical and lumbar spines have become the focus of increased attention recently with the advent of surgical procedures of the spine, such as disc repair and replacement, which may impact facet responses. Accordingly, this review summarizes the relevant anatomy and biomechanics of the facet joint and the individual tissues that comprise it. In order to better understand the physiological implications of tissue loading in all conditions, a review of mechanotransduction pathways in the cartilage, ligament and bone is also presented ranging from the tissue-level scale to cellular modifications. With this context, experimental studies are summarized as they relate to the most common modifications that alter the biomechanics and health of the spine—injury and degeneration. In addition, many computational and finite element models have been developed that enable more-detailed and specific investigations of the facet joint and its tissues than are provided by experimental approaches and also that expand their utility for the field of biomechanics. These are also reviewed to provide a more complete summary of the current knowledge of facet joint mechanics. Overall, the goal of this review is to present a comprehensive review of the breadth and depth of knowledge regarding the mechanical and adaptive responses of the facet joint and its tissues across a variety of relevant size scales.
1. Introduction
The zygapophyseal, or facet, joints are complicated biomechanical structures in the spine, with complex anatomy, mechanical performance and effects on overall spine behavior and health. At each spinal level, there is a pair of facet joints located on the postero-lateral aspects of each motion segment, spanning from the cervical to the lumbar spine (Fig. 1 ). These facet joints are typical diarthrodial joints with cartilage surfaces that provide a low-friction interface to facilitate motion during normal conditions in a healthy spine. Owing to the anatomy of the spine, the mechanical behavior of the facet joint is both dependent on the responses dictated by the overall spine's response and also can directly affect the spine's response, via its relationship to the intervertebral disc, its anatomic orientation, and its own mechanical behavior. The kinematics and mechanical properties of the facet joint and its tissue components have been studied extensively for a variety of different loading conditions [1–11]. Recently, there is growing interest in the facet joint—its biomechanics and physiology—with the advent of disc arthroplasty and there has been increased attention to the relationship between spinal degeneration and its effects on the mechanical environment of the different tissues in the facet joint [12–16]. Therefore, it is the primary goal of this review to present an updated perspective of the anatomy and global mechanics of the spinal facet joint and its individual tissue components in conjunction with their loading during physiologic and nonphysiologic motion. In addition, this review will summarize the mechanotransduction processes by which mechanical loading to the specific tissues of the joint translate into signals that drive physiologic responses in health, injury and trauma, and spinal degeneration. Computational models of the facet joint are also reviewed since there has been quite a bit of work in this area to complement and expand findings from biomechanical experiments and to provide insight about facet joint mechanics otherwise not measureable in typical cadaveric studies. Overall, this review focuses on synthesizing this anatomical, biomechanical and physiological information to give an overview of the facet joint's response to mechanical loading from the macroscopic to the cellular scale, with implications and perspective for future studies of this spinal joint.
Fig. 1.
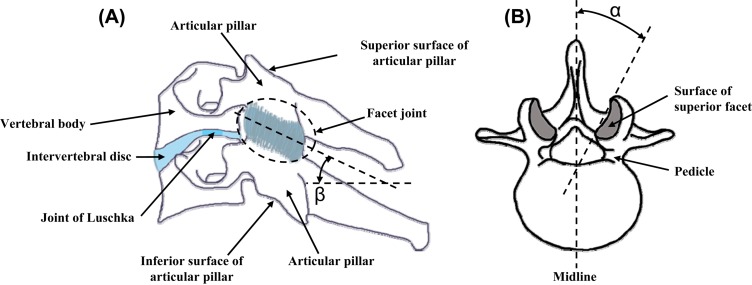
Lateral view of a cervical (a) and axial view of a lumbar (b) vertebra showing the overall anatomy and the facet joints, articulations, and orientation relative to its angle with each of the axial plane (β) and of the sagittal plane (α)
2. Anatomy and Tissue Mechanics
The facet joints, together with the intervertebral discs and spinal ligaments, connect the adjacent vertebrae of the spine at all regions and provide support for the transfer and constraint of loads applied to the spinal column. These articulations insure the mechanical stability and also overall mobility of the spine, while protecting the spinal cord running through it. At each spinal level, the bilateral facet joints are positioned symmetrically relative to the mid-sagittal plane in the postero-lateral regions of the spine (Fig. 1(a)). Because the facet is a diarthrodial synovial joint, cartilage covers the sliding surfaces and ligamentous capsules guide, couple, and limit the relative translations and rotations of adjacent vertebrae. Broadly, the facet joint is made up of a variety of hard and soft tissues: the bony articular pillars of the lateral mass provide the opposing surfaces that are covered by cartilage, the synovium which is a connective tissue lining that maintains lubrication for the articular surfaces and enables their frictionless motion, and a ligamentous capsule that envelops the entire joint [17–20]. The bony articular pillars support compressive loads and the facet capsular ligament resists tensile forces that are developed across the joint when it undergoes rotations and translations [1,6,21]. Together, this collection of tissues functions to transfer the different loads across the joint during a variety of loading modes for the spine. Here, we provide a more detailed presentation of the facet anatomy in order to describe the response to mechanical loading for each of the soft and hard tissues composing the facet joint.
2.1. Bony Articular Pillars.
The articular pillars are the bony protuberances that extend superiorly and inferiorly from the lamina of each vertebra along the long-axis of the spine (Fig. 1(a)). They are located at the junction between the lamina and the lateral masses in the cervical region of the spine; whereas, in the thoracic and lumbar regions, they are joined to the vertebral body via the bony pedicles. At each intervertebral joint along the spine, the adjacent articular pillars are aligned to establish two postero-lateral columns that provide mechanical support for axial loading along the spine, together with the anterior column comprised of the vertebral bodies joined by their interconnected intervertebral discs [22–23]. In general, the inclination angle of the articular surfaces of the facet joint in the sagittal plane ranges from 20°–78° in the cervical region, 55°–80° in the thoracic region, and 82°–86° in the lumbar region (angle β in Fig. 1(a)). The angle between the articulating surfaces in the axial plane range from 70°–96°, 85°–120°, and 15°–70° off of the midline in the cervical, thoracic, and lumbar regions, respectively (angle α in Fig. 1(b)), with increasing orientation angles moving towards the lower levels in the lumbar spine [24–27]. Lastly, the superior articular surfaces transition from having a postero-medial orientation in the cervical region to a more postero-lateral orientation in the thoracic region, although asymmetrical orientations have also been reported [26].
The facet joint is formed by two adjacent vertebrae with the inferior facet of the superior vertebra meeting the superior facet of the inferior vertebra (Fig. 1(a)). As such, each articular pillar of a vertebra has both a superior and an inferior articulating surface. The surfaces of the pillars that form the articulation of the joint have elliptically-shaped faces that are covered by cartilage (Fig. 2). The morphometry of these surfaces also differs between the regions of the spine, as well as at each vertebral level [26,28–32]. The superior facet of the inferior vertebra is rather flat in the cervical and thoracic regions and more convex in the lumbar region [26]. The opposing inferior facet of the superior vertebra is concave and forms an arch with its apex pointing towards the vertebral body [20,33–36] (Fig. 2). Articular surfaces are more horizontally-oriented in the cervical and upper thoracic spinal regions [26,36], which enables the great degree of coupling of axial rotation and lateral bending that exists in the cervical spine [37–39]. In the lower thoracic and lumbar regions of the spine the facets gradually become more vertically-oriented [25], which also limits the flexibility of the spine in both lateral bending and rotation in these regions. But, this decrease in flexibility protects the intervertebral discs and spinal cord from nonphysiological kinematic and kinetic exposures that could cause injury and/or create pathological conditions [6].
Fig. 2.
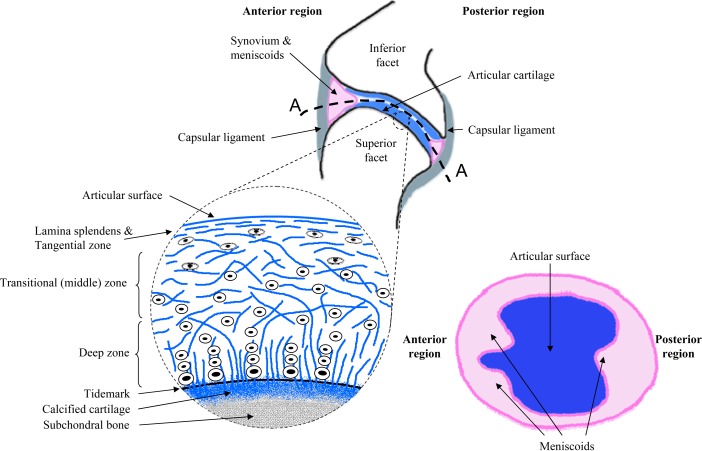
Schematic drawings of the facet joint and the primary tissues that compose it, as well as the cartilage and menisci of the facet articulation. The blowup illustrates the different zones of the articular cartilage layer with the collagen fibers and chondrocytes orientations through its depth. A cut through of the facet joint (A-A) is also drawn to show the elliptically-shaped inter-articular surfaces with the cartilage surface on the inferior facet, the synovium, and meniscoids. Adapted collectively from Martin et al., 1998, Pierce et al., 2009, and Bogduk and Engel, 1984 [48,49,73].
2.2. Cartilaginous Articular Surfaces.
An avascular layer of hyaline cartilage, with varying thickness across spinal regions and the genders, covers the articulating surfaces of each facet [19,40]. The cartilage is thinner at the edges of the opposing surfaces and gradually increases to its thickest (∼1 mm) towards the center of the articulating joint, in both the antero-posterior and medio-lateral regions of the joint [41]. Based on experimental studies, the thickness of cervical facet cartilage has been described to have a half sinusoidal shape with a maximum thickness (tmax) at its center and thinning out along its radius (r) towards the facet perimeter (rperim), according to Eq. (1) [41]:
| (1) |
where both the maximum thickness and the shape coefficient (k, ranging 0.38–0.63) were both determined by minimizing the difference between the experimental and theoretical thickness distributions [41].
Further, reports have found that the bony extremity of the pillars is not always completely covered by a cartilage layer, leaving a region of exposed subchondral bone at the outermost edges of the bony pillar [19,41]. Yoganandan et al. [19] reported the gap of exposed subchondral bone to be nearly three times wider in the upper region of the cervical spine than in the lower cervical spine, especially in the posterior and anterior regions of the facet articulating surfaces. The cartilage layer may be thinner in these regions because of the presence of synovial folds and meniscoids, which also provide additional protection from compressive and shear loads across the joint (see Sec. 2.1 for more details) [19]. However, subchondral bone can be exposed to mechanical compression during some loading scenarios. This not only presents the possibility for direct trauma to the bone but has also been hypothesized to lead to pain in some cases [42]. The gap in cartilage coverage is greater in females [19], which may play a role in the greater susceptibility of women to suffer neck traumas [43,44].
2.2.1. Cartilage Composition.
The cartilaginous layers covering the articulating facet surfaces enable frictionless motion between the adjacent vertebrae, while also bearing compressive, tensile, and shear loads. Such mechanical capabilities are due to the specific structure of the cartilage tissue and the mechanical properties of the matrix of the cartilaginous layer. The cartilage matrix consists of collagen fibers, glycosaminoglycans (GAGs), proteoglycans, and chondrocytes [45–47]. This matrix is actually a laminate composed of three main zones along the depth of the cartilage, with the outermost surface (lamina splendens) being the region where contact with the articulating surface of the opposing pillar occurs and the innermost region being at the subchondral bone of the articular pillar (Fig. 2). Each cartilage zone has a different structural organization, as well as variable and specific mechanical properties [47–49]. The superficial zone contains relatively few flattened chondrocytes and collagen fibers that are oriented tangentially to the surface of the cartilage; the horizontal fiber alignment provides resistance to both the tensile and shear stresses that develop during the relative sliding developed in the joint between the opposing articular surfaces when the spine bends or rotates [47,48]. In the middle or transitional zone, more chondrocytes are interspersed between larger collagen fibers with a pseudorandom arrangement [48–50]. Finally, in the deep zone, chondrocytes align in columns perpendicular to the articular surface and parallel to the collagen fibers. In the transitional and deep zones, the concentration in proteoglycans embedded in the chondrocytes and collagen structure increases with the depth. These proteoglycans trap water and increase the incompressibility of the structure, thereby supporting compressive and hydrostatic stresses that may be developed in the joint. At the bottom of the deep zone a tidemark separates the deep zone from a zone of calcified cartilage that transition into subchondral bone, making the change in elastic modulus from cartilage to bone more gradual [46–49].
2.2.2. Cartilage Mechanics.
As with articular cartilage of any diarthrodial joint (knee, hip, facet joint), the specific mechanical properties are heavily dependent on the cartilage composition (water content, collagen fiber orientations), the specimen's age and relative health, and the specific loading conditions that the joint undergoes [51–53]. Although the facet joint cartilage has been described macroscopically, there have been little-to-no specific investigations of its mechanical responses. However, there have been extensive reports for cartilage of the knee; those are provided briefly here, as they are relevant to the broader context of joint cartilage. The equilibrium modulus (H) of human patella samples subjected to confined compression has been reported to decrease linearly with age, structural disorganization (I), and water content (WAT), according to the set of linear equations (Eqs. (2), (3), and (4)) that were optimized to fit experimental data [51]. According to those relationships, the equilibrium modulus was found to depend more on the water content than with the other two parameters, which is expected since water is incompressible and a greater volume of it retained in the cartilage structure would stiffen it.
| (2) |
| (3) |
| (4) |
For example, a 30–40% decrease in water content leads to a 161% increase in the equilibrium elastic modulus of cartilage explants subjected to axial compression [52]. The stress-strain relationship is anisotropic as it depends on the dimensions and organization of the collagen fibers, and the cellular and proteoglycans content that differ across the depth of the cartilaginous layer. In addition, articular cartilage deformation results from a reorganization of its collagen structure and loss of fluid during loading. Fluid loss is a much slower process than the polymer network re-arrangement and so an initial deformation occurs first without any volume change, and a second deformation then results from a change in volume due to fluid loss which produces a nonlinear load-displacement response that is exhibited during unconfined compression [54,55]. This type of behavior highlights the biphasic and time-dependent mechanical properties of articular cartilage in diarthrodial joints [56,57]. Accordingly, creep studies have also demonstrated that the time constant (T) of cartilage to reach equilibrium under maintained compressive loading is a function of the thickness (h), the equilibrium modulus (E), and specific properties of the cartilage, as well as the applied load (π) [51,56]. This equilibrium time constant also depends on porosity (Φf), permeability (k), and the drag coefficient (K) as described in Eq. (5):
| (5) |
In contrast, because of the interactions between the collagen fibers and the proteoglycans during uniaxial confined compression, the relationship between axial and radial stresses (σ) and axial strain (ɛ) is defined by a linear isotropic constitutive relation (Eqs. (6) and (7)) [58]. In this relationship, the compression axial modulus (HA) and the chemical stress (β) imposed by the surrounding milieu, as well as the Lamé constants (λ and G), all depend on the concentration (c) of the environment.
| (6) |
with
| (7) |
Articular cartilage is a composite material composed of fluid (water) and solid (chondrocytes, collagen, proteoglycans) phases that has anisotropic nonlinear mechanical properties and load-bearing capacity [59]. The difference in response time of the two phases contained in articular cartilage makes its mechanical response dependent on the rate of loading. The dynamic stiffness of the cartilage lining in diarthrodial joints increases with strain rate [60–62]. For example, in a study of cartilage impacts during knee graft implantation, fissures in the cartilage matrix were produced for both single high energy impacts (over 25 MPa) and repetitive impacts (26–35 MPa) across a variety of human, bovine, and porcine species [60,63]. Chondrocyte viability was also reduced by up to 60% for impacts of 1 J compared to a 5% and 20% decrease in cell viability for impacts of 0.25 J and 0.5 J, respectively [52,62,63]. Fissures in the articular surface can allow the enzymes that are contained in the synovial fluid in the joint, such as collagenase and hyaluronidase, to penetrate and break down the cartilage matrix [48]. At the same time, an increase in chondrocyte death can also impair the subsequent synthesis of cartilage proteins that are required for the proper maintenance of the avascular cartilage matrix [63]. A damaged cartilage matrix cannot effectively support compressive loads, distribute pressure, or resist stresses because fissures can penetrate as far as the transition zone and disrupt the matrix structure [62]. In addition, the repair of the cartilaginous matrix and its functionality are compromised by the death of the chondrocytes because the production of molecules imperative for matrix regeneration is reduced, which is then followed by the denaturation of the collagen fibers and the release of proteoglycans, which are needed to retain water and to provide compressibility for the damaged cartilage structure [48,63]. Impact(s) on the articular cartilage can; therefore, cause significant loss of mechanical properties and cellular damage which also may provide the stimulus for the onset of degeneration in that tissue and/or can also accelerate it [52,64,65]. However, the energy transferred to the facet joint cartilage during physiologic and/or nonphysiologic loading of the spine remains to be measured.
Explicit experimental studies of facet joint cartilage are limited. Currently, there is only one investigation of canine lumbar facet cartilage, reporting its aggregate modulus to be 554 kPa at equilibrium after an indentation with a 1 mm flat-ended porous-tip [66]. That study also found that the aggregate modulus of cartilage from the facet joint was similar to the modulus of cartilage from other canine diarthrodial joints such as the knee lateral condyle, patellar groove, and shoulder, suggesting that the similarities between human and canine articular cartilage could also extend to facet joint cartilage [66]. It was also reported that human cartilage from the knee and the hip has a compressive stiffness comparable to that of the distal femur in canine models and the proximal femur in baboon models, respectively [67,68], which suggests that the mechanical properties of articular cartilage may be similar among any diarthrodial joints in the body. However, further characterization of human facet joint tissue is needed to verify if the mechanical properties of facet joint cartilage are similar across species as well.
2.3. Synovium, Menisci and Capsular Ligament.
Extending from the superior to the inferior articular pillar, two superposed membranes, the synovium and the ligamentous capsule, maintain the articular surfaces in a low-friction environment and provide mechanical resistance to their separation and relative motion. The synovium of the facet joint is a thin and soft periarticular connective tissue [17] with two main layers that secrete synovial fluid components involved in the maintenance of the synovial fluid used to lubricate and nourish the cartilaginous articular surfaces [69,70]. The synovium also regulates the exchanges between the blood and synovial fluid, and contains macrophage cells that phagocytose cell debris and waste contained in the joint cavity [70]. Although the functional role of this structure has been investigated at the cellular level [70,71], it has not been investigated mechanically, most likely because it is difficult to isolate since it is very thin and its outermost layer is intimately merged to the inner surface of the capsular ligament [71]. For the same reasons, and also because the innermost synovial layer is loose, the synovium also likely does not play a substantial role in the mechanical behavior of the joint as a whole. Although, the synovial membrane is very thin, its loose innermost layer bulges into the joint cavity in some areas, forming folds that wedge between the opposing articular surfaces of the facet joint [72–74].
The synovial folds, or meniscoids or menisci, are intra-articular structures that protect the articular cartilage when opposing articulating surface glide on each other during joint motion [75]. This protection is realized since the meniscoids compensate for the incongruence of the joint's articular surfaces, guiding and smoothing their relative motion, and distributing the load over a greater surface area [72,76,77]. Three main types of menisci have been identified in the facet joints across all of the regions of the spine: adipose tissue pads, fibro-adipose meniscoids, and connective tissue rims (Fig. 2). The adipose pads and meniscoids are located mainly at the periphery of the articular surface in the anterior and posterior region of the joint, where they only partially extend circumferentially along the rim of the articular pillar. These tissues are crescent-shaped and have a triangular cross-section in the sagittal plane (Fig. 2), with the base being attached to the capsule and the point extending as much as 9 mm inward toward the interior of the joint [72,75,78]. The connective rims of the synovial tissue are ring-shaped, wraparound the edge of the bony pillar, and are tapered inward towards the center of the joint [72,74,75,77,78]. The meniscoids are composed of fat, fibrous connective tissue and/or a mix of fat and fibers covered by a cellular synovial lining [72–75,77,79]. Although the meniscoids are known to cover the gap of exposed subchondral bone at the articular surface in order to reduce friction during articular motion, their mechanical role is still unclear [77]. They have been speculated to moderate the load transferred to the cartilage when the articular surfaces engage in compression during any joint motion by distributing the pressure as they move freely in and out of the inter-articular space during motion [72,75–77,80]. This putative function is probably linked to the meniscus entrapment theory that was developed to explain how low back pain symptoms could be caused, and then treated by simple manipulation [73,81]. Although this may be the case under normal loading, these intra-articular folds can become torn at their base under combined substantial compression and shear loading which can also lead to subcapsular hemorrhage and entrapment of the torn pieces in the joint, eventually inducing further physiologic dysfunction and even pain [73,74,82]. Although postmortem and in vivo MRI studies provide evidence of the presence of meniscoids in the spinal facet joints and help to characterize their dimensions and composition, the role of these structures in the biomechanical behavior of the whole facet joint remains unclear.
As in the other joints in the body, such as the knee and the hip, the facet capsular ligament covers the synovium to fully enclose the facet joint, enveloping it in the superior-inferior direction and with nonuniform thickness. For instance, the lumbar facet capsule has been reported to be 2.0 mm thick in the posterior region, and as much as 3.2 mm thick in the anterior region, whereas the superior and inferior regions are approximately 2.4 mm thick [83].The capsular ligament is comprised of dense collagen fiber bundles linked by proteoglycans, with elastin fibers and fibroblasts interspersed [18,84]. The collagen and elastin fibers extend between the laminae of adjacent vertebrae connecting to the ligamentum flavum both in the antero- and postero-medial regions of the facet joint, and completely surrounding the joint's articular surfaces in three dimensions. The collagen fibers are oriented differently along the superior-inferior axis of the capsular ligament [84] and they are crimped [18]. The crimped collagen fibers allow the capsule to undergo substantial excursions without reaching its mechanical limit or inducing local injury. Under load, the fibers can become uncrimped which allows the overall joint to translate and rotate without offering any mechanical resistance.
The capsule, as well as the subchondral bone, synovium and folds, are richly innervated with mechanoreceptive, proprioceptive and nociceptive nerve endings [21,85–88].Therefore, mechanical loading of any of those innervated tissues in the facet joint could activate nerve endings and modulate the signals in the nervous system to initiate the development and maintenance of pain and/or cellular dysfunction. The nervous system is also involved in modulating the overall mechanical response of the facet joint and its tissues since the intensity and frequency of the mechanical stimuli experienced by these nerve endings also provide feedback to the central nervous system which is used to adjust the activity of the surrounding muscles and correct the loading of the joint in real-time [89–91].
3. Facet Joint Macromechanics
3.1. Facet Joint and Spinal Stability.
The facet joints guide and constrain the motion of the vertebrae, while also facilitating the transmission of the loads applied to the spine [2,21,66,92]. The facet joints also contribute to and help maintain the stability of the spine. A structural column, like the spine, is considered mechanically stable when the sum of the forces and moments applied to it equals zero. Mechanical stability of the spine is achieved when the paraspinal musculature effectively counteracts the external loads via modification of the shape of the vertebral column. Clinically, the term ‘spinal stability’ has taken on the definition of the spine's ability to maintain its alignment and to provide protection to the neural structures it encloses during physiologic loading [93]. The clinical assessment of spinal stability/instability is required for a variety of different clinical scenarios, including degeneration with altered kyphosis or lordosis, surgical management or when motions become painful. The assessment is performed using imaging to measure the relative position of the vertebrae, and to detect any malalignment [93,94].
White and Panjabi [24] defined clinical instability of the spine as the spine's loss of ability to maintain its normal motions under physiologic loads which leads to initial or additional neurologic deficit [24]. Although most clinicians agree on the clinical definition of spinal instability, there is still ambiguity in using the term “spinal stability” because its quantitative assessment remains challenging and subjective in the clinical setting [93,95]. Currently, clinicians consider the spine as a three-column system in their assessment of spinal instability with the first column containing the anterior longitudinal ligament and the anterior half of the body and discs, the middle column contains the posterior half of the vertebral body and disc, and the posterior column contains the interspinous ligaments, spinous processes, pedicles, and the facets [93] (Fig. 1). The spine is considered unstable when two of the three columns are not intact. This rule is substantiated by the more complex system implemented by White and Panjabi [24] in which the spine is judged unstable when translations are greater than 3.5 mm and rotations greater than 20 degrees in the sagittal plane during flexion-extension bending [24]. Although injured or damaged facet joints do not a priori dictate that the spine is mechanically unstable, the proprioceptive and nociceptive nerve endings in the facet joint can respond to overload, damage, or injury to alter the musculature feedback and control for providing support to the spinal column. Moreover, injured nerves can also become nonresponsive to loading or motion or exhibit dysfunctional performance, both of which can result in abnormal sensory feedback for the central nervous system's coordination of the various spinal tissues and paraspinal muscles to insure mechanical stability [94].
3.2. Mechanical Contributions of the Facet Joint.
The role of the facet joints in the mechanical stability of the spine has been established from biomechanical and mathematical studies. The facet joints prevent two adjacent vertebrae from engaging in relative motions that could overload and damage the surrounding spinal structures, such as the intervertebral disc, the nerve roots that exit the spinal column, and the spinal cord. Consequently, the facet joint tissues are themselves mechanically loaded. For example, Yang and King [2] reported that between 75–97% of the compressive load applied to the lumbar spine is borne by the intervertebral discs, and they estimated that 3–25% is carried by the posterior elements of the vertebral column in what they referred to as “facet force” [2]. In similar experiments using lumbar motion segments, Adams and Hutton [96] measured that under 2 degrees of extension and 560–1030 N of compression, 16% of the load is borne by the facet joints) [96]. Pal and Routal [4] assumed the spine to be mechanically equivalent to three columns; an anterior column composed of the vertebral bodies and discs, and two posterior columns consisting of vertically-connected articular processes. Those authors considered that any compressive load applied to the spine was distributed over the whole vertebral body and areas of the entire facet joints and that the ratio of the articular facet area to vertebral body area could be used as a metric of the load-sharing between the anterior and posterior columns [4]. Using an analysis of detailed facet joint morphology (facet articular area, vertebral body horizontal cross-section area, lordosis angle) Pal and Routal [4] computed that 23% of any axial compressive load is transmitted by the facet joints in the cervical and upper thoracic regions of the spine [4]. They reached the same conclusion in a matched study using the lower thoracic and lumbar regions of the spine, in which their anatomical observations and cross-sectional measurements of the vertebrae showed that the posterior vertebral elements were actually connected over a single larger area formed by the stacking of laminae instead of the two smaller areas formed by the articular pillars as in the cervical spine [5].
In addition to transmitting compressive loads along the spine, facet joints also provide torsional stiffness, and resistance to shear, lateral and antero-posterior vertebral translation, and joint distraction [24,97]. The specific contribution of the facet joint in resisting these mechanical scenarios has been most widely studied and demonstrated in facetectomy studies, in which the facet joints are surgically removed either in total or partially [98–104]. For example, the shear strength of cadaveric cervical motion segments was shown to diminish by 29% after 70% of the facet joints were removed bilaterally [98]. In a later investigation, Raynor et al. [99] found that a partial bilateral facetectomy in which only 50% of the cervical facet joints were removed significantly reduced coupled motions. The lateral translation, axial rotation, and superior-inferior translation all decreased when a lateral bending moment of 3.4 Nm was applied to the head. Also, both lateral translation and rotation were smaller when a lateral force of 89 N was applied to the head, as compared to the intact condition [99]. These changes in coupled motion following facetectomy led to the conclusion that more force must be applied to reach the same degree of neck motion. However, the degree of vertical distraction in response to a tensile load increased after facetectomy, in comparison with the intact condition, suggesting there is greater risk for facet dislocation when a tensile load is combined with a model of loading that also opens the facet joint, like flexion, since less force is required to further separate the facets in that combined loading scenario [99].
3.3. Capsular Ligament Mechanical Properties.
Because the facet capsular ligament is composed of elastin and collagen fibers, that structure can only support tensile and/or shear loading. Accordingly, it can only provide mechanical resistance when the vertebrae that it encloses undergo relative translations and rotations (Fig. 1). As such, the capsule can contribute to limiting the motions of the facet joint. This role was demonstrated in studies using C3-C7 cadaveric spine segments subjected to 100 N of compression coupled with either 2 Nm of sagittal bending or 5 Nm of axial torsion, before and after graded bilateral removal of the capsular ligament [105]. After a removal of 50% of the facet capsule, axial rotation increased by 19% when torsion was applied while the vertical distance between the C4 and C6 spinous processes increased by 5% under flexion [105]. The extent of axial torsion and posterior displacement increased by 25% and 32%, respectively, after 75% of the facet capsule was removed [105]. In a recent investigation using cadaveric cervical motion segments we also measured a significant increase in rotation in flexion after even a unilateral transection of the facet capsule [106]. The increase in the range of motion observed after capsule transection or removal supports that this ligament provides substantial contribution to constraining vertebral motion, particularly in flexion and lateral bending or torsion when the capsular fibers are stretched.
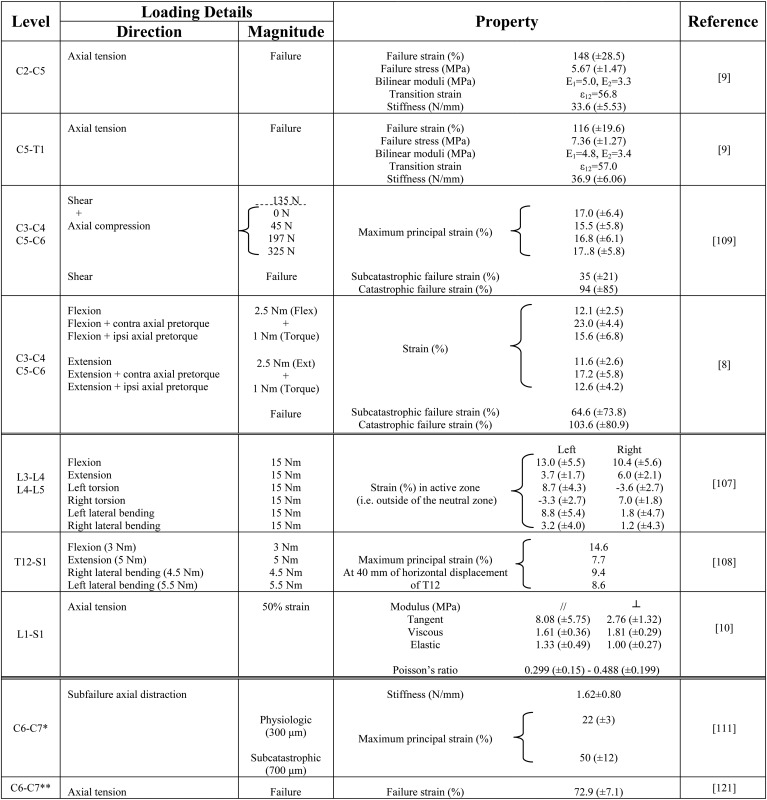
Strains are measured because they inform on the strength, stiffness, and deformability of the capsule and also help to quantify both the failure mechanisms and thresholds of the facet capsular ligament. In particular, the tensile stiffness, ultimate tensile strength, and failure strain of the facet capsular ligament have been measured from isolated cervical and lumbar facet joints in tension to estimate the risk of capsular injury during physiologic bending [8] and also to characterize the anisotropic viscoelastic properties of the ligament for inputs for computational models [9,10]. Both Winkelstein et al. [8] and Yoganandan et al. [9] reported average failure strains ranging from 100 to 150% for the cervical capsular ligament. Little and Khalsa [10] subjected isolated lumbar facet joints to tensile stretch in directions parallel and perpendicular to the principal orientation of the collagen fibers up to a strain of 50% of their measured length in order to characterize the static and dynamic mechanical properties of the capsule. An exponential strain-stress relationship was determined for the capsules stretched in the direction parallel to the collagen fibers, while a linear relationship was obtained for those loaded in the direction perpendicular to the collagen fibers [10]. In these relationships, ε represents the strain and σ the stress for the viscous (V) and elastic (E) cases:
| (8) |
| (9) |
| (10) |
| (11) |
Both Yoganandan et al. [9] and Little and Khalsa [10] reported a nonlinear relationship between the strain and the stress with moduli of the same magnitude (>10 MPa) although the capsules were from cervical and lumbar spines, respectively (Table 1). The mechanical properties of the capsular ligament do not seem to vary across the spinal regions despite different demands for their loading throughout the different regions of the spine and their varied anatomy and orientation of the facets in those regions. The similar mechanical properties could indicate that the mechanical role of the facet capsule is not specific to each spinal region and is secondary to that of the intervertebral disc and supplemented by the other more-robust spinal ligaments in restricting vertebral motions.
Table 1.
Summary of experimental studies of the facet capsule material and mechanical properties
|
3.4. Regional Capsular Strains During Spinal Loading.
Capsular strains measured in human cervical and lumbar spinal segments subjected to flexion, extension, lateral bending, and axial rotation moments have been shown to not be uniform over the entire capsule surface and also that they can reach very large values without being associated with any macroscopic evidence of tissue damage or failure [8,107,108]. Panjabi et al. [107] subjected lumbar motion segments to 15 Nm moments about each of the three anatomical axes and measured the strains in the capsule as an average of the change in distance between five pairs of points materializing the ligament superior and inferior attachments. The strains measured outside of the neutral zone, when vertebral rotations reached approximately 5 degrees, were up to 13% in flexion, 6% in extension, 8.7% in axial rotation, and 8.8% in lateral bending in the left and right facet capsules [107]. Capsular strains have also been measured in cervical motion segments subjected to a 2.5 Nm sagittal moment with and without a 1 Nm axial pretorque but defining full-field strains [8]. Using an array of 30 miniature beads affixed to the lateral region of the right capsule, maximum principal strains were 12% in flexion and extension. Further, in that study, when an axial pretorque was applied directed away from the facet joint being studied for strain, the maximum principal strain significantly increased to 23% in flexion and 17% in extension. For an axial pretorque towards the facet joint, strains increased to 16% in flexion and to 13% in extension but were not significantly greater than in the loading condition without pretorque [8]. In a companion study, flexibility tests on human cadaveric cervical motion segments found that the maximum capsular strain under 135 N of posterior shear was independent of any combined axial compressive loading, and stayed around 17%, with a primary direction oriented along the antero-posterior axis under combined shear, bending, and compression [109]. Under shear loading of isolated facet joints, the maximum principal strain in the capsular ligament reached 35 ± 21% and 94 ± 85%, corresponding to the conditions when the facet joints underwent sufficient loading to induce minor “subcatastrophic” and frank “catastrophic” failures, respectively [109]. Using an array of 6–9 infrared markers implemented on the left and right capsules of the lumbar vertebrae, Ianuzzi et al. [108] implemented the same approach as Winkelstein (2000) and Siegmund (2000) to measure principal strains in the capsular ligament of T12-S1 lumbar specimens during flexion, extension, and lateral bending [8,109]. The maximum principal strains were generally smaller than those measured in the cervical spine, reaching 15% in flexion, 8% in extension, and 9% in lateral bending [108]. The differences between the studies in terms of spinal region, number of vertebral levels tested, and magnitude and application method of the moment, do not permit a direct comparison of the capsular strains reported (Table 1). Yet, the large capsular strains measured at failure during shear loading indicate that the facet capsule can elongate significantly when it is loaded. However, the small capsular strains reported for simple loading conditions such as pure flexion or axial torsion, further demonstrate that the capsule is very strong in resisting deformation and opposing vertebral rotation and translation. This ability also explains the significant increase in vertebral range of motion observed in the experimental studies employing capsulotomy [105,106].
Straining of the fibers in the facet capsule not only results from vertebral motion but also from the activation of the muscles that can occur during mechanical loading of the spine as the outer surface of the capsular ligament is covered by the surrounding paraspinal muscles [110]. As such, the individual fibers of the capsule can become stretched when the muscles that insert on it contract [83,88,110]. In fact, muscle insertions have been found to cover nearly 23% of the capsule area in the cervical spine with a nonuniform spatial distribution [110], which can give rise to unequal capsular strains and stresses in all the regions (posterior, anterior, lateral) of the capsule when different muscles are activated to stabilize the spine during loading. An inhomogeneous mechanical loading environment (either in direction and/or magnitude) of the capsular fibers can also occur due to vertebral motion alone in the absence of any muscle activation. For instance, during flexion, the fibers of the posterior capsule region are stretched while the fibers in the anterior region of the capsule remain lax [8]; in contrast, during extension this pattern is reversed. Variation in strains in the capsule has also been observed in the facet capsule of the rat, in association with painful and nonpainful mechanical loading conditions imposed to the vertebral bones. Upon facet distractions that are considered to be physiologic, the strains reached 21 ± 4% in the posterior region, 17 ± 4% along the postero-lateral ridge, and 18 ± 4% in the lateral region of the C6-C7 facet capsule. Similar differences in strains of the capsule regions were also reported for painful facet joint distractions in that study [111]. Quantitative polarized light imaging was used to measure fibers kinematics during tensile loading of the capsule [111,112] and it was found that distractions of the joint that correspond to those producing pain also produced a significantly greater fiber dispersion in the posterior (23.0 ± 4.9°) than in the lateral (16.8 ± 2.6°) regions of the capsule [111]. Therefore, stretching of the capsular fibers in each region of the capsular ligament depends on the type of loading and on the extent of muscle insertion in that area.
3.5. Capsular Stretch and Neural Activity.
Strains in the capsular ligament and stiffness have also been defined in animal models in which a distraction was imposed across the facet joint in the cervical spine in order to investigate pathomechanisms of facet-based pain [113–123]. In these approaches, an array of 25 to 35 miniature beads was placed on the exposed capsule to calculate strains during cervical distraction. Specifically, Quinn et al. [111] reported a maximum principal strain of 50% for a 700 μm subcatastrophic distraction of the rat C6-C7 facet joint that did not tear the capsule but did produce sustained behavioral hypersensitivity [111]. Kallakuri et al. [121] reported a strain of 73% at failure of the C5-C6 ligament that occurred between 12 and 30 mm tensile stretch in the goat [121]. The capsular strain of 50% measured in the rat model [111] is slightly larger than the 35% strain measured at the first sign of tissue rupture in the human capsule under shear [109] but compares well with the analogous measurement of 65% strain for the human capsule under tension [8]. Similarly, the tensile failure strain of 73% reported for the goat model [121] compares well with the failure strains of 94 ± 85% and 104 ± 81% reported by both Siegmund and Winkelstein for the human capsule [8,109] (Table 1). The similarity between the capsular strain values in the human and animal specimens may be a reflection of their similar mechanical function and composition.
When the capsule is stretched, the nerve afferents that innervate it are also stretched, which has been shown to trigger the generation of neuronal signaling to the central nervous system (CNS) in cases of noxious stretch [113,116,117,123]. Lu et al. [116] stretched the C5-C6 facet joint in a goat model and quantified capsule strains as well as the associated activation of afferents from the joint. They found that the capsule contained afferents that responded with firing at both low- and high-thresholds of strain (10% and 47%, respectively) and also that afferents of both types exhibited persistent generation of afterdischarge for up to 5 mins after the release of the applied strain (39–57%) that did not produce tissue rupture [116]. That work strongly implicated afferent injury in the capsule as a possible mechanism of pain because the afterdischarges were hypothesized as potentially having long-term effects in the CNS. Using a rodent model, Lee et al. [113] distracted the C6-C7 facet joint along the long-axis of the spine and measured a three-fold increase in behavioral hypersensitivity, as well as a significant sustained increase in astrocytic activation in the spinal cord in the absence of any ligament failure. Activated astrocytes modulate immune activation, neuronal synapses and play a role in pain signaling [113]. Using the same rodent model, we have found that after a high-rate facet joint distraction, expression of a glutamate receptor is also elevated in the spinal cord and positively correlated with both the degree of strain in the capsule and the amount of behavioral sensitivity [123]. Collectively, the integration of biomechanics with physiological and behavioral outcomes in these in vivo studies indicate that the loading environment of the afferents in the capsular tissue may be responsible for signaling injury and dysfunction (i.e., pain) in that tissue of the facet joint. In fact, from that combined work it has been suggested that the strain threshold for sustained painful capsular distraction may be between 20 and 47% [113,116,117,123,124].
3.6. Facetectomy Alters the Motion Segment Mechanical Response.
Cusick et al. [100] reported that both unilateral and bilateral cervical facetectomies produced a loss of strength by as much as 32% and 53%, respectively. In those same cadaveric studies, rotations increased by 18% and joint distraction increased by 19% for application of combined compression-flexion [100]. Zdeblick et al. [102] showed that progressive bilateral facetectomy in multisegment cervical spine specimens subjected to 100 N of compression and 5 Nm of torsion significantly decreased torsional stiffness from 0.37 Nm/degree in an intact specimen to nearly half (0.18 Nm/degree) after a complete C5-C6 facetectomy [102]. When the specimens were subjected to 2 Nm of flexion they measured a 25% increase in the vertical distance between the C4 and C6 spinous processes after a 75% facetectomy, which was not significant but did show an increase in C4-C6 rotation [102]. Nowinski et al. [103] proceeded with a similar graded facetectomy procedure on C2-C7 segments after a C3-C6 laminoplasty had already been performed. Applying moments of up to 1.5 Nm about all three axes, they measured an increase of 7 degrees in sagittal rotation, 9 degrees in axial rotation and 3 degrees in lateral rotation [103]. They also measured an increase in translation but no significant change in coupled motion, after 25% or more facetectomy, which is in disagreement with the results reported by Raynor et al. [99].
In the lumbar spine, partial stepwise and total facetectomies also significantly increase rotation in flexion and axial rotation in motion segments loaded in compression (200 N) and subjected to 8 Nm about the three axes [101]. Tender et al. [104] resected the L5 pars interarticularis followed by a total unilateral facet removal on L5-S1 cadaveric motion segments subjected to 280 N of compression and 7.5 Nm of axial torsion. They found that the unilateral facetectomy significantly increased ipsilateral axial rotation by 1.4 degrees and overall axial ROM by 3 degrees. The increase in rotation, the loss of strength, and the decrease in stiffness in the spinal motion segment following facetectomy demonstrate that the facet joint contributes to spinal mechanical stability in a variety of directions and loading scenarios by limiting the linear and rotational motions during physiological loading [104]. The restriction of motion and the assurance of spinal stability provided by the facet joint stem from the biomechanical properties of the capsular ligament, articular cartilage, and bony pillars that together facilitate the functions of the joint as a whole.
3.7. Cartilage Mechanical Properties.
Since the capsule provides support to help keep the facet joint intact during physiologic motions of the spine, the articular surfaces also remain in contact during those normal conditions. During such joint motions, the superficial layer of the cartilage is exposed to both tensile and compressive stresses as the cartilage of the opposing facet makes contact, glides over it and applies compression [49]. With increasing tensile strain, the collagen fibers untangle and straighten to exhibit nonlinear-to-linear σ-ɛ behavior (Eq. (12)), referred to as the fiber-recruitment model [56].
| (12) |
Although the tensile strength of cartilage is provided by collagen fibers, its compressibility depends on the water content [64,66,125,126]. Since joint cartilage contains both fluid and solid elements it exhibits viscoelastic properties [125–130]. This response has been demonstrated in pure-shear tests during which a cartilage specimen is subjected to a sinusoidal angular displacement, while measuring the torque that is generated. From such studies, the dynamic viscoelastic shear modulus (G*) of cartilage has a complex value that has been described by a sinusoidal function of the phase shift angle (δ) between the applied angular displacement and the torque [56]. The viscoelastic nature of cartilage is highlighted by the storage (G’) and loss moduli (G”) that compose the complex shear modulus (G*). The magnitude of the complex shear modulus depends on the amplitude of the angular displacement input (θo), the torque (To), the thickness (h) and polar moment of inertia (Ip) of the specimen (Eq. (13)):
| (13) |
Although many studies have reported the mechanical properties of cartilage from other diarthrodial joints ([64] provides a summary from several investigations), the mechanical properties of healthy facet cartilage tissue have not been well-studied. In fact, only one investigation reports a Young's modulus of 10.08 ± 8.07 MPa and an ultimate strength of 4.44 ± 2.40 MPa for dog-bone-shaped specimens of canine lumbar facet cartilage under tensile loading [66]. Even less has been defined regarding the compressive properties of facet cartilage. The surface of the human facet articular cartilage is curved and has a nonuniform thickness (with a maximum thickness of only approximately 1 mm), making it challenging to harvest. Although the techniques employed by Elder et al. [66] provide a potential method to collect human facet cartilage for compressive and tensile testing, further biomechanical investigations of human facet cartilage are currently lacking. Such techniques may soon enable additional testing to provide a more complete understanding of this tissue's properties in the human.
3.8. Facet Forces and Pressures.
Because the compressive force between articular facets in the joint is transferred to the underlying bone, pressure measurements are important to identify the loading experienced by the cartilaginous matrix. Further, certain loading conditions may place the cartilage matrix at risk for damage and the underlying bone at risk for compressive fracture. However, direct measurement of the contact pressure between the articular cartilage surfaces in the intact facet joint is quite challenging without rupturing the capsule and altering the macroscale mechanics of the joint. Since pressure and force are related by contact area, facet contact pressure has been measured indirectly using proxies such as force applied to the facet. Such force measurements have been made during different modes of loading (compression, extension, flexion, lateral bending, and rotation) in spines from different species. Lumbar and cervical facet forces have been estimated using strains measured on the articular pillar and lamina during flexion, extension, lateral bending, and axial rotation with applied moments varying from 1 to 7.5 Nm with a 100 N axial preload [11,131,132]. In that approach, uniaxial strain gauges were aligned along the major axis of the articular pillar (supero-inferior direction; Fig. 1) and the measured strains from the gauges were used to indirectly interpolate the force transferred through the joint [11,131]. After testing in the motion segment, the facet joint was removed en bloc and tested using loads that were applied at different locations on the exposed articular cartilage, perpendicular to the surface, to establish a strain-force relationship that correlated the strains measured during testing to the actual compressive load develop in the joint at these locations [11,131]. Using that approach, average facet forces of 74 N were estimated for the canine lumbar spine under 2 Nm of extension [131]. Chang et al. [132] reported 205 N under a 10 Nm extension moment combined with 190 N of axial compression, and Sawa et al. [11] reported 51 N under a 7.5 Nm extension moment of human lumbar segments. Although the investigations by Buttermann et al. [131] and Sawa et al. [11] differed in the specifics of the testing methods and specimen species, the facet force in both studies was found to increase when an axial compressive load was superposed on the primary loading vector. Also, both studies identified contraletral axial rotation away from the joint being investigated as the loading condition generating some of the highest facet forces (Table 2). However, further comparisons cannot be made because of the differences in the testing methods of these investigations. In addition, a recent study using the same technique as Buttermann et al. (1991) [131], with strain gauges mounted on the lamina of an isolated cadaveric lumbar vertebra, showed that considerable error can stem from determining facet force from extra-articular strains in all loading configurations except axial rotation [133]. Nevertheless, this strain gauge technique for the evaluation of the facet force preserves the facet capsule and enables comparison of load transfer through the facet joint before and after implantation of a medical device such as a fusion cage or an artificial disc
Table 2.
Summary of estimated facet forces and pressures during loading of intact specimens
| Loading Details | ||||||
|---|---|---|---|---|---|---|
| Level | Direction | Magnitude | Property | Technique | Reference | |
| C4-C5 | Compression | 80 N | Force (N) | 30 − 38 | FEM | [323] |
| C5-C6 | Pressure (MPa) | 0.13 − 0.19 | ||||
| C4-C5 | Flexion | 1.3 (±0.3) Nm | Pressure (MPa) | 0.086 (±0.012) | Pressure paper between | [106] |
| C6-C7 | Extension | 1.7 (±0.5) Nm | 0.092 (±0.014) | facet surfaces | ||
| Flexion | 1.3 (±0.3) Nm | −0.047 (±0.057) | Tip-mounted pressure sensor | |||
| Extension | 1.7 (±0.5) Nm | 0.158 (±0.040) | in posterior region of superior facet | |||
| C5-C6 | Flexion | 2.7 (±0.3) Nm | Pressure (MPa) | 0.010 (±0.010) | Tip-mounted pressure sensor | [146] |
| Extension | 2.4 (±0.3) Nm | 0.068 (±0.027) | in posterior region of superior facet | |||
| C5-C6 | Compression | 73.6 N | Force (N) | 4.2 N | FEM | [322] |
| + | ||||||
| Flexion | None | |||||
| Extension | 1.8 Nm | 37.6 | ||||
| Axial torsion | 28.5 | |||||
| SL CS HE FL | FEM | [331] | ||||
| Cervical | Compression | 4.5 mm | Compressive | 0.29 0.55 0.39 0.05 | ||
| Flexion | stress (MPa) | 0.04 0.23 3.98 0.28 | ||||
| Extension | 18 deg | 0.29 0.30 4.90 0.23 | ||||
| Lateral bending | 0.02 0.14 3.81 0.23 | |||||
| T12-L2 | Flexion | Force (N) | 46.1 (±41.3) | Uniaxial strain gages on the | [11] | |
| Extension | 51.5 (±39.0) | outer lateral portion of L2 | ||||
| Axial torsion − ipsi | 31.3 (±33.4) | superior articular processes | ||||
| Axial torsion − contra | Up to 7.5 Nm | 70.3 (±43.2) | ||||
| Lateral bending − ipsi | (1.5 Nm increments) | 32.0 (±44.4) | ||||
| Lateral bending − contra | 30.6 (±29.1) | |||||
| Axial compression | 400 N | 45.5 (±40.4) | ||||
| + | ||||||
| Flexion | Unspecified | 46.6 (±41.9) | ||||
| Extension | Unspecified | 75.4 (±39.0) | ||||
| L1-L2 | Neutral position | Pressure (MPa) | 4.5 (±1.6) | Pressure paper between facet surfaces | [137] | |
| L2-L3 | ||||||
| L3-L4 | Flexion | 4 deg | 3.7 (±1.3) | |||
| L4-L5 | ||||||
| L5-S1 | Extension | 4 deg | 5.8 (±1.6) | |||
| 6 deg | 6.1 (±1.9) | |||||
| L1-L5 | Compression | 500 N | Force (N) | 43 (@ L2-L3) | FEM | [268] |
| + | ||||||
| Extension | 7.5 Nm (@ L1) | 86 (@ L2-L3) | ||||
| Extension | 20 deg (@ L1) | 117 (@ L4-L5) | ||||
| L2-L3 a | Axial compression | 100 N | 23 (±16) | Uniaxial strain gages on the | [131] | |
| + | outer lateral portion of | |||||
| Flexion | 1 Nm | Force (N) | None | right L3 superior articular process | ||
| Extension | 2 Nm | 74 (±23) | ||||
| Axial torsion | 4 Nm | 92 (±27) | ||||
| Lateral bending − ipsi | 1 Nm | 40 (±32) | ||||
| Lateral bending − contra | 1 Nm | 54 (±19) | ||||
| Axial torsion | 2.3 Nm | 32 | Pressure paper between | |||
| 6.0 Nm | 210 | facet surfaces | ||||
| Left Right | [136] | |||||
| L2-L5 | Flexion | Force (N) | 2 (±5) 4 (±4) | Pressure film between | ||
| Extension | 7.5 Nm | 13 (±14) 14 (±10) | facet surfaces | |||
| Lateral bending | 11(±11) 16 (±14) | |||||
| Axial torsion | 56 (±17) 55 (±18) | |||||
| L4-L5 | Flexion | None | FEM | [271] | ||
| Extension | 7.5 Nm | Force (N) | 50 | |||
| Lateral bending | 36 | |||||
| Axial torsion | 105 | |||||
| L5-S1 | Axial compression | 650 N | Force (N) | Pressure film between | [134] b | |
| Shear | 550 N | facet surfaces | ||||
| + | Group 1 Group 2 | |||||
| Flexion | 40 (±13) 45 (±10) | |||||
| Extension | 6 deg | 54 (±18) 65 (±18) | ||||
| Lateral rotation − ipsi | 50 (±13) 54 (±19) | |||||
| Lateral rotation − contra | 9 (±4) 33 (±10) | |||||
Note: C-Cervical, T-Thoracic, L-Lumbar; FEM − Finite Element Model; SL-slideline model, CS-contact surface model, HE-hyperelastic model, FL-incompressibe fluid model of articular cartilage; ispsi − ipsilateral, contra – contralateral.
canine
reported here from non-tabular data of two separate test groups.
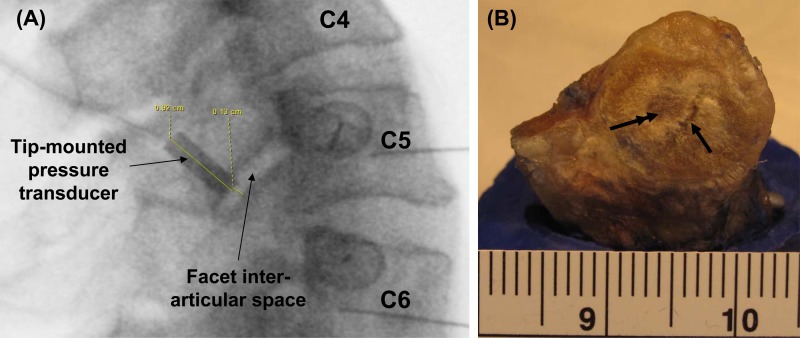
Thin and flat pressure-sensitive paper or sensors can also be inserted between the articular surfaces of the joint after capsule transection to measure facet force [134–136] and contact pressure [137–139]. Using pressure-sensitive paper Dunlop [137] was one of the first to localize the regions and maximal magnitudes of contact pressure that are established in the human cadaveric lumbar facet joint during combined loading, with sagittal bending coupled with a 1000 N compressive load and a 200–400 N shear load applied to motion segments. Contact pressures of up to 3.7 MPa and 6.1 MPa were noted in the central-medial and central-inferior (dorsal) regions of the articular surface near its periphery for 4 degrees of flexion and 6 degrees of extension, respectively [137]. Later, Wiseman et al. [138] measured mean (0.93 MPa) and peak (3.73 MPa) pressures with the same technique in lumbar joints under more aggressive loading scenarios (a combined 700 N axial compression and 15 Nm extension) [138]. More recently Niosi et al. [136] implemented a flat electroresistive pressure sensor array in the L3-L4 facet joints of lumbar motion segments subjected to a 7.5 Nm moment (with and without a 600 N compressive preload) in sagittal bending, lateral bending, and axial torsion. The calibrated sensor measured facet forces of 4 N in flexion, 14 N in extension, 16 N in lateral bending, and 56 N in axial torsion [136]. Using pressure-sensitive paper and a tip-mounted pressure probe fitted through the superior articular facet, our group has measured facet contact pressure in cervical motion segments subjected to 0.8–1.7 Nm extension moments [106] (Fig. 3). The pressure paper localized the area of articular contact in the posterior region of the facet near the periphery of the joint and measured an average pressure of 92 kPa, while the pressure transducer measured an average pressure of 158 kPa [106] (Table 2). Together, all of these investigations showed that contact is not uniform over the articular surface and that the location of contact varies during different loading conditions likely owing to the shape and incongruence of the facet surfaces.
Fig. 3.
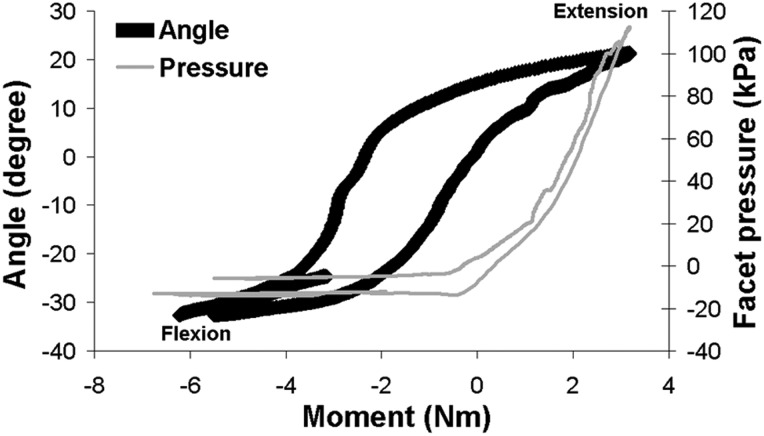
Representative data quantifying the spinal rotations and pressure responses in the facet of a multisegment (C2-T1) cadaveric cervical spine during a range of bending moments applied in continuous flexion-extension. The pressure response in the C5-C6 facet joint increases with applied extension as contact is developed in the articulating facets, but exhibits a different pattern than the rotation angle. In contrast, during flexion, when the joint opens up there is no pressure detected.
Although the flat pressure sensors enable spatial mapping of the location of contact between the articular surfaces of the facet joint during spinal motion, that approach does require that the capsule be cut in order to insert the sensor in the joint. Capsule transection has been shown to contribute to hypermobility of the facet joint [105] and can be hypothesized as also potentially inducing nonphysiological joint loads and/or articular contact, and in locations that are not usually loaded in an intact joint. Capsule transection does not likely affect the joint's behavior in extension since the capsule is not stretched and does not bear load during that direction of loading. But, force measurements in flexion, lateral bending, and axial rotation can be biased since the joint's overall mechanical behavior is modified by the capsule transection itself [105,139–141]. This could explain why the facet force values extrapolated from pressure sensor measurements in the study by Niosi et al. [136] were much smaller than those obtained from strain gauge measurements (Table 2). Furthermore, in any loading condition, the pattern and magnitude of contact are modified by the presence of the sensing device [142–144]. A similar, but less-invasive, method was developed by el-Bohy et al. [145] that maintains the integrity of the facet capsule. In that approach, a 1.5 mm-diameter pressure gauge implemented at the tip of a 13-gauge steel tube was positioned below the posterior bony tip of a lumbar inferior facet just above the cartilage covering the lamina of the vertebrae below [145]. Contact pressures of up to 0.3 MPa were measured at the edge of the articular surface of the lowest vertebra when a combined 600 N compression and 15 Nm flexion loading was applied to three-vertebrae lumbar segments. A comparable sparing-capsule technique was recently implemented in cadaveric cervical motion segments to determine average facet pressures of 10.3 ± 9.7 kPa and 67.6 ± 26.9 kPa for 2.7 Nm flexion and 2.4 Nm extension moments, respectively [144] (Fig. 3).
4. Mechanotransduction
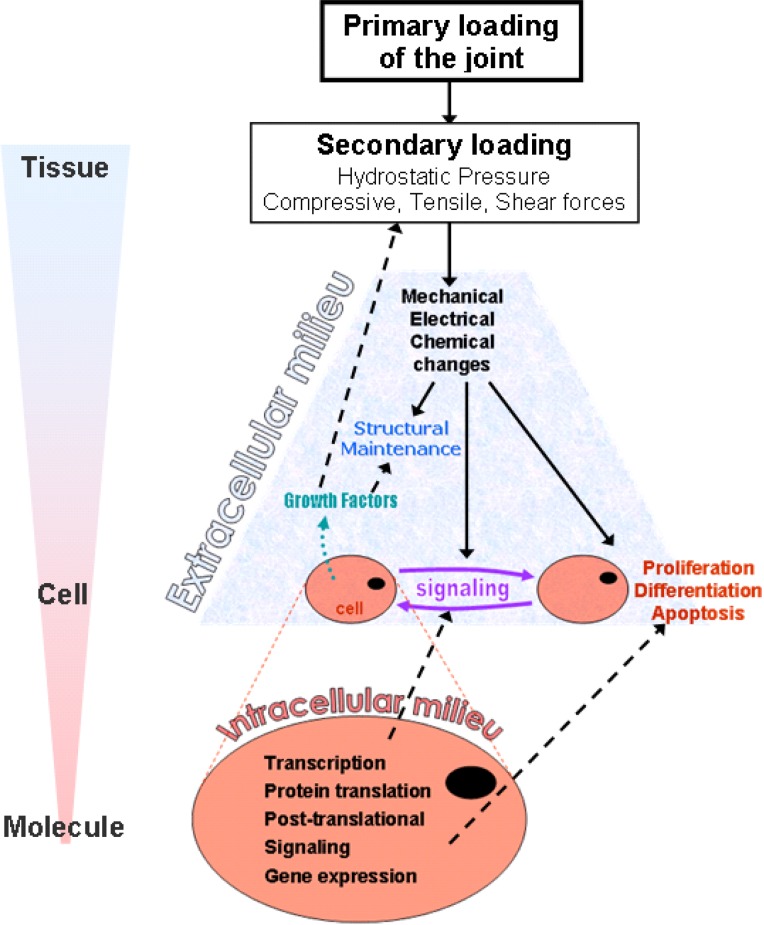
Since a portion of the spine's mechanical loading is supported by the facet joint, a variety of mechanical, physical, and chemical cascades are initiated in response to loading of the individual tissue components comprising the facet joint. These physiologic responses occur across several scales, ranging from the macroscopic tissue-level, to cellular and molecular levels via many mechanotransduction mechanisms. Although mechanotransduction can control and contribute to maintenance of the tissues in the joint [57,147–149], this process can also lead to and/or accelerate tissue degeneration and dysfunction [150,151]. The mechanisms of mechanotransduction in articular cartilage, ligaments, and bone have been described in other synovial joints. Broadly, as the first step the external primary spinal input (load or motion) is transformed into a secondary tissue-specific loading profile (Fig. 4). Then, the tissue-specific loads elicit a host of cascading mechanical, electrical, and chemical responses from the various elements that compose the tissue. These responses trigger further chemical changes that affect the intracellular milieu (protein translation, gene transcription, post-translational signaling) and the intercellular signaling (Fig. 4). Both the initial mechanical, electrical, and chemical changes and the modification of the intracellular milieu alter the intercellular signaling as well as the cellular activity (i.e., proliferation, differentiation, apoptosis). Modification of cellular activity can result in the release of chemical agents and electrical signals that influence the maintenance of the extracellular milieu, but can also alter the secondary tissue-specific loading (Fig. 4). Together, these physiological responses can modify the mechanical behavior of the tissue and lead to further changes in its response to mechanical loading and degeneration. Although this cascade has been well defined through a large body of elegant work, very few articles specifically address and detail these processes in the tissues of the facet joint. Therefore, this section reviews the mechanotransduction mechanisms known for the facet joint tissues and also provides a more global review of such mechanisms in similar tissues from other synovial joints.
Fig. 4.
Schematic representation of the generalized processes of mechanotransduction in synovial joints, across the scales ranging from tissue to molecule
4.1. Mechanotransduction in the Facet Joint and its Tissues
4.1.1. Capsular Ligament.
Since spinal loading and motion are both guided and constrained by the facet joints the primary mechanical loading of the facet joint induces primarily capsular ligament stretch and compression of the cartilaginous articular surfaces and the subchondral bone. In the capsular ligament under stretch, the collagen fiber structure and the nerve endings embedded in that network [152] and cells (fibroblasts, macrophages) are all distorted and activated [153]. Accordingly, capsular deformations of certain magnitudes can trigger a wide range of neuronal and inflammatory responses [124,154,155]. Neurophysiologic studies with a goat model have shown that the nerve endings in the capsule possess different stretch thresholds for activation [116]. Although most of the proprioceptive and nociceptive afferents have a low-strain threshold (∼10%) for activation, a few receptors have a high-strain threshold (42%) for signal generation via neural discharge. In addition, capsular strains greater than 47% activate nociceptors with pain signals transmitted directly to the central nervous system [116]. Among both the low- and high-strain threshold neural receptors in the capsular ligament a few sustain their firing even after the stretching of the capsular ligament is released [116,154]. This persistent afterdischarge evident for strains above 45% constitutes a peripheral sensitization that may lead to central sensitization with long-term effects in some cases [154]. Also, in vivo stretch of goat cervical capsule until its rupture (up to ∼30 mm) showed that the strains in the capsule averaged 73% and were sufficient to induce changes in axons taken as indicators of dysfunction (i.e., swelling, retraction beads, vacuolations). The effect of the capsule distraction on axonal changes was significant, with the ratio of abnormal to normal axons being greater in the stretched (94/186) than in unstretched capsules (29/108) [121]. Such axonal changes can be a source of hyperexcitability, spontaneous firing, and persistent pain [156] since that axonal dysfunction subsequently disrupts gene transcription of substance P, a neuropeptide protein involved in pain signaling [157]. In addition, inflammation in the facet joint also increases the discharge rate of multiunit nerves, sensitizes the nerves to mechanical stimulation, and activates previously inactive nerves [158].
The neural signals from the capsule travel via the primary afferents to the dorsal root ganglion (DRG) and spinal cord, and can induce several hallmarks of neuroinflammation, including glial activation [155] and cytokine upregulation [159,160]. These inflammatory responses have been reported after failure of the facet capsular ligament and also after its subfailure distraction in a rat model [161]. In response to the injurious stimuli, neuropeptides involved in pain signaling, such as substance P, are also modified. Substance P protein expression in the DRG after painful capsule distractions was twice that of nonpainful distractions of controls [115,124]. Although no gross damage of the capsule was observed after a painful distraction, spinal astrocytic activation was 61% greater and pain symptoms were also increased [113].
Capsular strains causing damage to the ligament structure can also activate fibroblasts directly or indirectly for structural repairs. While strains causing excessive failure of the collagenous ligament structure trigger an inflammatory-driven cellular response, subfailure strains elicit a fibroblast-mediated remodeling response to restore integrity to the damaged collagen structure [153]. Complete tissue tearing elicits an inflammatory response of the tissue that results in macrophage infiltration in order to clear any debris from the damaged collagen fibers and matrix. During the phagocytosis of the debris these cells release molecules that also trigger the recruitment of additional fibroblasts with increased collagen expression and this response can also lead to the formation of a provisional collagenous scar [153]. In the case of a subfailure loading scenario, no inflammatory response is observed and an increase in proteoglycans (decorin, fibromodulin) might actually help to modulate the fibrillogenesis of newly synthesized collagen by the fibroblasts [153].
4.1.2. Cartilage.
Compression of the articular cartilage in the joint can occur during any mechanical loading of the facet joint [136]. Although compressive load is transferred via the facets between adjacent spinal levels and contact pressure develops in the facets' articular cartilage, contact is not uniform and the facet surface presents both load-bearing and nonload-bearing regions [162–165]. Given the difference in material properties between the various zones of the same tissue, the mechanisms by which mechanical signals modulate physiologic responses likely also lead to different spatial distributions of the responses in the affected tissues. However, the particular relationship between the mechanical, chemical, and cellular responses to compression in the cartilaginous matrix of the different zones remains largely unreported for the human spinal facet joint. Nevertheless, damage to the cartilage structure elicits an inflammatory response [43,166], which itself can also elicit not just osteoarthritis of the joint but can modulate pain signals from other regions of the joint. For example, one study showed that the inflammatory cytokines IL-6 and IL-1β were present in the facet cartilage retrieved from patients undergoing surgery for lumbar spinal canal stenosis and disc herniation [160]. This result led to the conclusion that pain symptoms might be due not only to mechanical tissue insults but also to chemical irritation of the tissue from the inflammatory agents leaking from the facet joint into the spinal space.
4.2. Mechanotransduction Processes in Articular Cartilage of other Synovial Joints.
Mechanical stimuli elicit a cascade of multistep responses including mechanocoupling, mechanotransduction, intracellular conversion, and cellular response from articular cartilage (Fig. 4) [165]. These steps differ between the thick, proteoglycan-rich load-bearing areas and the mechanically weaker nonload-bearing areas of the articular cartilage layer because the extracellular environment (collagen fibers, proteoglycan and water content) varies along the depth of the cartilage layer (Fig. 2). These structural and compositional variations imply that the cellular responses to mechanical loading vary within each zone of the cartilage layer as well [46,167].
Tensile stresses that arise in the more superficial zone of the cartilage layer and hydrostatic pressure increases in the transitional and deep zones are converted at the tissue and cellular levels into electrical, chemical, and biomechanical stimuli [168]. Distortion of the chondrocyte membrane and nucleus, changes in membrane potential, electric stimulation from streaming potentials and changes in matrix water content, ion concentrations and pH are all likely involved in the metabolic changes of compressed cartilage [168].
Chondrocytes are embedded in the collagen matrix of cartilage and deform with it under compressive and shear strain [46,168–170]. Round chondrocytes cultured in a bioengineered cartilage have been shown to become polygonal, doubling their area, and spread after cyclic compressive load (1800 cycles at 1 Hz); they resumed their initial round shape and size within six hours after the cessation of stimulation [170]. Changes in chondrocytic shape and spreading are linked to an increase in their secretion of matrix metalloproteinases that augment the accumulation of newly synthesized proteoglycans [170], and maintain the tissue function by synthesizing matrix molecules such as aggrecans and type II collagen [169,171]. When chondrocytes are deformed, ion channels present on their membrane are activated [165,172]; the levels of intracellular calcium Ca2+ have been shown to increase under hydrostatic pressure [173]. An increase in calcium concentration can inhibit the accumulation of cyclic adenosine monophosphate (cAMP), a second messenger used for intracellular signal transduction. This inhibition and reduction in cAMP may induce cell proliferation [173]. Under mechanical loading, chondrocytic proliferation and differentiation [46,165] result in a greater number of cells for the synthesis of extracellular matrix (ECM) components (collagen, proteoglycans). For example, intermittent tensile stresses applied to chondrocytes from the rat rib growth plate increased both DNA and proteoglycan synthesis by about 1.5-fold [174]. Newly formed collagen is used for the maintenance and repair of damaged extracellular matrix, while providing a support medium for the proteoglycans to trap water in order to resist compression. However, if the hydrostatic pressure is too high (5–50 MPa) cytoskeletal elements of the chondrocyte, such as the Golgi apparatus and microtubules, can disorganize, and there may be a reduction in protein synthesis and inhibition of membrane transport [175]. Changes in chondrocytic growth and cellular division are modulated by mechanical loading as the physicochemical mechanisms within the cells in which the coding for protein synthesis occur via gene transcription, protein translation, and post-translational modifications [41,147,165,176].
The mechanical loading of cartilage also influences tissue metabolism indirectly through electric stimulation from streaming potentials. Streaming potentials develop when the cations contained in the synovial fluid penetrate the matrix to interact with the increasing concentration of proteoglycans that bear negative charges, as fluid flows in and out of the cartilage matrix during compression [46]. Streaming potentials are likely associated with an electric potential jump across the chondrocytes' membrane [172], which can stimulate the biosynthesis of these cells during dynamic compression [177]. Considering the electrokinetic transduction taking place during cyclic compression of cartilage led to the development of a solid-fluid interaction relationship (see Eq. (14)) in which the total area-averaged fluid velocity (U) and the current density (J) both depend on the electric potential generated across the specimen (V) and the fluid pressure of the surrounding bath (P) via a combination of the circuit hydraulic permeability (k11), the electrokinetic coupling coefficients (k12 = k21) and the electrical conductivity (k22) of the specimen [177].
| (14) |
Upon this electrical stimulation, as well as a shape alteration caused by a volume change in the surrounding extra-cellular matrix, chondrocytes synthesize proteins involved in the maintenance of the cartilaginous matrix other than collagen and proteoglycans. Neu et al. [178] reported that transforming growth factor (TGFβ) mediates the secretion of lubricin, a glycoprotein synthesized by the chondrocytes of the superficial zone, in bovine condylar explants subjected to shear loading. Lubricin is a lubricative glycoprotein that maintains the tribological properties of the synovial joint and inhibits synovial cell overgrowths. Knowing its regulatory mechanisms can provide insight on the progression and potentially treatment of degenerative processes of cartilage [178].
Generally, the tissue and cellular responses to mechanical stimulation in the joint depend on the frequency, amplitude, and rate of loading. The rate of matrix synthesis decreases as the static hydrostatic pressure increases up to 50 MPa [46,179]. In contrast, cyclic loading can stimulate matrix synthesis [165,174]. However, physiologic responses to cyclic loading have been shown to be temporally- and spatially-dependent since extracellular osmolality is not homogeneous across the depth of each cartilage layer [46,50,165,167,178,180]. However, it is not possible to fully define the tissue and cellular responses of cartilage to cyclic compression because there are also variations and inhomogeneities in the extracellular oxygen content, water content, pH [48,167] and in the magnitude, frequency, duration, and location of the applied loads [41]—all of which complicate these responses. The mechanotransduction processes and their synergistic or antagonistic interactions in articular cartilage are not yet fully understood. However, from a global perspective, mechanical energy is converted into a type of energy useful for the cells to proliferate, differentiate, communicate, and synthesize proteins for the maintenance of the extracellular-matrix in response to loading. The mechano-electrochemical processes that take place in cartilage are thus very similar to those that take place in the surrounding bone and ligaments. However, such mechanotransduction mechanisms could be limited in cartilage because, unlike those other tissues, it is both avascular and aneural [48].
4.3. Mechanotransduction Processes in Bone and Ligament.
In bone, as in cartilage, a multistep process including mechanocoupling, biochemical coupling, transmission of intercellular signal, and effector cell response (Fig. 4) was identified between mechanical strains and the tissue response [181]. Briefly, mechanocoupling defines how mechanical energy is detected by the bone cells in the tissue (osteocytes, osteoblasts). The process consists in a transformation of mechanical strains into fluid pressure in the canaliculae, which generate fluid shear stresses on the osteocytes' membranes. Fluid flow also generates streaming potential, an electrical energy, which stimulates bone cells for remodeling and repair as proven by bone fracture healing from exposure to electromagnetic fields [182–185]. Both the magnitude and the frequency of the mechanical stimulation, and also the strain rate, influence the bone cells response. Turner et al. [186] reported that mechanically induced bone formation was not increased in the rat tibia subjected to bending until the loading frequency increased over 0.5 Hz [186]. Biochemical coupling likely takes place at the binding interface between the cells' membrane and the extracellular matrix. This attachment of the cell generates tensile forces on the cytoskeleton that alter the shape of the cell, its phenotypic expression, the binding of protein to the cell's membrane, and the activation of ionic channels on the cell's membrane. Release of prostaglandins and nitric oxide by the osteocytes activates the proliferation of and matrix synthesis by osteoblasts [187]. The complex biochemical interactions between the ECM and the bone cells and within the cells are quite complicated and can be the focus of a review themselves. Since this is not the central focus of this review and they have been elegantly detailed in recent specialized reviews [148,187], they are only broadly presented here. In essence, several peptides and proteins, such as insulinlike growth factor (IGF), epidermal growth factor (EGF), bone morphogenic protein (BMP), transforming growth factor (TGFβ), bind to the osteoblastic membrane and modulate kinase activity within the cell which induces expression of activator protein 1 (AP-1) in the cell nucleus. Intracellular chemical signaling is also modulated by the entrance of extracellular calcium ions into the intracellular milieu. Ultimately, the mechanotransduction mechanisms permit the adaptation and maintenance of the bone structure to mechanical loading by acting on bone-regulating genes contained in the nucleus of bone cells leading to their proliferation, differentiation, and survival [148,187].
Mechanical loading of the facet capsule imparts structural changes to the collagen structure that can result in the degradation of its mechanical properties, loss of function, and pain-activating protein generation [88,112,155]. But ligaments also contain fibrogenic cells that are directly and indirectly affected by mechanical loading as chondrocytes in cartilage [188]. Fibroblasts deform when the tissue in which they are embedded deforms. Matyas et al. [189] observed that the nucleus of fibroblasts contained in the rabbit medial collateral ligament were 4 μm longer and 1 μm thinner when the ligament was under 6% tensile strain than at rest. Accordingly, the nuclear roundness decreased from 0.4 to 0.19 [189]. Upon deformation of the cell membrane, stretch-activated ion channels might be activated which permits the penetration and increase of cation concentration in the intracellular milieu and can eventually alter cellular activities as was described above for bone and cartilage cells. Studies of the periodontal ligament showed that mechanical stimuli also indirectly affect fibroblastic activity via trans-membrane and intracellular signaling as for osteoblasts. Disturbance of the ECM homeostasis leads to an intracellular conversion of the mechanical signal into a biochemical one via the transduction of focal adhesion molecules such as FAK and MAP-kinases in the fibroblast [190]. The cells then synthesize and release matrix metalloproteinase in the ECM for the regulation, modification, or degradation of ECM components [169], to modulate the mechanical loading state of the tissue. Similarly, loading of periodontal ligament fibroblasts was shown to activate various kinase proteins (ERK, JNK, p38) that communicate with the inner cellular milieu and activate AP-1 in the cells' nucleus. AP-1 can up-regulate the COL I gene in the nucleus of the fibroblasts, stimulating collagen expression by these cells [191]. Collagen expression is used to either maintain or repair the extracellular matrix.
Fibroblast activity and interaction with the extracellular environment is directly and indirectly affected by mechanical loading and tissue deformation. Such mechanotransduction mechanisms are similar to those described for cartilage and bone. Although the general mechanisms of mechanotransduction in bone, ligaments, and cartilage have been identified, as illustrated in Fig. 3, they remain to be elucidated more specifically for the facet joint tissues.
5. Injury and Trauma of the Facet and Other Synovial Joints
Facet joint injuries result most-often from motor vehicle and sports trauma, such as skiing, snowboarding, cycling, and diving [82,192–195], and include a wide range of bony and ligamentous lesions depending on the extent and type of tissue trauma. Interestingly, unilateral and bilateral facet injuries make up nearly 6% of all cervical injuries, with undisplaced fractures, subluxations, and dislocations being the most commonly reported facet injuries [192,193,195,196]. Facet injuries often directly damage the hard and soft tissues that compose that joint (Fig. 2) [8,197–200]. In addition, facet trauma is also associated with the occurrence of damage to other soft tissues of the spine, such as disc tearing, spinal cord trauma, and/or nerve root compression, all of which can also lead to a transient or even permanent loss of mechanical and neurological function of the facet joint, spinal column and/or physiologic sequelae [193,201–206].
5.1. Facet Joint Injuries.
Because the facet joints comprise the integrative biomechanical structure of the spine, any violation of their mechanical integrity as can be caused by injury or trauma directly affects the mechanical behavior of a motion segment or even the overall spinal region [195,206]. For example, a unilateral locked facet at C5-C6 produced by combined lateral bending and flexion of the cervical spine has been reported to significantly reduce the segmental range of motion (ROM) by 2.7 – 3.6 degrees in all modes of loading except in ipsilateral axial rotation [206]. Once unlocked, the ROM of the C5-C6 motion segment was further increased compared to its preinjury intact condition by an additional 3.5 – 8.0 deg. This report, suggests that the capsular ligament had been damaged due to the facet injury. Indeed, Crawford et al. [206] also reported that the laxity of the capsular ligament was significantly increased after that locked facet condition compared to that in the intact condition. Also, the increase in laxity was associated with some ligament tearing, supporting both the hypothesis that the ligament sustained damage and exhibited altered mechanical properties [206]. Taken with reports of instability following facet dislocation [193,201,207], these findings imply that there may be a continuum between the degree of instability and trauma to the facet, with greater instability for more severe facet trauma, including dislocation and the more-extreme fracture.
In a clinical study of patients with cervical unilateral lateral mass facet fractures, Lee and Sung (2009) found that the degree of axial rotation and the segmental kyphosis were significantly greater in those patients whose facet was both fractured and dislocated than in those sustaining only a facet fracture [195]. Despite these differences, both types of injury were associated with instability for the cervical spine in rotation; surgical treatment was required to sufficiently restore stability, again demonstrating the role of the facet joint in limiting spinal motions, in particular rotation. Also, unilateral fracture of the facet joint has been shown to lead to spondylolisthesis, an anterior translation of the superior vertebra, sometimes associated with an axial rotation of the superior vertebra around the intact contralateral mass [195,208]. Such a fracture injury can lead to a variety of neurological disorders since the motion segment is unstable and can compress the spinal cord and/or nerve roots during certain motions. In these cases there is also the potential for capsule injury when the fractured vertebra exhibits abnormal kinematics during physiologic motions that can also impose nonphysiologic compressive stresses on either or both the capsule and cartilage of the contralateral facet joint [208]. In the same way, excision of the capsule and cartilage during a surgical procedure has been shown to increase the sagittal and axial ranges of motion by 38% and 57%, respectively [209]. Most simply, fractures of the articular pillar or lateral mass impose an overt disruption of the facet joint's mechanical properties since they eliminate the joint's ability to support any load and, in so doing, can cause spinal instability and neurological impairment.
5.2. Surgical Treatments of Facet Injuries and Effects on Facet Biomechanics.
Fractures of the bones of the facet joint leading to joint separation, comminution, split, and traumatic spondylolysis, require surgical treatment to reduce the anterior translation and axial rotational deformity associated with these injuries [195,210]. However, the type of treatment varies with the type and severity of the fracture [210]. A separation fracture that isolates the entire lateral mass can be treated with a pedicle screw that provides stability and strength while also encouraging bone growth [210]. However, if the separation fracture is also associated with disc and/or ligamentous damage, a one-level reduction and stabilization is recommended to avoid any slippage of the vertebra; fractures which can also result in the development of multiple bone fragmentations and traumatic spondylolisthesis also require only a single-level stabilization to treat the unstable anterior translation of the superior vertebra. Split and severe fractures have been shown to be successfully treated with two-level posterior fixation that resolves both the spinal instability and restores the spinal alignment [210]. In the most severe cases, the articular surface of the facet joint can become completely obliterated and the articulation so disrupted that the constraining and guiding functions of the facet joint cannot be restored; in that case, fusion is necessary.
Surgical fusion can relieve many of the physiologic symptoms caused by facet fracture but the mechanical function of the joint is not fully returned to normal. In fact, the normal range and pattern of motions in the whole vertebral motion segment are altered substantially [192,195,210]. In biomechanical studies of cervical and lumbar fusions, vertebral rotations were shown to decrease at the fused level and increase at the adjacent levels [211,212]. Akamaru et al. (2003) reported that sagittal rotation significantly decreased by over 80% at the fused L4-L5 level and by 100% at the adjacent level superior to it [211]. Similarly, Finn et al. [212] reported that sagittal rotation decreased by 78% at the fused C4-C5 level, but increased by only 10–34% at all of the unfused levels in a multisegment (C2-C7) cadaveric specimen. The trend was similar in lateral bending and axial rotation at the unfused levels [212]. The overall spinal stiffness is also increased by fusion, to differing degrees depending on the antero-posterior positioning of the fusion construct [213]. Those authors also found that the center of rotation of the L5-S1 motion segment shifted supero-posteriorly with a posterior fusion, which increased the stress on the facets and the motion at the adjacent level. Further, because fusion restricts the motions at the level of intervention, it also modifies the kinematics and kinetics of the adjacent levels. Although biomechanical studies have evaluated the at- and adjacent-level biomechanics, there is growing clinical evidence from longer-term studies indicating that the mechanical demands on the spine must be met by the adjacent motion segments and imposes potentially-nonphysiologic and abnormal loading on the tissues of those joints leading to their dysfunction and/or damage [214–216].
5.3. Neurologic Disorders Associated with Facet Joint Injuries.
The proximity of the neural structures of the spine—the nerve roots and spinal cord—to the facet joint complicate treatment of any injury of the facet joint [203]. In fact, Hadley et al. [192] reported that 90% of patients with either a unilateral or bilateral cervical facet fracture-dislocation also presented with neurological injuries. Although unilateral facet injuries might not induce any neurological disorders [192], these injuries can cause severe chronic pain without local pathology [193]. Radiculopathy, myelopathy, spinal cord injury, and neck pain have all been reported in conjunction with facet injuries [195,207]; facet injuries have also been linked to neurologic deficit, vertebral artery injury, and avascular necrosis of the articular pillar [217–220]. The array of neurologic disorder and syndromes associated with facet injury show that the mechanical function of the facet joints is essential not only for spinal motion but also for the protection of neural structures important to the proper function of the nervous system.
Although a torn facet capsular ligament will lose its overall mechanical integrity, its mechanical failure can also induce pathophysiological responses, including nociception and pain. In fact, even subcatastrophic or “subfailure” loading of the facet capsule has been shown to induce pain in a variety of scenarios [113,118,123]. Perhaps the best example of subcatastrophic capsular distortions modulating physiological responses is highlighted by spinal loading and whiplash-associated disorders [82,116,111,123,221]. Whiplash-associated disorders are particularly challenging in terms of understanding the biomechanics of the tissue injury and the physiological consequences because there is often no radiologic or other imaging evidence that reveals any obvious indicators of tissue trauma [200, 222–223]. However, many volunteer and cadaveric studies have been performed to define the kinetics and kinematics of the cervical spine during vehicular rear impacts in order to define local tissue responses, their relationship to spinal kinematics, symptom and tissue injury risks, and tolerance for injury [8,194,197–199,224,225]. More specifically, these investigations have focused on measuring the deformation imparted to the spinal tissues (ligaments and disc, mainly) by the rear impact and the associated changes in their mechanical properties.
5.4. Capsule Damage, Laxity and Failure.
Simulations using cadaveric cervical spines mounted to a table-top sled and exposed to rear-end accelerations have been used to study the response of a variety of soft tissues in the cervical spine, including the facet capsular ligament. For simulations of whiplash exposures, the strains in the capsular ligament were found to be 2–5 times greater than those sustained during physiologic motions of the cervical spine [198]. In a similar but separate study, the facet joints of cervical spines that were previously exposed to a whiplash injury were then exercised under low-level tension and found to undergo elongations nearly three times greater than unexposed ligaments for the same tensile loads [194]. Those capsular ligaments were also found to exhibit greater laxity after the purported injury [194]. Since increased laxity may be linked to a reduction in the joint's ability to stabilize the motion segment during sagittal motion [226], this finding suggests that whiplash exposure may alter the structure of the individual tissues of the facet, such as the capsular ligament, and/or the mechanotransduction processes that could maintain and repair the ligamentous structure. Accordingly, such an injury exposure could initiate a variety of signaling cascades that prevent a full recovery of the mechanical properties of the tissues in the facet joint.
Collagen fiber damage and/or rupture have been hypothesized as a cause of facet capsule laxity [111,112,227]. This hypothesis has been investigated in a variety of different ex vivo studies, but recent studies in a rat model of pain have explicitly related such changes in mechanical properties and local reorganization of the collagen fibers to physiological outcomes following direct stretching of the capsule. In particular, painful distractions of the cervical capsule were found to induce greater laxity (7.30 ± 3.01%) and a greater decrease in tensile stiffness (1.47 ± 0.86 N/mm) than nonpainful distractions (0.99 ± 0.45%; 0.36 ± 0.22 N/mm) [111]. Evaluation of fiber disorganization was performed following these same conditions and the dispersion angle quantifying fiber alignment increased by 38% after painful loading of the ligament and was found to exhibit a spatial dependence of such changes occurring only in some regions of the capsule [111]. Furthermore, anomalous re-alignment of the collagen fibers in the rat facet capsule was detected real-time during its loading in ex vivo studies of that tissue [112]. Interestingly, fiber re-alignment was found to occur at loads and deformations preceding the tissue's yielding and at joint loading conditions corresponding to those that induce persistent pain in the rat [113]. Although the capsular ligament might not display any sign of macroscopic failure or tissue rupture, the changes in its mechanical properties can be explained by the anomalous realignment of the capsule fibers during tension, which results from microstructural damage [112,120]. By extension, injury of the ligament tissue that modifies the collagen fiber kinematics may also be sufficient to damage the cells contained in the matrix, leading to apoptosis and substantial changes in their gene expression [153]. Repair of a ruptured collagenous structure is compensated by the deposition of a provisional scar tissue that is later remodeled by fibroblast-mediated activity. However, these changes as well as those to the fibers and dispersed cells could provide a mechanism to explain the deficits in strength and laxity that have been measured in ligaments after subfailure [111,153].
Mechanical stretch of the capsule can produce both subcatastrophic and catastrophic injuries, which range from its yielding or a visible tear or rupture in the tissue. The thresholds for these types of injuries are different and vary with the mode of loading. For example, using similar cadaveric preparations the maximal strains at subcatastrophic failure in shear (35 ± 21%) were nearly half of those in tension (65 ± 74%) for the same cervical levels [8,109]. In contrast, the strains at tissue rupture were quite similar: 94 ± 85% in shear failure and 104 ± 81% in tensile failure [8,109]. The failure strains were similar to those strains reported by Yoganandan et al. (2000) for upper cervical specimens (148 ± 28%) and lower cervical specimens (116 ± 20%) (Table 1). By comparison, comparable strains are observed for failures of cervical capsules in the rat and goat under tension [113,111,112,119,121]. The similarities in the strains at failure across cervical levels and species bolster the use of animal models to investigate the biomechanical responses of these tissues and the relationship between mechanical loading and physiological outcomes. Accordingly, electrophysiological and pain studies in these same animal species have shown that capsular strains lower than the threshold for mechanical injury or failure can elicit acute modifications of the neuronal signaling as well as pain [112,114,116,124]. However, the mechanical thresholds for such modifications have not yet been identified or investigated in humans.
5.5. Structural Damage of Cartilage Alters its Mechanical Properties.
Facet articular cartilage also can be injured when the motion and loading of the joint gives rise to a nonphysiologic contact between the facets. For example, Pearson et al. [198] reported a three to five fold increase in compression of the articular surfaces in each facet at both the C2-C3 and C4-C5 levels of cervical spines exposed to 3.5 to 8 g impacts in whiplash simulations [198]. Although the injury threshold for facet cartilage is unknown, such an increase in facet compression could lead to an overloading of cartilage that causes its injury. The fact that a significant increase in facet compression was only observed at two vertebral levels demonstrates that spinal mechanics vary in the upper and lower cervical spine which correlates with the loading pattern associated with whiplash [198]. The potential for compressive loading to cause cartilage injury is further supported by the results of physiologic cyclic compression applied to this tissue. Fibrillation of the facet articular cartilage has been observed in the lumbar spine during torsional fatigue studies subjecting specimens to 10,000 cycles of either ±1.5 degrees of torsion or 11–45 Nm of axial torque [228].
Although the degree of disruption of the cartilage matrix depends on whether the articular surface is lacerated, penetrated, or impacted, as well as the extent of the lesion, the loading rate, and the fluid content of the cartilage layer [52,229,230], any changes to the cartilage matrix modify its mechanical properties. For example, sudden degradation of the cartilage structure of bovine ulna caused by a 1.1–2.8 J impact of tissue explants was sufficient to decrease the compressive stiffness from 687 kPa to 156 kPa at four weeks after the impact [231]. Kurz et al. [232] subjected bovine knee cartilage explants to 50% compressive strains and found a significant decrease in the compressive and shear stiffnesses for those explants tested at 1 sec−1 in comparison to those tested at a strain rate of 0.01 sec−1 [232]. Cyclic tensile strains and impact can generate cracks in the lamina splendens of the cartilage layer [52,228]; the cracks can extend deeper in the cartilage layer upon further compressive loading if the rate of degradation exceeds the rate of repair. Disruption of the collagen structure alters the process of fiber recruitment during loading but it also alters the matrix fluid pressure which is involved in fibril reinforcement during creep and relaxation [233]. In turn, modification of the creep and relaxation times can result in higher stresses being maintained longer in the cartilage matrix which can also lead to its degradation over time [234]. The results from all of these investigations show that as soon as the structural integrity of facet cartilage is violated, its mechanical properties also become weakened and the response of the cartilage matrix to compression is further modified, which can change the mechanical environment of the chondrocytes and the mechanical response of these cells as well.
5.6. Chondrocytic Activity After Joint Injury.
In addition to structural damage, both fibrillation and impact of articular cartilage also decrease the cellular activity that is necessary for tissue repair and the maintenance of the native properties of that tissue. Piperno et al. [235] measured that the degree of chondrocytes adherent to fibronectin (an ECM component) was nearly one-half of that of healthy cells for those obtained from fibrillated cartilage of human femoral heads; this could indicate the cells' dysregulation and could explain the development of osteoarthritis [235]. Impact injury has a more pronounced effect than fibrillation on chondrocytes and provokes these cells to undergo necrosis and apoptosis as highlighted by studies on cell viability [55,65,231,232,236–238]. Despite using cartilage explants from joints of different species and regions (canine and bovine humeral head; bovine elbow and knee; rabbit patella) and subjecting them to different impact scenarios (single, cyclic, magnitude), these studies all report an increase in cell death after impact. Torzilli et al. [237] reported that for compressive strains less than 50%, cell death was restricted only to the superficial zone, while it progressed deeper but never reached the deep zone for strains greater than 60%. Because cartilage is an avascular tissue, its repair is slow and performed by diffusion mechanisms and the surviving chondrocytes which have a limited metabolic activity [229,231]. When a cartilage injury leads to cell death, less collagen is synthesized and therefore less is available to repair the damaged structure. Moreover, the repaired matrix has properties of fibrocartilage rather than those of hyaline cartilage [229]. Therefore, any modifications of the mechanical environment of those cells inhibit their proper functioning and can lead to further degradation of the mechanical function of the cartilage. Finally, chondrocytes derive their nutritive energy from the synovial fluid so their function and survival can be hindered by leaks of synovial fluid through tears or ruptures of the synovial membrane that have been reported to occur with facet joint and capsule stretch [228,238]. A loss of synovial fluid; therefore, means that the joint can lose its lubricative environment and friction can be increased between the articular surfaces of the joint, thereby potentially accelerating degradation of the cartilage matrix. But, the loss of fluid also means that the cellular activity (synthesis of collagen for matrix maintenance and repair) will similarly also not be as potent.
In each of the cartilage, capsule, synovium, and bone of the facets, the threshold for injury changes overtime, with age, metabolic activity, disease, the nature of injury, and the extent of the lesion [229]. Repair of any injured tissues usually takes place for minor lesions and the joint can regain its functionality over time, but likely never reaching its pretrauma state [153]. However, minor damage such as altered fiber alignment in the capsule [112] can also lead to more-permanent metabolic changes that can elicit or accelerate degeneration and dysfunction of the whole joint. The most dramatic injuries can produce a complete loss of mechanical function and mobility [210], with the potential to affect the mechanical integrity of adjacent spinal levels [214]. In addition, nonphysiologic acute loading below that needed to induce a fracture of the facet can also lead to osteoarthritis [234,239,240] and the development of degenerative joint disease [52,55]. In fact, facet joint degeneration can be triggered or accelerated by an injury or a trauma to the facet or its surrounding tissues.
6. Degenerative Conditions of the Facet Joint
Degeneration is a progressive condition that often proceeds from modifications of the material, biochemical, and structural properties of many tissues. The degenerative process also alters the material and mechanical properties of the joint which eventually lead to further damage of the material integrity of the affected tissues. Since the mechanical behavior of the facet joints and intervertebral disc are inter-dependent, degeneration of the facet joint will also affect the mechanical behavior of the whole vertebral motion segment, and similarly, disc degeneration can impact the overall spinal degenerative cascades. Tissue degradation occurs at the structural and cellular levels during degeneration and that process can result from and/or be associated with aging [241–244], injury [92,229,242,245,246], and also infection or inflammation (septic arthritis, synovitis, and rheumatoid arthritis) [247–250].
6.1. Intervertebral Disc and Facet Joint Degeneration.
Degeneration of the spine and its facet joint impacts all of the tissues of that joint (bony pillars, capsular ligament, synovium, cartilage), but its primary effects are on the cartilage which can undergo osteoarthritis. The most prominent signs of degeneration are signs of pathology, including cartilaginous loss, wear, tears, and necrosis, fibrillation, ulceration, sclerosis, exposure of subchondral bone, osteophytes, subchondral cysts, and capsular calcification [43,242,243,251,252] (Fig. 5). Kettler and Wilke [253] have identified grading schemes to describe lumbar facet joint degeneration involving the use of either or both computer tomography (CT) and magnetic resonance imaging (MRI) [253]. Pathria et al. [251] proposed a grading system for facet joint degeneration based on CT imaging that has been widely adopted for research use. According to Pathria et al.'s system, a facet joint with a narrowing space represents grade 1-degeneration. If additional signs of sclerosis or hypertrophy are present, it is taken as grade 2-degeneration; and if osteophytic formations are also detected it is defined as grade 3-degeneration [251]. In addition, more recently, an MRI-based system was developed by Fujiwara et al. [242] to specifically classify lumbar facet joint osteoarthritis in conjunction with disc degeneration [242]. The degeneration of the bony pillars (hypertrophy, osteophytes) and capsule (calcification) can be assessed with radiographic and CT imaging (Fig. 5) because of the sharp contrast obtained with these techniques (Fig. 5). However, defining degeneration of the articular cartilage is more challenging and necessitates the use of MRI because this tissue is enclosed in the joint and it is “transparent” to X-rays. The existence of the different schemes to define degeneration in the facet joint based on varied imaging modalities highlights the need to employ complementary techniques to best assess the different tissues in the facet.
Fig. 5.
(a) Lateral radiographic of a healthy spine, indicating a healthy C5-C6 facet joint. A tip-mounted transducer has been inserted in the superior articular facet to measure the contact pressure developed in the facet joint during experimental studies inducing spinal bending. (b) A photograph of the facet surface of an exposed C5 facet from a 65 year old male donor demonstrating hallmarks of a degenerated articular surface: fissure (single arrow) and eroded cartilage area (double arrow).
The intrinsic structural and chemical complexity of the biologic tissues of the spine also makes the degenerative cascades and responses of the facet joints and intervertebral discs susceptible to many independent, common, and/or correlated degenerative processes [254]. Both degeneration of the intervertebral disc and degeneration of the facet joints have been reported to be observed clinically in concert and independently [245] and there is still controversy over the time course of these events and which tissue degenerates first or follows. Margulies et al. [255] found a significant correlation between the presence of lumbar degenerative disc disease and facet arthrosis and proposed a degeneration process by which the disc disease follows facet degeneration in small underweight persons presenting with osteoporosis. In that schema, the proposed pathologic process starts as osteoporosis that creates microfractures in the vertebral bodies which result in the facets' malalignment and then leads to cartilage wear and tear. This degeneration of the facet leads to the mechanical instability of the vertebral motion segment that increases the mechanical stresses supported by the disc, damaging its tissues and causing its progressive degeneration [255]. However, several studies have concluded that in most cases, facet joint degeneration is always associated with and preceded by adjacent disc degeneration [92,242,245,256]. Despite differences in interpretation of the clinical observations of disc and facet joint degeneration, the degenerative outcomes in both tissues are considered related when they are observed to coexist.
6.2. Artificial Disc Implants.
Although the relationship between disc and facet degeneration is still unclear, clinical investigations reported that degeneration of the intervertebral disc is more pronounced in the lower lumbar spine [257] where the degree of facet degeneration is also the most pronounced [258]. Degeneration of the intervertebral disc can lead to morphological changes in the spine, such as decreased disc height and/or spondylolisthesis, which can directly and indirectly alter facet joint mechanics by shifting the load transfer and/or modifying joint motions. Recently, a great deal of interest has been placed on developing and implementing spinal devices for disc treatment that restore the mobility of the spinal motion segment. Disc arthroplasty (i.e., arthroplasty) has been the focus of biomechanical and computational modeling, as well as clinical investigation [13,14,16,134,200,259–282]. Although there are many different designs for disc replacements, they are generally comprised of some sort of motion preserving component, such as a ball-and-socket, together with a motion limiter to mimic the normal functions of the disc. However, biomechanical investigations have shown that the ranges of motion of spines with disc replacement can be substantially different from those of the intact spine [13,259–262]. These clinical and cadaveric investigations reported that the range of motion in the sagittal plane for the affected segments (cervical or lumbar) increase after disc implantation, with some slight differences depending on the type of implant tested. In only one of these studies [261] were changes at adjacent levels for one type of implant reported; sagittal rotation increased after implantation but was decreased at the superior adjacent level and unchanged at the lower adjacent levels [261]. It is; therefore, likely that the device design (height, radius of rotation), as well as the placement and orientation of it in the spine also alter the mechanics of the facet joints at both the index and adjacent levels [132,134,263]. Ongoing studies are beginning to focus on these effects for the spine. Given the facet joint's important role in spinal biomechanics and its relationship to disc degeneration, as well as mechanotransductive pathways leading to cartilage degeneration following its altered biomechanical loading, future studies must focus on the effect of such interventions on facet joint biomechanics and health.
6.3. Spinal Morphology and Tropism.
Morphological abnormalities of this diarthrodial joint [252] and/or a different coronal orientation [283,284] between the left and right facet joints can create an asymmetrical stress distribution in the disc and zygapophyseal tissues. After testing cadaveric lumbar motion segments in combined compression and shear, Cyron and Hutton [285] concluded that a relative axial rotation caused by facet asymmetry places higher compressive load on the facet that has the smallest coronal orientation and could add rotational stress on the contralateral side of the annulus fibrosus and in the contralateral facet capsule [285]. Such a mechanical loading imbalance could accelerate the local degradation of the tissue and trigger degenerative processes. Any asymmetrical loading of the facet joints contributes to the development of facet osteophytes, cartilage erosion, fibrillation, or denudation, as well as narrowing of the joint space and neural foramen, and formation of synovial cysts [286]. In addition, facet orientation and tropism, an asymmetrical orientation of the bilateral facets, also appear to be predisposing factors for the development of degenerative spondylolisthesis [287] and facet joint degeneration [288], especially in the lumbar spine. Dai et al. (2001) reported that the average facet tropism in patients with a lumbar L4-L5 degenerative spondylolisthesis was significantly greater (12.9 ± 9.6 deg) than in the control group (10.6 ± 7.2 deg) and was also significantly correlated with the degree of disc degeneration [287]. However, several other investigations have reported facet tropism to not be associated with disc herniation, disc degeneration or degenerative spondylolisthesis [114,283,287–292]. Regardless of the relationship between facet tropism and disc-related degenerative processes, morphological abnormalities of the facet joints and the associated mechanical consequences of that on the overall motion segment can both affect the tissues of the facet.
6.4. Metabolic Changes in Degenerated Cartilage.
At the tissue and cellular levels, degeneration takes place through structural and metabolic changes that modify the composition of the tissue and, accordingly, its mechanical properties. Ziv et al. [254] reported that the water and proteoglycan content of lumbar facet cartilage increase during the first three decades of life, whereas the collagen content decreases. They also observed the same proportion of ulcerated and fibrillated facet joints in the spines of young and old adults. Based on these observations, those authors concluded that facet cartilage destruction is primarily caused by a disruption of the collagen network and that facet degeneration is not age-related [254]. However, an investigation of the various causes leading to facet cartilage degeneration remains to be performed to establish whether age is a main influencing factor. In an earlier biomechanical study of human patellar cartilage explants Armstrong and Mow (1982) also measured an increase in water content in the cartilage matrix that was linearly related to a decrease in the cartilage equilibrium modulus, implicating there to be less solid matrix, especially proteoglycans, to resist confined compression and possibly that the cartilage layer had degenerated [51]. Those authors also found that the equilibrium compression modulus decreased with both age and degeneration, suggesting that the degree of cartilage degeneration increases with age [51]. The hypothesis that metabolic changes are related to the degradation of the tissue structure is in agreement with more recent studies that have identified increased hydration and a loss of proteoglycans as characteristics of the first stage of osteoarthritis [64,293]. These metabolic changes of articular cartilage may also explain why the static and dynamic moduli of osteoarthritic cartilage are less than 80% and 30% of the corresponding moduli from healthy cartilage [64].
6.5. Facet Joint Degeneration and the Capsule.
The degeneration of the facet joint cartilage alters the mechanics of the joint because of the erosion of the articular layer; that thinning can also be linked to a faster rate of degradation of the capsular ligament because of the altered joint mechanics. Fujiwara et al. [294] investigated the change in spinal motion associated with facet cartilage degeneration and found that lateral rotation significantly increased with cartilage degeneration in the female lumbar motion segments while in male segments both axial and lateral rotations increased as the degree of cartilage degeneration increased up to Grade-3 but decreased for a Grade-4 degree of degeneration. They concluded that degenerative thinning of the cartilage together with spondylolisthesis can cause hypermobility of the spinal segment and laxity of the facet capsule [294]. The laxity of the capsule could be due to the development of scar tissue that establishes to reconnect collagen fibers that were ruptured while resisting higher tensile stresses caused by the segment's hypermobility. Failure of the capsular ligament, though, can also damage the fibroblasts that are involved in the remodeling of its damaged structure [153]. The weaker repairing capsular ligament can; therefore, be at a greater risk for further damage, degeneration, and loss of mechanical integrity because its mechanical environment has changed during this process. Fewer intact collagen fibers support the ligament loads which can also increase their risk of damage. At the same time, an increase in the stresses on the fibroblasts can also induce their synthesis of aberrant proteins that are less appropriate for the repair of the ligament extracellular matrix to maintain its mechanical function. The remodeling of the damaged tissue that might occur during degeneration of the capsular ligament has been described by Boszczyk et al. [295] in an immunohistochemical study of the extracellular matrix of the posterior facet capsules obtained from degenerative lumbar spines. The posterior capsule region was found to be hypertrophied and to show evidence of extensive fibrocartilage proliferation in comparison to the capsule from healthy motion segments [295]. Although capsule degeneration may have resulted from the axial rotational instability caused by a degenerated L4-L5 disc, that work supported the notion that resistance to an increased mechanical demand can trigger molecular responses that lead to cellular differentiation and fibrocartilaginous adaptation in the capsule. Degenerative processes in the facet capsule do not necessarily result in its weakening but in a transformation of the tissue structure in response to a modified mechanical environment, and a change in the tissue mechanical function, thereby affecting the normal mechanotransductive processes of the normal tissue (Fig. 4).
6.6. Facet Joint Degeneration and the Bony Pillars.
Eventually, degenerative mechanisms can also propagate from the cartilage and capsule to the bone of the articular pillars themselves. Along with the hypertrophy of the posterior capsule, Boszczyk et al. [295] identified osseous spurs in the degenerated lumbar motion segments. Several investigations have also reported that osteophytes form along the posterior capsular attachment where the compressive and tensile stresses can become excessive in the hypermobile degenerative joint [1,296]. Furthermore, the posterior region of the facet joint is also found to be the most frequent location of cartilage defects [297], which may mean that nonphysiologic mechanical stresses develop at this location during extension when the intervertebral disc height decreases (because of disc degeneration or disc arthroplasty), making the posterior edge of the superior facet collide with the lamina of the inferior vertebra. Furthermore, an increase in the mechanical stress experienced by the bony articular pillar can also initiate or exacerbate degeneration by overstimulation of bone cells activity. Osteoblastic activity is stimulated to synthesize and add bone in the form of osteophytes or spurs and to enlarge the facet surface in an effort to restore the joint's stability and resist the associated increases in strain and stress [243,294]. In fact, Fujiwara et al. [294] reported a decrease in lateral bending and extension motions in female osteophytic cadaveric lumbar motion segments and in flexion-extension motion in male osteophytic segments [294]. However, those trends were not significant which suggests that the degree of osteophytic degree alone, may not modify the flexibility of lumbar motion segments.
6.7. Neurologic Implications of Facet Joint Degeneration.
The degeneration of tissues in the facet not only affects the mechanical function and properties of the joint but can also have neurologic consequences. Cartilage delamination and erosion occur in the progression of osteoarthrosis and eventually lead to the exposure of the subchondral bone under the two facets comprising the joint (Fig. 5(b)). With the loss of the cushioning cartilage layers, vertebral motion can elicit pain as the bone rubs on the opposing bone. As motion of a degenerated facet joint still takes place, the mechanical stresses induced in the degenerated capsule can also tear it which can allow leakage of inflammatory cytokines into the intraspinal space. Those caustic chemical agents can irritate the nerve root and trigger pain signaling [298]. In addition, defect or rupture of the capsule, as a consequence of osteoarthritis or degenerative spondylolisthesis, may also trigger synovial cyst formation from the herniation of the synovial lining. Cysts can also promote additional neurologic deficits, such as sciatica or acute radicular pain, as they compress the nerve root or the thecal sac, or hemorrhage upon trauma [299,300]. But most severely, cysts may also detach from the synovium and migrate within the joint's space [301], presenting the potential for indenting and damaging the articular cartilage when they get trapped between the two articular surfaces that are compressed together. Finally, osteophytes can form on the periphery of the facet surface and have the potential to reduce the neural foramen and compress the nerves or dorsal root ganglia, which can induce pain and modify local inflammation at the nerve root as well as modify inflammation in the dorsal root ganglion and spinal cord [302–305]. Although rarely reported, cervical facet osteophytes can also generate a symptomatic compression of the vertebral artery, reducing the brain blood supply and inducing neurologic disorders such as vertigo [306].
Over time, the material properties of any biologic tissue will degrade due to aging and/or nonphysiological processes. This degradation can be accelerated by injury, trauma, or infection. Local material degradation leads to a weakening or a loss of mechanical properties of the tissues. That local mechanical deficiency can affect the surrounding tissue that are required to compensate and either mechanically adapt or fail. The change in the mechanical stresses and strains supported by the neighboring tissues and their cells can themselves lead to cellular dysfunction and morphological changes of the structure, such as hypertrophy and/or disorganization. Osteophyte formation, articular hypertrophy, articular cartilage thinning, formation of synovial and subchondral cysts, and calcification of the joint capsule have all been imaged in association with low back pain, sciatica, and lumbar facet joint osteoarthritis syndromes [21,307]. The development of these abnormalities illustrate that the degeneration of a facet tissue is never in isolation but is both affected by and impacts its surrounding vertebral tissues. Therefore, the effects of facet degeneration on the mechanical behavior of the facet joint and spine as whole cannot be measured directly. But, experimental determination of the degenerated tissue properties could be used in finite element models to evaluate the mechanical impact of degeneration.
7. Mathematical and Finite Element Models
Finite element (FE) models have been developed to model individual tissues, motion segments and multisegmental spines in both the cervical and lumbar spinal regions in order to provide otherwise unavailable information about the internal stress and strain distributions in the tissues of the spine, as well as to model a variety of clinical scenarios, such as degeneration and the effects of spinal instrumentation [308–310]. Mathematical and finite element models offer great utility for investigating spinal tissue responses because they provide an alternative to experimental approaches that can present a wide variety of challenges owing to a scarcity of specimens and a potential poor tissue quality due to advanced age or degeneration. In addition, mathematical models enable an infinite number of model conditions and variations that can be set up to investigate the influence of biological and mechanical conditions that alter tissue properties [310,311], or to compare effects of surgical procedures such as laminectomy, facetectomy, or disc arthroplasty [264–266,274,312–314] on the mechanical behavior of the spine. Computational approaches can extend beyond experiments that can be limited in their utility if restricted to cadaveric tissue only.
Although a modeling approach requires assumptions be made about tissue properties owing to a paucity of specific or relevant data [308], finite element models do permit the evaluation of the load-sharing between different elements of each vertebra and between spinal levels, as well as facilitate evaluation of the loads and deformations of the various facet joint tissues during physiologic and nonphysiologic spinal loading. To this end, sensitivity analyses have been performed to quantify the influence of changes in material properties on model performance [97,315]. The most accurate analyses for facet joint mechanics and kinematics will require detailed and specific experimental quantification of the tissue properties of that joint for use in computational models. Despite the need for experimental validation, the predictions provided by finite element models are useful for examining and explaining the variations observed for different cadaveric test scenarios and for extending experimental work beyond the lab [315]. Models also enable specific investigations to understand many of the very issues discussed earlier in this review, such as degeneration [311]. Although the earlier computational models were limited by computing power and a lack of experimental data for their construction and validation, there are now more anatomically specific models that include detailed anatomy and geometry of the ligaments, bone and muscles and that integrate more sophisticated constitutive equations to simulate tissue properties to predict mechanical behavior at a much smaller scale. In this section we review mathematical and finite element models that have been developed to study spinal biomechanics, with an emphasis on those models that are mainly related to facet joint mechanics; we do not aim to provide a comprehensive review of all models of the spine.
7.1. Early Vertebral Models and Their Limitations.
Employing a geometric model of an L5 vertebra Wu and Chen [316] considered the vertebral body, intervertebral disc, and ligaments as porous media and derived the associated constitutive equations of motion to develop a poroelastic mixed FE model to evaluate deformation and failure stresses in those tissues. Specifically, that model required that the facet surfaces maintain contact during joint deformation. The predicted deflections of the vertebral body and disc and the amount of disc bulge were compared to predictions from an axisymmetric model of the spinal motion segment. The poroelastic mixed FE model predicted that the highest von Mises stresses would develop in the anterior region of the facet during axial compression and under a compressive pressure of 0.43 MPa and in lateral bending for an unspecified moment or lateral rotation [316]. The axisymmetric model predicted the von Mises stress contours to be different on the facet surfaces while the degree of disc bulge and vertebral deflection were greatly reduced in all loading configurations [316]. The asymmetry in the stress contours and the reduction of the deformations suggested that the spinal motion segment could not be modeled as an axisymmetric structure and that the facet joints play a role in limiting the deformation of the loaded spinal motion segment. Accordingly, those authors concluded that the facet joints should be included in any FE model and analysis [316].
At the same time, Yoganandan et al. [308] introduced a finite element model of a three-vertebra (C4-C6) cervical spine segment. They initially validated its mechanical behavior by comparing the overall force-deformation response of the FE model to that of cadaveric specimens subjected to a 1 mm axial compression using data from Shea et al. [317]. The FE model predicted the stress distribution established in different anatomic structures in the vertebrae and predicted stresses three times higher in the posterior elements than in vertebral bodies or disc at the C4-C5 level. The following year, Clausen et al. [312] employed a three-dimensional nonlinear finite element model of a C5-C6 motion segment to evaluate the involvement of the uncinate processes and joints of Luschka (Fig. 1) in the vertebral bodies in coupling of motions in the lower cervical spine. Their model predicted that the uncinate processes reduce primary sagittal rotation by 5%, axial rotation by 24%, and lateral bending by 16% while also reducing the coupling of lateral bending to axial rotation by 14% and of axial rotation to lateral bending by 25%. In contrast, the joints of Luschka were found to increase the amount of primary motion by 14% in flexion-extension, 17% in axial torsion, and 36% in lateral bending [312]. The mechanical role of the facet joints, uncinate processes, and Luschka joints highlighted in these early computational studies demonstrate that such anatomical features of the spine that have orientations in several planes (Fig. 1) and are involved in coupling motions must be included in finite element models for the most accurate development and utility of such models for understanding spine biomechanics.
Although those studies demonstrated the importance of modeling these and other anatomical features of the spine, the facet joint was modeled using overly-simplified approaches for its geometry and mechanical behavior, without incorporation of the various tissues that compose this joint (Fig. 2). Using different approaches, the facet articulation has been modeled by nonlinear contact elements [318], sliding contact elements [310], or surface-to-surface elements [319]. Models using these simpler approaches to model the articulation likely selected them because they reduce computation time. In a more refined approach, the facet articular cartilage has been modeled using 8-noded solid elements, the synovial fluid by incompressible fluid elements, the synovium by membrane elements, and the capsule by nonlinear tension-active elements [97,309,320]. However, the material properties of the individual tissues of the facet joint have not been completely defined (see Secs. 2 and 3), so it is difficult to accurately model the facet tissue properties [97]. Despite this limitation, finite element models of the spine have been developed and used to evaluate spinal kinematics [310], spinal stability [318,319,321], and loads and pressure in the intervertebral disc during spinal motions [309,320].
7.2. Models Specifically Including Facets.
FE models have also been created to evaluate the patterns and extent of load sharing between the anterior (intervertebral disc) and posterior (facet joints, pedicles, laminae) elements of the spine during physiologic loading conditions [322–324]. In their model of a C5-C6 motion segment, Goel and Clausen [322] oriented the articular facets at 45 degrees off of the axial plane and modeled the two articular layers and the gap between them using 40 “gap” elements; the fissure of the joint of Luschka was modeled by 20 “gap” elements while the capsular ligament was modeled by 221 tension-only nonlinear cable elements [322]. That model used experimental data from tests using cadaveric specimens reported by Moroney et al. [325] as inputs and predicted 12% of a 73.6 N axial compressive load to be transmitted through the facets [322]. With the further addition of 1.8 Nm of moment applied in the sagittal, right lateral and left axial torsion directions, the facets were found to be relieved of compressive stress in flexion but to each carry 51% of the compressive load in extension. In right lateral bending and left axial torsion, the right facet carried 41% and 37% of the load, respectively, while the left facet was relieved of compressive load [322]. The model also predicted that no strain developed in the facet capsule during the initial axial compression, but that during the 1.8 Nm of axial torsion, strain in the capsule ranged from 1.2 to 3.4% and reached a maximum of 13% in the left capsule during left axial rotation. Kumaresan et al. [323] also examined the facet forces that develop during an axial compression with their C4-C6 model that used 8-noded isoparametric solid elements to represent the facet articular cartilage, tension-only rebar elements for the capsule, membrane elements for the synovium, and incompressible fluid elements for the synovial fluid [323]. In that work, the bilateral facets were predicted to carry together a similar portion of the load as Goel and Clausen [322] found: 38.5 N (48%) for the C4-C5 facets and 30.7 N (38%) for the C5-C6 facets, when an 80 N axial compressive force was imposed. Although the cervical models developed by Goel and Clausen [322] and Kumaresan et al. [323] were subjected to similar loading scenarios, the discrepancy in the predicted facet loads may be due to the different types of elements used to model the articular cartilage and the greater influence of the boundary conditions on the one motion segment model compared to the two-motion segment model [97]. Panzer and Cronin [324] developed a geometrically specific model of a cervical motion segment with an isotropic elastic constitutive model for the facet cartilage that exhibited ranges of motion similar to published experimental data [38,322] for extension, lateral bending, and axial torsion moments of 0.3 Nm and 1.0 Nm and flexion moments up to 3.5 Nm [324]. That model also predicted that the articular pillars and facet joints carried approximately 10% of the load in each of axial torsion and lateral bending; further, in extension 100% of the load was supported by the facets though they were found to be completely unloaded in flexion [324]. The differences between the predictions of facet forces from all of these studies are likely due to the fact that the models defined different spinal regions to study and subjected them to different loading scenarios.
7.3. Models Simulating Clinical Procedures.
Although no models have been developed to specifically evaluate facet joint biomechanics during any physiologic spinal loading, the contribution of the facet joint to overall spine biomechanics has been evaluated for a variety of clinically-relevant pathological and surgically-modified conditions that can exist. For example, models of intact spines were modified by changing the constitutive relationships and/or the material properties of the various spinal structures to investigate the effect of disc degeneration and surgically-imposed laminectomy and facetectomy on the overall spinal kinematics, stresses that develop in the intervertebral disc, and loads transmitted across the facet during flexion, extension, lateral bending, and axial torsion [97,313,318,323,326–328]. Kumaresan et al. [323] modified the material properties of the nucleus as well as the fiber content and material properties of the annulus at C5-C6 in their multilevel cervical model to represent slight, moderate, and severe disc degeneration. Using that approach, segmental stiffness at that level was predicted to double in the severely degenerated condition, yet remained unchanged at the un-modified C4-C5 level. In contrast, facet load at the intact and degenerated levels was not found to be changed by the extent of disc degeneration. However, facet pressure did slightly decrease at the degenerated level in the worst degeneration condition under axial compression [323].
Other investigations using a greater number of discretized elements to represent the facet joints have focused on simulating the effect of surgical interventions, such as facetectomy, by selectively and in a controlled fashion removing those elements of the relevant anatomy that define the facet joint [313,327,328]. All of the models of facetectomy predict that a total bilateral facetectomy significantly increase the stress in the intervertebral disc and the flexibility of the cervical spine, especially in extension, lateral bending, and torsion, making the spinal column unstable. The increase in stress in the disc can have long-term consequences as it can lead to disc degeneration, which will eventually affect the facet mechanics (as presented in earlier sections). Models of the lumbar motion segments [313,328] reported that a total unilateral facetectomy increased the load on the contralateral facet by 58% and that a total bilateral facetectomy increased axial rotation by 120% while nonsignificantly increasing the other motions. The facetectomy studies with both the FE models [313,328] and cadaveric specimens [100–104] demonstrated the role of the facet joints in limiting intersegmental rotation and providing stability to the spine. This agreement suggests that the FE models of spinal segments can be used to predict spinal kinematics in the major physiologic loading conditions, despite the limited material properties data being available only for certain spinal tissues.
In the same way as elemental modification is used to model facetectomy, computational models have also been modified by replacing the intervertebral disc elements with those modeling the geometric and material properties of disc implants in order to evaluate the influence of disc arthroplasty on spinal kinematics and facet loads [200,264,266–268,270,273,274,314,329,330]. Those FE models are useful because they can be altered to study the influence of parameters like implant size or its placement on the mobility of the motion segment and the contact pressure developed in the facet articular cartilage. Cadaveric studies do not permit such permutations. Chen et al. [200] modeled a lumbar L2-L4 segment with a ProDisc-L at L3-L4 and found the facet forces to increase by 150% during extension compared to the un-implanted condition [200]. In their model, they defined the contact pressure (p) as an exponential function of the ratio between the initial gap distance between the facets (c) and the facet gap closure (h), taking the initial pressure (p0) to be equal to 3500 MPa for facets in contact (i.e., with h = 0) (Eq. (15)).
| (15) |
Similarly, the L1-L5 FE model developed by Schmidt et al. [273] predicted facet force to increase during extension by 24% on average with two disc implants at any two levels, and by 38% with four implants at each level [273]. In addition, the antero-posterior placement of the implant has been shown to modify the relative segmental motions because it separates or compresses the facet articular surfaces [314]. Similarly, geometric parameters such as the height or the radius of curvature of the implant's ball-and-socket joint influence facet contact during flexion/extension, lateral bending, and axial torsion [314]. Womack et al. [269] used a C3-C7 FE model with a 3-element thick extruded cartilage model to define the superficial, middle, and deep zones of the articular cartilage layer; load sharing between the disc and the facets at the implant level was found to be inversely correlated with the height of the implant, but the presence and size of the disc implant did not affect the facet load at the adjacent levels [269,330]. In that approach, a hyperelastic model was used to define the cartilage strain-energy density (Eq. (16)) from which the facet force was calculated [269].
| (16) |
The hyperelastic coefficients (C10, C20, D1, D2) employed in the strain-energy density function were determined by comparing the theoretical model to experimental data of cartilage specimens subjected to compression, with a known deformation gradient (J) and first strain invariant () [269].
Furthermore, Kang et al. [270] investigated the influence of different artificial discs on facet load during bending in a single-level (C5-C6) motion segment model and found that with increased rigidity of the disc implant, the facet load decreases [270]. Ahn et al. [267] also incorporated different disc designs in their C5-C6 model and reported that facet loads increase when the center of rotation of the spherical joint of the implant is constrained. Additional studies find that the antero-posterior placement of an artificial disc also affects the flexural stiffness of the motion segment at the level of the implant and the loads transferred to the facet during all modes of loading [264,266,268,274]. In general, all of these studies have found that a posterior position of a disc implant can unload the facets although the influence of this parameter should be considered in conjunction with the influence of other parameters such as the implant design, anatomical modeling, and constitutive equations employed in the individual finite element models. The influence of the antero-posterior position of the disc implant on the facet loads and spinal segment mechanics also highlight the fact that the predictions obtained from FE models strongly depend on the quality of the geometrical and morphological modeling.
7.4. Models Predicting Facet Forces
7.4.1. Physiologic Loading.
The modeling of the implant depends on the specification of the disc implant itself such that many combinations of the geometrical, morphological, and implant modeling likely result in different predictions from the FE model. Rohlmann et al. [314] calculated facet forces for simulations combining the effects of disc implant position, ball radius, facet gap size, and the presence of ligamentous scar tissue in a model of L3-S1. Their simulations predicted that facet forces increase in 37% of the scenarios in flexion and that the maximum facet force reached 533 N for a small facet gap and a large implant ball radius. Also, the facet gap, ball radius, and the presence of scar tissue on the anterior longitudinal ligament were all found to influence the forces that developed in the facet during bending and axial torsion [314]. Similarly, Kumaresan et al. [331] and Womack et al. [269] showed that using different approaches to model the tissues of the facet joint produced differences in the prediction of the magnitude and location of the compressive stress in facet cartilage during bending [269]. Kumaresan et al. [331] simulated the facet joint in four different models, in two of which the articular cartilage and synovial fluid were not included and in the other two the synovial fluid was simulated by 8-noded incompressible hyperelastic solid or hydrostatic incompressible fluid elements. Maximum compressive stresses in the facet cartilage during extension were 16 times smaller in the models that did not incorporate the cartilage and fluid than in the hyperelastic model but were similar to those in the hydrostatic fluid model. Despite the difference in stress magnitude, both of the simplified models and the hyperelastic model predicted the maximum compressive stresses to develop along the posterior edge of the joint whereas it was predicted to be uniform over the whole articular surface in the fluid model [331]. Womack et al. [330] investigated four different representations of the articular surface geometry, two anatomy-based thickness distributions, a constant thickness and a flat surface model. Although the contact force profiles in each of sagittal bending, lateral bending, and axial torsion were similar for all of the models, the pressure distribution on the articular surface varied: it was uniform in the flat model, along the anterior, posterior, and lateral edges in the constant thickness model, and along the postero-central edge in the anatomy-based models [269]. The results of all of these investigations highlight the fact that validated experimental properties of the facet joint tissues are necessary to develop accurate models for reliable predictions.
Despite a lack of data describing the facet tissue properties, finite element models have been specifically developed to investigate the intrinsic loading of the facet tissues (mainly the cartilage and capsular ligament) during flexion-extension in intact, degenerated, or injured conditions or in traumatic loading throughout the spine [141,268,269,271,272,311,314,321,331,332]. Both Sharma et al. [321] and Kumaresan et al. [331] modeled the facet articular surface areas as rectangles or squares, respectively, and they partitioned these areas in to zones in which facet contact occurs in order to evaluate contact pressure distributions in the facet joint during flexion, extension, and lateral bending. Those authors found that facet contact occurred along the posterior edge of the facet during extension and along the superior edge during flexion [321,331]; coinciding with reports of nonhomogeneous and regional contact measured in cadaveric studies of the joint [137,139]. Schmidt et al. [271] evaluated the relationship between the instantaneous center of rotation of L4-L5 and the associated facet loads during extension. The center of rotation was found to move posteriorly, with facet forces up to 50 N being established. In lateral bending, the center of rotation migrated posteriorly and ipsilaterally giving rise to 36 N of force in the facets. Lastly, in axial rotation the center of rotation moved ipsilaterally towards the direction of rotation, establishing the greatest facet forces of 105 N [271]. Cook and Cheng [141] recently investigated the percentage of the facet's articular surface over which contact occurs during flexion and extension and termed that relative proportion as the contact area ratio. They reported that the average contact area ratio between the articular surfaces of the lumbar facets was 0.68 in flexion and 0.82 in extension; that study also highlighted that the facet contact varies between spinal levels. Specifically, the percentage of articular surface over which contact occurred was found to be as much as 70% at L1-L2 and 65% at L4-L5 in flexion, and was even higher at 79% at L2-L3 and 86% at L3-L4 in extension [141]. Using a poroelastic C5-C6 finite element model that simulates the nonlinear mechanical behavior of the tissues caused by the fluid contained in the pores of the tissue structure Hussain et al. [311] predicted that moderate and severe disc degeneration would decrease rotation motion by 2–43% in flexion, 20–80% in extension, 14–55% in torsion, and 5–71% in lateral bending. On the contrary, disc degeneration was predicted to increase facet loads by 47–125% in compression, 38–63% in extension, 55–169% in axial rotation, and 37–58% in lateral bending in comparison to the intact model [311]. Although the magnitude of facet force predicted in all of these studies might be biased by the accuracy with which the mechanical behavior of the facet cartilage is modeled, they do provide a better understanding of facet mechanics that can help to define more appropriate experimental testing procedures for further comparisons and validations against these computational approaches.
7.4.2. Nonphysiologic Loading.
Finite elements models have also been developed to evaluate facet loads and deformations during nonphysiologic loading conditions such as vehicle impacts [333,334]. Kitagawa et al. [333] used a human finite element model with simulated facet capsules to investigate capsular ligament elongation during simulations of rear-end vehicle impacts, based on the hypothesis that capsular stretch is a mechanism for initiating pain. Their model calculated capsular strains that were then integrated as a criterion in the design of cushioning seats aimed at reducing the severity of rear-end impacts on neck distortion [333]. In addition, El-Rich et al. [334] developed a finite element model of a lumbar motion segment to investigate the effect of the rate of loading on load-sharing and ligament stress during sagittal bending in frontal and rear impacts. They found high rates of sagittal rotation to be associated with a faster increase in facet force and a higher peak facet force that can result in facet surface fracture at small rotations, in comparison to rates of rotation that were one to two orders of magnitude smaller [334]. In that same model, the stresses in the joint's capsule increased from 2 to 6 MPa at the time of the facet fracture but reached only a maximal stress of 8 MPa at 5 degrees of rotation that was comparable to the stress achieved at the lower rates of loading in extension. The fact that the maximum stress in the capsule predicted in extension was unchanged across the different rates of rotation suggests that either the material properties used to model the capsule may be incorrect or that in that particular mode of loading, the ligament experienced similar load environments at all rates. However, the capsule stress was more than doubled at the fastest loading rate in flexion as compared with the other two lower rates of loading for 5 degrees of rotation, which might indicate that tearing of the capsule does not necessarily depend on the amount of rotation but on the rate of rotation [334]. Despite the need for experimental validation of such predictions, these finite element models provide a useful means to understand better the effects and consequences of traumatic loading of the spine and the facet joints at the tissue level and provide data which are currently otherwise unobtainable using experimental approaches.
Although the different approaches to model the spine do not permit explicit comparison of the outcomes and conclusions drawn by the many different mathematical models of the spine, the results obtained from these validated models do collectively inform about the mechanics and kinematics of the facet joint. The increase in the calculating power of computers and the refinement of image analysis now enable more accurate and complex modeling. A single motion segment model contained about 10,000 elements [308] in the mid-1990s whereas, current ones contain up to 190,000 elements [334]. However, the material properties of the facet joint tissues will first need to be thoroughly defined in experimental investigations to be incorporated in such complex finite element models and make them truly accurate in terms of the range of motion and load-sharing but also regarding disc and facet pressures and ligament strains.
8. Biomechanical Insights, Implications and Future Trends
The facet joint has a complicated anatomy that provides its unique biomechanical functions of supporting loads and coupling motions in all regions of the spine (Figs. 1 and 2). This anatomy, together with the complex loading scenarios that the facet joint and its tissues undergo in normal and pathological conditions, contributes to the need for continued work in defining the biomechanical and physiological mechanisms of its functions (Fig. 6). Further, understanding the factors that directly and indirectly affect physiologic performance of the joint as a whole and its individual tissue components can help guide development of interventions to prevent its injury and/or tissue degeneration, as well as to develop targeted tissue engineering and surgical approaches for the facet joint and the entire spine.
Fig. 6.
Schematic illustrating the multifaceted approach to understanding the form, function and physiological balance of mechanisms in the facet joint. A variety of experimental and computational techniques are needed to complement the existing knowledge of the factors that directly and indirectly affect the physiologic performance of the facet joint, as well as its function in health, injury, aging and spinal interventions.
8.1. Insights and Implications.
Generally, it is clear that the mechanical responses of the joint are highly dependent on the specific loading parameters—i.e. rate, magnitude, and direction (Tables 1 and 2). Throughout the regions of the spine there are spinal loading scenarios that are most typical and have been studied extensively at both the whole-joint and tissue levels. For those cases, the biomechanical response of the facet joint has been well defined based on cadaveric experiments, with most studies focused on the joint kinematics and capsular ligament responses. That collection of work has defined not only some of the tissue and material properties for the facet joint tissues, but also identified potential thresholds for injury and nonphysiologic responses. Although such biomechanical studies have defined the macromechanical behavior of the facet joint (Table 3), specific descriptions of the mechanical response of the facet cartilage remains unstudied. Certainly, it is possible that the facet cartilage simply behaves as the cartilage of other joints does; yet, this has not been definitively determined. Moreover, there is a growing need to understand the specific facet joint cartilage loading profiles throughout the spine, as well as the physiological consequence of such loading, given the advent and increased use of total disc replacements and the potential impact of those surgical treatments on the facet joint biomechanics and degeneration (Table 4). Such innovative technology for spine treatment may directly affect the function of the facet joint and disrupt the normal function and balance between mechanics, signaling and physiologic function (Fig. 6).
Table 3.
Summary of major findings regarding facet joint biomechanics and associated key references
| Tissue Components |
| Facet cartilage is not uniform in thickness [41]. |
| The capsular ligament exhibits anisotropic viscoelastic properties [9,10]. |
| Capsular strains are not uniform and vary with the joint loading scenario [8,107–109,111]. |
| Failure strains of the human cervical capsular ligament are large: |
| 94 ± 85% in shear [109] |
| 104 ± 81% to 148 ± 28% in tension [8,9] |
| Structural damage occurs in the capsule at strains of 51 ± 12% of those required for its failure [120]. |
| Facet Joint Loading |
| The facets carry between 3–25% of the spinal load in axial compression [2,4,5,96,322–324]. |
| The joints of Luschka increase the amount of primary motion while the uncinate processes reduce it [312]. |
| The forces/pressures in the facet joint are non-uniform and vary spatially in different spinal regions and with the loading scenario [11,106,131,132,137– 139,141,162–165,271,331]. |
| Lumbar stresses in the facet capsule are predicted to double when the rate of rotation increases by two orders of magnitude during 5 deg of flexion [334]. |
| Facetectomy decreases the stiffness and increases the mobility of the spinal motion segment in all modes of loading [98–104,313,327,328]. |
| Segmental mobility increases after capsulotomy [105,106,195,206,209]. |
| Disc arthroplasty modifies facet loading at index and adjacent spinal levels [132,134,200,263,269,270,273]. |
| Geometric parameters of a disc implant influence facet joint contact during loading [269,314,330]. |
| Mechanotransduction Processes Identified in Conjunction with Facet Joint Loading |
| Physiologic capsular stretch of the cervical spine is associated with neuroinflammatory processes in the dorsal root ganglion and the spinal cord and afferent modulation in animal models of pain [116,124,155]. |
| Injection of an anesthetic into the facet joint relieves pain symptoms [154]. |
| Facet Joint Injury and Spine Biomechanics |
| Facet joint injury increases spinal mobility and weakens its mechanical properties [193,195,201,207]. |
| Spinal fusion increases segmental rotation at adjacent levels, which can lead to increased loading of the facet joints [211–216]. |
| Subcatastrophic stretching of the facet capsule induces pathophysiological responses in the dorsal root ganglion and the spinal cord in animal models [111,113–119,123,124]. |
| Non-physiologic loading of the cervical spine leads to facet capsule damage that induces laxity [120,194]. |
| Facet Joint Degeneration |
| Intervertebral disc and facet joint degeneration have many independent, common, and/or associated processes [245, 254]. |
| Spinal asymmetry might be associated with a greater risk of spinal degeneration [192,283,285–292]. |
| Osteophytes decrease lumbar segmental motion [294]. |
| Local hypertrophy and extensive fibrocartilage metaplasia of the facet joint tissues result from greater mechanical loading in degenerated lumbar spines [295]. |
| Leakage of cytokines from the degenerated joint into the intraspinal space can initiate pain cascades [298]. |
Table 4.
Issues and future research directions for human facet joint biomechanics
| Topic Area | Issues | Areas of Needed Research | Potential Future Implications |
|---|---|---|---|
| Tissues | |||
| Facet Cartilage | • Small/thin structure | • Clearer definition of anatomy and geometry of these tissues | • Define healthy and unhealthy |
| Meniscoids | • High spatial variability | • Coupling of tissue biomechanics with physiologic function | • Better understanding of mechanical role of these tissues in biomechanical and physiologic functions of facet joint |
| • Difficult to image | • Comparative analysis relative to other diarthrodial joints | • Define material properties of these tissues for more accurate modeling | |
| • Difficult to access physically without damaging the joint | • Noninvasive technologies for measuring relevant kinematics & kinetics | • Context to integrate with findings from other joints | |
| • Loading depends on other tissues in the joint | • Understanding of mechanotransduction processes | ||
| • Little known about mechanical properties; assumed to be similar to other diarthrodial joints | |||
| Mechanotransduction | |||
| Normal Facet | • Despite extensive work in other joints, mechanisms not defined for facet | • Clearer definition of physiologic cascades of injury, degeneration, aging and pain | • Develop better preventive strategies |
| Developing Facet | • Effects of cyclic loading not defined | • Better understanding of relationships between global & local milieus | • Design treatments to address spinal health problems |
| Aging Facet | • Cellular responses in tissues remote to those under load | • Clearer understanding of local effects of specific tissue interventions for global spine performance | |
| Pain Signaling | • Impact of biomechanics on physiology | • Expanded utility of computational & animal models | |
| • Impact of physiology on biomechanics | |||
| Model Systems | |||
| Computational | • Despite tremendous advances, integration of material and biologic properties of facet tissues remain limited | • More sophisticated models to incorporate degenerative processes, pathologies and dysfunctions | • More realistic models |
| Animal | • Context for human relevance | • Ethical and useful human studies | • Inform about pain/dysfunction |
| Human | • Relevant input parameters | • Appropriate contextualization | • Leverage in vivo imaging to study biomechanics of mechanotransduction |
| • Understanding mutual influence of facet joint and its surrounding structures | |||
| • Investigate effects of current and future surgical interventions | |||
With the advent of biomechanical modeling in vivo and in vitro, it is possible to continue to advance the understanding of relationships between the macro- and micro-scale loading events and the physiological cascades that are initiated (Figs. 4 and 6). For example, culture models provide a means to mimic the loading exposures to tissue explants and specific cell populations, enabling the precise definition of the mechanical, electrical, structural, and chemical cascades that develop to translate signals about mechanical loading in order to preserve and/or adapt tissue functionality and integrity (Fig. 4). However, it is clear also that once such physiological cascades are initiated, they can become unbalanced and lead to either injury or degeneration. Although there is a great body of very elegant research on the mechanotransduction processes in the hard and soft tissues of other synovial joints, such investigations of the facet joint tissues are still lacking and are necessary since they might reveal mechanotransduction mechanisms that are specific to the facet joint (Table 4). Further, coupling such local biomechanics with physiological function, as can be done in animal models, provides a rich opportunity to specifically link cellular and sub-cellular responses to overall system function, to evaluate outcomes like function and pain. As with any modeling, care must be taken to identify and accommodate any disparities between the species differences since most animal models are in quadripedal animals whose spines undergo loading profiles quite different from the upright human. Nonetheless, such models have begun to, (see Table 3), and can continue to provide invaluable physiological context for the local biomechanical loading of normal, injury and treatment conditions. Many such models leverage the utility of strain measurements across species as they can offer a normalized measurement of the mechanical response of tissues. Such a metric provides great utility for setting thresholds for injury and/or dysfunction that can also be implemented in computational models.
Computational models of the spine and facet have provided a tremendous tool for expanding experimental investigations to include scenarios that are challenging to model experimentally, as well as for determining otherwise difficult measurements such as stress and pressure distributions in the facet (Table 3). Looking forward, as the relationship between mechanics and physiologic sequelae becomes clearer it will be useful and advantageous for such models to incorporate methods to set thresholds for physiological responses, not simply to predict fracture, tissue rupture, or gross failure (Table 4). For example, the facet capsule contains both collagen and nerve fibers, and it is known that strains corresponding to subfailure distraction of the capsule are sufficient to induce persistent pain [123]. Therefore, it is possible to integrate data from in vivo studies, together with biomechanical tests, to establish criteria for pain production in a computational model. With the current and future introduction of more sophisticated experimental and computational techniques, it will be possible to develop models which predict tissue dysfunction and not simply mechanical failure. As the detection and treatment of spine injury and pathology continue to similarly become more sophisticated, the traditional biomechanical foci for studies of this joint will also require incorporation of physiological measurements and the direct definition of mechanotransduction processes in this joint under normal, injury, aging and treatment conditions (Fig. 6; Table 4).
8.2. Future Trends.
There has been substantial research contributing to a variety of major findings related to the spinal facet, its biomechanics, and its contribution to spinal responses in health, injury and disease (Table 3). However, despite this growing body of literature there is an equally large list of new issues that need to be addressed and facet-specific biomechanical and physiologic mechanisms that need to be defined, highlighting possible trends for future biomechanical research (Table 4). For example, owing to advances in technology, it is now possible to make measurements across size scales and in noninvasive approaches that have not yet been feasible. Because of these advances, it is now possible to measure the anatomical, geometrical, material and mechanical properties of the individual tissues of the facet joint, at spatiotemporal resolutions necessary to make relevant conclusions for spinal tissue mechanics. In addition, with those data, it is also highly likely that more specific and relevant models can be constructed, validated, and utilized, as the computing power and modeling techniques continue to become more advanced. Similarly, as biomechanics of the facet capsule has begun to incorporate and integrate biomechanics with physiologic responses that relate to dysfunction and not only failure, it is possible (and necessary) to incorporate tolerance criteria for cellular dysfunction and physiological responses (i.e., dysfunction, degeneration, and pain) in finite element and computational models. However, in order to do that, additional experimental work is needed to define such criteria, for the spine, the facet joint, its individual tissue components, and for a variety of relevant spinal loading scenarios.
The facet capsular ligament appears to have been the most heavily studied tissue of the facet joint to date, perhaps due to its ease of accessibility and the ability to perform clearly delineated mechanical studies. However, the other tissues of the facet joint, including the cartilage and synovial folds, that were previously considered either too small, too difficult to access, or require modification of the joint itself to access, must be investigated (Table 4). For instance, the layer of cartilage covering the facets has a thickness that varies and is much smaller than in the other diarthrodial joints. This has hindered the ability to study it; yet, imaging technology has now become sophisticated enough to easily enable sensitive and specific definition of its anatomic and geometric characteristics, and even its mechanical responses (Table 4). The same technology can also be used in conjunction with micro-mechanical testing and biological modeling and assays to define relationships between mechanical loading and the tissue and cellular responses of that tissue.
Studies to provide insight on the mechanotransduction mechanisms in healthy, developing, aging and degenerating tissues are crucial in order to better understand the consequences of technological developments for spinal care for the facet tissues. Certainly, the advent of advanced and more “lifelike” spinal tissue replacements, such as artificial discs, has also hastened the requisite investigations in to how joint and spinal loading affect the pathology and/or remodeling of these same tissues in the joint (Table 4). Integrating all of these factors together, a clear understanding of the mechanical and physiological responses of tissues and their cellular constituents is imperative for future designs of implants, treatments and management of the natural degenerative processes in the spine (Table 4). For instance, studies will need to investigate if the increase in laxity that develops in the facet capsule after injury, even a noncatastrophic one, can be overcome overtime or if even transient changes in its mechanics are sufficient to lead to further damage in the capsule and other spinal tissues.
With defined mechanical responses of the individual tissues in the facet joint and the mechanotransductive processes, it will then be necessary to integrate and relate them to overall spinal function and dysfunction, both biomechanically and physiologically (Table 4). This is perhaps one of the major challenges moving forward, yet an imperative and very necessary undertaking since the facet joint itself influences each of its component tissue responses and the surrounding structures of the spine, such as the intervertebral disc, ligaments and muscles. The complexity of such investigations makes experimental work almost impossible but computer modeling offers an appropriate alternative for such studies (Table 4). Moreover, such an approach also facilitates addressing a host of conditions experienced by the spine during natural life processes. As addressed in this review, computer models can also provide valuable platforms to investigate surgical interventions such as total disc replacement or laminectomy. Perhaps more importantly, they enable investigations of the interventions that have not yet been identified but that may result from the next generation of biomechanical studies.
In summary, in many ways, the state-of-the-art in terms of facet joint biomechanics lags behind other joints in the body. However, this is not surprising given the fact that it has only recently been viewed as anything more than an extension of the bony vertebrae of the spine. Conversely, given the challenges in studying this joint complex and its complicated tissues, there is quite a bit of important foundational knowledge about its mechanics, tolerance, failure and physiological capacities (Table 3). The field is well-poised to expand on that foundation in a variety of directions, using a variety of approaches, and to provide important insight to prevent facet joint injury, model pathophysiologic responses, intervene for treatment, and inform decision making (Table 4).
Acknowledgment
This material is based on work supported by the National Science Foundation under Grant No. 0547451. Additional support was provided by the Catharine Sharpe Foundation and the National Institutes of Health (Grant No. T32-NS-043126 awarded to the Department of Neurosurgery at the University of Pennsylvania).
Contributor Information
Nicolas V. Jaumard, e-mail: njaumard@mail.med.upenn.edu
William C. Welch, Dept. of Neurosurgery, University of Pennsylvania, HUP - 3 Silverstein, 3400 Spruce Street, Philadelphia, PA 19104
Beth A. Winkelstein, Dept. of Neurosurgery, University of Pennsylvania, HUP - 3 Silverstein, 3400 Spruce Street, Philadelphia, PA 19104; Dept. of Bioengineering, University of Pennsylvania, 210 S. 33rd Street, Room 240 Skirkanich Hall, Philadelphia, PA 19104, e-mail: winkelst@seas.upenn.edu.
References
- [1]. Adams, M. A. and Hutton, W.C. , 1983, “The Mechanical Function of Lumbar Apophyseal Joints,” Spine, 8, pp. 327–330. 10.1097/00007632-198304000-00017 [DOI] [PubMed] [Google Scholar]
- [2]. Yang, K. H. and King, A. I. , 1984, “Mechanism of Facet Load Transmission as a Hypothesis for Low-Back Pain,” Spine, 9(6), pp. 557–565. 10.1097/00007632-198409000-00005 [DOI] [PubMed] [Google Scholar]
- [3]. Schendel, M. J. , Dekutoski, M. B. , Ogilvie, J. W. , Olsewski, J. M. , and Wallace, L. J. , 1995, “Kinematics of The Canine Lumbar Intervertebral Joint. An in Vivo Study Before and After Adjacent Instrumentation,” Spine, 20(23), pp. 2555–2564. 10.1097/00007632-199512000-00015 [DOI] [PubMed] [Google Scholar]
- [4]. Pal, G. P. and Routal, R. V. , 1986, “A Study of Weight Transmission Through the Cervical and Upper Thoracic Regions of the Vertebral Column in Man,” J. Anat., 148, pp. 245–261. [PMC free article] [PubMed] [Google Scholar]
- [5]. Pal, G. P. and Routal, R. V. , 1987, “Transmission of Weight Through the Lower Thoracic and Lumbar Regions of the Vertebral Column in Man,” J. Anat., 152, pp. 93–105. [PMC free article] [PubMed] [Google Scholar]
- [6]. Ahmed, A. M. , Duncan, N. A. , and Burke, D. L. , 1990, “The Effect of Facet Geometry on the Axial Torque-Rotation Response of Rumbar Motion Segments,” Spine, 15(5), pp. 391–401. 10.1097/00007632-199005000-00010 [DOI] [PubMed] [Google Scholar]
- [7]. Onan, O. A. , Heggeness, M. H. , and Hipp, J. A. , 1998, “A Motion Analysis of the Cervical Facet Joint,” Spine, 23(4), pp. 430–439. 10.1097/00007632-199802150-00005 [DOI] [PubMed] [Google Scholar]
- [8]. Winkelstein, B. A. , Nightingale, R. W. , Richardson, W. J , and Myers, B. S. , 2000, “The Cervical Facet Capsule and its Role in Whiplash Injury: a Biomechanical Investigation,” Spine, 25(10), pp. 1238–1246. 10.1097/00007632-200005150-00007 [DOI] [PubMed] [Google Scholar]
- [9]. Yoganandan, N. , Kumaresan, S. , and Pintar, F. A. , 2000, “Geometric and Mechanical Properties of Human Cervical Spine Ligaments,” J. Biomech. Eng., 122(6), pp. 623–629. 10.1115/1.1322034 [DOI] [PubMed] [Google Scholar]
- [10]. Little, J. S. and Khalsa, P. S. , 2005, “Material Properties of the Human Lumbar Facet Joint Capsule,” J. Biomech. Eng., 127(1), pp. 15–24. 10.1115/1.1835348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Sawa, A. G. and Crawford, N. R. , 2008, “The Use of Surface Strain Data and a Neural Networks Solution Method to Determine Lumbar Facet Joint Loads During in Vitro Spine Testing,” J. Biomech., 41(12), pp. 2647–2653. 10.1016/j.jbiomech.2008.06.010 [DOI] [PubMed] [Google Scholar]
- [12]. Botolin, S. , Puttlitz, C. , Baldini, T. , Petrella, A. , Burger, E. , Abjornson, C. , and Patel, V. , 2011, “Facet Joint Biomechanics at the Treated and Adjacent Levels After Total Disc Replacement,” Spine, 36(1), pp. E27–E32. [DOI] [PubMed] [Google Scholar]
- [13]. Demetropoulos, C. K. , Sengupta, D. K. , Knaub, M.A. , Wiater, B. P. , Abjornson, C. , Truumees, E. , and Herkowitz, H. N. , 2010, “Biomechanical Evaluation of the Kinematics of the Cadaver Lumbar Spine Following Disc Replacement With the ProDisc-L Prosthesis,” Spine, 35(1), pp. 26–31. 10.1097/BRS.0b013e3181c4eb9a [DOI] [PubMed] [Google Scholar]
- [14]. Kafchitsas, K. , Kokkinakis, M. , Habermann, B. , and Rauschmann, M. , 2010, “Effect of Lumbar Disc Replacement on the Height of the Disc Space and the Geometry of the Facet Joints: a Cadaver Study,” J. Bone Jt. Surg. Br. Vol, 92(4), pp. 595–601. 10.1302/0301-620X.92B4.23175 [DOI] [PubMed] [Google Scholar]
- [15]. Siepe, C. J. , Zelenkov, P. , Sauri-Barraza, J. C. , Szeimies, U. , Grubinger, T. , Tepass, A. , Stäbler, A. , and Mayer, M. H. , 2010, “The Fate of Facet Joint and Adjacent Level Disc Degeneration Following Total Lumbar Disc Replacement: a Prospective Clinical, X-ray, and Magnetic Resonance Imaging Investigation,” Spine, 35(22), pp. 1991–2003. 10.1097/BRS.0b013e3181d6f878 [DOI] [PubMed] [Google Scholar]
- [16]. Takigawa, T. , Espinoza Orías, A. A. , An, H. S. , Gohgi, S. , Udayakumar, R. K. , Sugisaki, K. , Natarajan, R. N. , Wimmer, M. A. , and Inoue, N. , 2010, “Spinal Kinematics and Facet Load Transmission After Total Disc Replacement,” Spine, 35(22), pp. E1160–E1166. 10.1097/BRS.0b013e3181e5352d [DOI] [PubMed] [Google Scholar]
- [17]. Vasseur, P. B. , Saunders, G. , and Steinback, C. , 1981, “Anatomy and Function of the Ligaments of the Lower Cervical Spine in the Dog,” Am. J. Vet. Res., 42(6), pp. 1002–1006. [PubMed] [Google Scholar]
- [18]. Yahia, L. H. and Garzon, S. , 1993, “Structure on the Capsular Ligaments of the Facet Joints,” Ann. Anat. Pathol. (Paris), 175(2), pp. 185–188. [DOI] [PubMed] [Google Scholar]
- [19]. Yoganandan, N. , Knowles, S. A. , Maiman, D. J. , and Pintar, F. A. , 2003, “Anatomic Study of the Morphology of Human Cervical Facet Joint,” Spine, 28(20), pp. 2317–2323. 10.1097/01.BRS.0000085356.89103.A5 [DOI] [PubMed] [Google Scholar]
- [20]. Varlotta, G. P. , Lefkowitz, T. R. , Schweitzer, M. , Errico, T. J. , Spivak, J. , Bendo, J. A. , and Rybak, L. , 2011, “The Lumbar Facet Joint: a Review of Current Knowledge: Part 1: Anatomy, Biomechanics, and Grading,” Skeletal Radiol., 40(1), pp. 13–23. 10.1007/s00256-010-0983-4 [DOI] [PubMed] [Google Scholar]
- [21]. Kalichman, L. and Hunter, D. J. , 2007, “Lumbar Facet Joint Osteoarthritis: a Review,” Semin. Arthritis. Rheum., 37(2), pp. 69–80. 10.1016/j.semarthrit.2007.01.007 [DOI] [PubMed] [Google Scholar]
- [22]. Louis, R. , 1985, “Spinal Stability as Defined by the Three-Column Spine Concept,” Anat. Clin., 7(1), pp. 33–42. 10.1007/BF01654627 [DOI] [PubMed] [Google Scholar]
- [23]. Pal, G. P. and Sherk, H. H. , 1988, “The Vertical Stability of the Cervical Spine,” Spine, 13, pp. 447–449. 10.1097/00007632-198805000-00001 [DOI] [PubMed] [Google Scholar]
- [24]. White, A. A. and Panjabi, M. M. , 1990, Clinical Biomechanics of the Spine, 2nd ed. Lippincott, New York. [Google Scholar]
- [25]. Panjabi, M. M. , Oxland, T. , Takata, K. , Goel, V. , Duranceau, J. , and Krag, M. , 1993, “Articular Facets of the Human Spine. Quantitative Three-Dimensional Anatomy,” Spine, 18(10), pp. 1298–1310. 10.1097/00007632-199308000-00009 [DOI] [PubMed] [Google Scholar]
- [26]. Pal, G. P. , Routal, R. V. , and Saggu, S. K. , 2001, “The Orientation of the Articular Facets of the Zygapophyseal Joints at the Cervical and Upper Thoracic Region,” J. Anat., 198(4), pp. 431–441. 10.1046/j.1469-7580.2001.19840431.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Ebraheim, N. A. , Patil, V. , Liu, J. , Haman, S. P. , and Yeasting, R. A. , 2008, “Morphometric Analyses of the Cervical Superior Facets and Implications for Facet Dislocation,” Int. Orthop., 32(1), pp. 97–101. 10.1007/s00264-006-0286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Berry, J. L. , Moran, J. M. , Berg, W. S. , and Steffee, A. D. , 1987, “A Morphometric Study of Human Lumbar and Selected Thoracic Vertebrae,” Spine, 12(4), pp. 362–367. 10.1097/00007632-198705000-00010 [DOI] [PubMed] [Google Scholar]
- [29]. Zindrick, M. R. , Wiltse, L. L. , Doornik, A. , Widell, E. H. , Knight, G. W. , Patwardhan, A. G. , Thomas, J. C. , Rothman, S. L. , and Fields, B. T. , 1987, “Analysis of the Morphometric Characteristics of the Thoracic and Lumbar Pedicles,” Spine, 12(2), pp. 160–166. 10.1097/00007632-198703000-00012 [DOI] [PubMed] [Google Scholar]
- [30]. Krag, M. H. , Weaver, D. L. , Beynnon, B. D. , and Haugh, L. D. , 1988, “Morphometry of the Thoracic and Lumbar Spine Related to Transpedicular Screw Placement for Surgical Spinal Fixation,” Spine, 13(1), pp. 27–32. 10.1097/00007632-198801000-00007 [DOI] [PubMed] [Google Scholar]
- [31]. Panjabi, M. M. , Duranceau, J. , Goel, V. , Oxland, T. , and Takata, K. , 1991, “Cervical Human Vertebrae. Quantitative Three-Dimensional Anatomy of the Middle and Lower Regions,” Spine, 16(8), pp. 861–869. 10.1097/00007632-199108000-00001 [DOI] [PubMed] [Google Scholar]
- [32]. Panjabi, M. M. , Takata, K. , Goel, V. , Federico, D. , Oxland, T. , Duranceau, J. , and Krag, M. , 1991, “Thoracic Human Vertebrae, Quantitative Three-Dimensional Anatomy,” Spine, 16(8), pp. 888–901. 10.1097/00007632-199108000-00006 [DOI] [PubMed] [Google Scholar]
- [33]. Van Schaik, J. P. , Verbiest, H. , and Van Schaik, F. D. , 1985, “The Orientation of Laminae and Facet Joints in the Lower Lumbar Spine,” Spine, 10(1), pp. 59–63. 10.1097/00007632-198501000-00009 [DOI] [PubMed] [Google Scholar]
- [34]. Bogduk, N. and Twomey, L. T. , 1991, Clinical Anatomy of the Lumbar Spine, Churchill Livingstone, Melbourne, Australia. [Google Scholar]
- [35]. Tulsi, R. S. and Hermanis, G. M. , 1993, “A Study of the Angle of Inclination and Facet Curvature of Superior Lumbar Zygapophyseal Facets,” Spine, 18(10), pp. 1311–1317. 10.1097/00007632-199308000-00010 [DOI] [PubMed] [Google Scholar]
- [36]. Pal, G. P. and Routal, R. V. , 1999, “Mechanism of Change in the Orientation of the Articular Process of the Zygapophyseal Joint at the Thoracolumbar Junction,” J. Anat., 195(2), pp. 199-209. 10.1046/j.1469-7580.1999.19520199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Panjabi, M. M. , Crisco, J. J. , Vasavada, A. , Oda, T. , Cholewicki, J. , Nibu, K. , and Shin, E. , 2001, “Mechanical Properties of the Human Cervical Spine as Shown by Three-Dimensional Load-Displacement Curves,” Spine, 26(24), pp. 2692–2700. 10.1097/00007632-200112150-00012 [DOI] [PubMed] [Google Scholar]
- [38]. Ahn, H. S. and DiAngelo, D. J. , 2007. “Biomechanical Testing Simulation of a Cadaver Spine Specimen: Development and Evaluation Study,” Spine, 32(11), pp. E330–E336. 10.1097/01.brs.0000263331.78903.61 [DOI] [PubMed] [Google Scholar]
- [39]. Kozanek, M. , Wang, S. , Passias, P. G. , Xia, Q. , Li, G. , Bono, C. M. , Wood, K. B. , and Li, G. , 2009, “Range of Motion and Orientation of the Lumbar Facet Joints in Vivo,” Spine, 34(19), pp. E689–E696. 10.1097/BRS.0b013e3181ab4456 [DOI] [PubMed] [Google Scholar]
- [40]. Dunn, W. , DuRaine, G. , and Reddi, A. H. , 2009, “Profiling MicroRNA Expression in Bovine Articular Cartilage and Implications for Mechanotransduction,” Arthritis Rheum., 60(8), pp. 2333–2339. 10.1002/art.24678 [DOI] [PubMed] [Google Scholar]
- [41]. Womack, W. , Woldtvedt, D. , and Puttlitz, C. M. , 2008, “Lower Cervical Spine Facet Cartilage Thickness Mapping,” Osteoarthritis Cartilage, 16(9), pp. 1018–1023. 10.1016/j.joca.2008.01.007 [DOI] [PubMed] [Google Scholar]
- [42]. Eisenstein, S. M. and Parry, C. R. , 1987, “The Lumbar Facet Arthrosis Syndrome. Clinical Presentation and Articular Surface Changes,” J. Bone Jt. Surg. Br., 69, pp. 3–7. [DOI] [PubMed] [Google Scholar]
- [43]. Côté, P. , Cassidy, J. D. , Carroll, L. , Frank, J. W. , and Bombardier, C. , 2001, “A Systematic Review of the Prognosis of Acute Whiplash and a New Conceptual Framework to Synthesize the Literature,” Spine, 26(19), pp. E445–E458. 10.1097/00007632-200110010-00020 [DOI] [PubMed] [Google Scholar]
- [44]. Stemper, B. D. , Derosia, J. J. , Yoganandan, N. , Pintar, F. A. , Shender, B. S. , and Paskoff, G. R. , 2009, “Gender Dependent Cervical Spine Anatomical Differences in Size-Matched Volunteers - Biomed 2009,” Biomed. Sci. Instrum., 45, pp. 149–154. [PubMed] [Google Scholar]
- [45]. Urban, J. P. , 1994, “The Chondrocyte: A Cell Under Pressure,” Br. J. Rheumatol., 33(10), pp. 901–908. 10.1093/rheumatology/33.10.901 [DOI] [PubMed] [Google Scholar]
- [46]. Silver, F. H. , Bradica, G. , and Tria, A. , 2001, “Relationship Among Biomechanical, Biochemical, and Cellular Changes Associated With Osteoarthritis,” Crit. Rev. Biomed. Eng., 29(4), pp. 373–391. [DOI] [PubMed] [Google Scholar]
- [47]. Leddy, H. A. and Guilak, F. , 2008, “Site-Specific Effects of Compression on Macromolecular Diffusion in Articular Cartilage,” Biophys. J., 95(10), pp. 4890–4895. 10.1529/biophysj.108.137752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Martin, R. B. , Burr, D. B. , and Sharkey, N. A. , 1998, Skeletal Tissue Mechanics, Springer, New York, pp. 50–55. [Google Scholar]
- [49]. Pierce, D. M. , Trobin, W. , Trattnig, S. , Bischof, H. , and Holzapfel, G. A. , 2009, “A Phenomenological Approach Toward Patient-Specific Computational Modeling of Articular Cartilage Including Collagen Fiber Tracking,” J. Biomech. Eng., 131(9), pp. 091006. 10.1115/1.3148471 [DOI] [PubMed] [Google Scholar]
- [50]. Leipzig, N. D. and Athanasiou, K. A. , 2004, The Encyclopedia of Biomaterials and Bioengineering, Wnek G., and Bowlin G., eds., Marcel Dekker, Inc., New York. [Google Scholar]
- [51]. Armstrong, C. G. and Mow, V. C. , 1982, “Variations in the Intrinsic Mechanical Properties of Human Articular Cartilage with Age, Degeneration, and Water Content,” J. Bone Jt. Surg. Am., 64(1), pp. 88–94. [PubMed] [Google Scholar]
- [52]. Morel, V. , Merçay, A. , and Quinn, T. M. , 2005, “Prestrain Decreases Cartilage Susceptibility to Injury by Ramp Compression in Vitro,” Osteoarthritis Cartilage, 13(11), pp. 964–970. 10.1016/j.joca.2005.06.016 [DOI] [PubMed] [Google Scholar]
- [53]. Adams, M. A. , Dolan, P. , and McNally, D. S. , 2009, “The Internal Mechanical Functioning of Intervertebral Discs and Articular Cartilage, and its Relevance to Matrix Biology,” Matrix Biol., 28(7), pp. 384–389. 10.1016/j.matbio.2009.06.004 [DOI] [PubMed] [Google Scholar]
- [54]. Mizrahi, J. , Maroudas, A. , Lanir, Y. , Ziv, I. , and Webber, T. J. , 1986, “The “Instantaneous” Deformation of Cartilage: Effects of Collagen Fiber Orientation and Osmotic Stress,” Biorheology, 23(4), pp. 311–330. [DOI] [PubMed] [Google Scholar]
- [55]. Morel, V. , Berutto, C. , Quinn, and T. M. , 2006, “Effects of Damage in the Articular Surface on the Cartilage Response to Injurious Compression in Vitro,” J. Biomech., 39(5), pp. 924–930. 10.1016/j.jbiomech.2005.01.026 [DOI] [PubMed] [Google Scholar]
- [56]. Mow, V. C. , Ratcliffe, A. , and Poole, A. R. , 1992, “Cartilage and Diarthrodial Joints as Paradigms for Hierarchical Materials and Structures,” Biomaterials, 13(2), pp. 67–97. 10.1016/0142-9612(92)90001-5 [DOI] [PubMed] [Google Scholar]
- [57]. Wong, M. and Carter, D. R. , 2003, “Articular Cartilage Functional Histomorphology and Mechanobiology: a Research Perspective,” Bone, 33, pp. 1–13. 10.1016/S8756-3282(03)00083-8 [DOI] [PubMed] [Google Scholar]
- [58]. Khalsa, P. S. and Eisenberg, S. R. , 1997, “Compressive Behavior of Articular Cartilage is Not Completely Explained by Proteoglycan Osmotic Pressure,” J. Biomech., 30(6), pp. 589–594. [DOI] [PubMed] [Google Scholar]
- [59]. Wu, J. Z. and Herzog, W. , 2002, “Elastic Anisotropy of Articular Cartilage is Associated with the Microstructures of Collagen Fibers and Chondrocytes,” J. Biomech., 35, pp. 931–942. 10.1016/S0021-9290(02)00050-7 [DOI] [PubMed] [Google Scholar]
- [60]. Repo, R. U. and Finlay, J. B. , 1977, “Survival of Articular Cartilage After Controlled Impact,” J. Bone Jt. Surg. Am., 59(8), pp. 1068–1076. [PubMed] [Google Scholar]
- [61]. Oloyede, A. , Flachsmann, R. , and Broom, N. D. , 1992, “The Dramatic Influence of Loading Velocity on the Compressive Response of Articular Cartilage,” Connect. Tissue Res., 27(4), pp. 211–224. 10.3109/03008209209006997 [DOI] [PubMed] [Google Scholar]
- [62]. Jeffrey, J. E. , Gregory, D. W. , and Aspden, R. M. , 1995, “Matrix Damage and Chondrocyte Viability Following a Single Impact Load on Articular Cartilage,” Arch. Biochem. Biophys., 322(1), pp. 87–96. 10.1006/abbi.1995.1439 [DOI] [PubMed] [Google Scholar]
- [63]. Whiteside, R. A. , Jakob, R. P. , Wyss, U. P. , and Mainil-Varlet, P. , 2005, “Impact Loading of Articular Cartilage During Transplantation of Osteochondral Autograft,” J. Bone Jt. Surg. Br., 87(9), pp. 1285–1291. 10.1302/0301-620X.87B9.15710 [DOI] [PubMed] [Google Scholar]
- [64]. Knecht, S. , Vanwanseele, B. , and Stüssi, E. , 2006, “A Review on the Mechanical Quality of Articular Cartilage − Implications for the Diagnosis of Osteoarthritis,” Clin. Biomech. (Bristol, Avon), 21(10), pp. 999–1012. 10.1016/j.clinbiomech.2006.07.001 [DOI] [PubMed] [Google Scholar]
- [65]. Isaac, D. I. , Meyer, E. G. , and Haut, R. C. , 2008, “Chondrocyte Damage and Contact Pressures Following Impact on the Rabbit Tibiofemoral Joint,” J. Biomech. Eng. 130(4), p. 041018. 10.1115/1.2948403 [DOI] [PubMed] [Google Scholar]
- [66]. Elder, B. D. , Vigneswaran, K. , Athanasiou, K. A. , and Kim, D. H. , 2009, “Biomechanical, Biochemical, and Histological Characterization of Canine Lumbar Facet Joint Cartilage,” J. Neurosurg. Spine 10(6), pp. 623–628. 10.3171/2009.2.SPINE08818 [DOI] [PubMed] [Google Scholar]
- [67]. Athanasiou, K. A. , Rosenwasser, M. P. , Buckwalter, J. A. , Malinin, T. I. , and Mow, V.C. , 1991, “Interspecies Comparisons of in Situ Intrinsic Mechanical Properties of Distal Femoral Cartilage,” J. Orthop. Res. 9(3), pp. 330–340. 10.1002/jor.v9:3 [DOI] [PubMed] [Google Scholar]
- [68]. Erratum: “Biomechanical Properties of Hip Cartilage in Experimental Animal Models,” [Clin Orthop Relat Res. (316), pp. 254-66]. [PubMed] [Google Scholar]
- [69]. Ghadially, F. N. and Roy, S. , 1983, Ultrastructure of Synovial Joints in Health and Disease, Butterworths, London: [Google Scholar]
- [70]. Iwanaga, T. , Shikichi, M. , Kitamura, H. , Yanase, H. , and Nozawa-Inoue, K. , 2000, “Morphology and Functional Roles of Synoviocytes in the Joint,” Arch. Histol. Cytol., 63(1), pp. 17–31. 10.1679/aohc.63.17 [DOI] [PubMed] [Google Scholar]
- [71]. Vandenabeele, F. , Lambrichts, I. , Lippens, P. , and Creemers, J. , 2001, “In Vitro Loading of Human Synovial Membrane with 5-Hydroxydopamine: Evidence for Dense core Secretory Granules in Type B Cells,” Arch. Histol. Cytol., 64(1), pp. 1–16. 10.1679/aohc.64.1 [DOI] [PubMed] [Google Scholar]
- [72]. Engel, R. and Bogduk, N. , 1982, “The Menisci of the Lumbar Zygapophysial Joints,” J. Anat., 135(4), pp. 795–809. [PMC free article] [PubMed] [Google Scholar]
- [73]. Bogduk, N. and Engel, R. , 1984, “The Menisci of the Lumbar Zygapophyseal Joints. A Review of Their Anatomy and Clinical Significance,” Spine, 9(5), pp. 454–460. 10.1097/00007632-198407000-00006 [DOI] [PubMed] [Google Scholar]
- [74]. Yu, S. W. , Sether, L. , and Haughton, V. M. , 1987, “Facet Joint Menisci of the Cervical Spine: Correlative MR Imaging and Cryomicrotomy Study,” Radiology, 164(1), pp. 79–82. [DOI] [PubMed] [Google Scholar]
- [75]. Mercer, S. and Bogduk, N. , 1993, “Intra-Articular Inclusions of the Cervical Synovial Joints,” Br J. Rheumatol., 32(8), pp. 705–710. 10.1093/rheumatology/32.8.705 [DOI] [PubMed] [Google Scholar]
- [76]. Taylor, J. R. and McCormick, C. C. , 1991, “Lumbar Facet Joint Fat Pads: Their Normal Anatomy and Their Appearance when Enlarged,” Neuroradiology, 33(1), pp. 38–42. 10.1007/BF00593331 [DOI] [PubMed] [Google Scholar]
- [77]. Friedrich, K. M. , Reiter, G. , Pretterklieber, M. L. , Pinker, K. , Friedrich, M. , Trattnig, S. , and Salomonowitz, E. , 2008, “Reference Data for in Vivo Magnetic Resonance Imaging Properties of Meniscoids in the Cervical Zygapophyseal Joints,” Spine, 33(21), pp. E778–E783. 10.1097/BRS.0b013e318182c399 [DOI] [PubMed] [Google Scholar]
- [78]. Schulte, T. L. , Filler, T. J. , Struwe, P. , Liem, D. , and Bullmann, V. , 2010, “Intra-Articular Meniscoid Folds in Thoracic Zygapophysial Joints,” Spine, 35(6), pp. E191–E197. 10.1097/BRS.0b013e3181c9b053 [DOI] [PubMed] [Google Scholar]
- [79]. Jones, T. R. , James, J. E. , Adams, J. W. , Garcia, J. , Walker, S. L. , and Ellis, J. P. , 1989, “Lumbar Zygapophyseal Joint Meniscoids: Evidence of Their Role in Chronic Intersegmental Hypomobility,” J. Manipulative Physiol. Ther., 12(5), pp. 374–385. [PubMed] [Google Scholar]
- [80]. Lewin, T. , Moffett, B. and Viidik, A. , 1962, “The Morphology of the Lumbar Synovial Intervertebral Joints,” Acta Morphol. Neerl. Scand., 4, pp. 299–319. [PubMed] [Google Scholar]
- [81]. Kos, J. and Wolf, J. , 1972, “Les Ménisques Intervertébraux et Leur Rôle Possible Dans les Blocages Vertébraux,” Annales de Médecine Physique, 15, pp. 203–218. [Google Scholar]
- [82]. Stemper, B. D. , Yoganandan, N. , and Pintar, F. A. , 2004, “Gender- and Region-Dependent Local Facet Joint Kinematics in Rear Impact: Implications in Whiplash Injury,” Spine, 29(16), pp. 1764–1771. 10.1097/01.BRS.0000134563.10718.A7 [DOI] [PubMed] [Google Scholar]
- [83]. Sato, S. , Oguma, H. , Murakami, G. , and Noriyasu, S. , 2002, “Morphometrical Study of the Joint Surface and Capsule of the Lumbar Zygapophysial Joint With Special Reference to Their Laterality,” Okajimas Folia Anat. Jpn., 79(1), pp. 43–54. 10.2535/ofaj.79.43 [DOI] [PubMed] [Google Scholar]
- [84]. Yamashita, T. , Minaki, Y. , Ozaktay, A. C. , Cavanaugh, J. M. , and King, A. I. , 1996, “A Morphological Study of the Fibrous Capsule of the Human Lumbar Facet Joint,” Spine, 21(5), pp. 538–543. 10.1097/00007632-199603010-00002 [DOI] [PubMed] [Google Scholar]
- [85]. McLain, R. F. , 1994, “Mechanoreceptor Endings in Human Cervical Facet Joints,” Spine, 19(5), pp. 495–501. 10.1097/00007632-199403000-00001 [DOI] [PubMed] [Google Scholar]
- [86]. Vandenabeele, F. , Creemers, J. , Lambrichts, I. , Lippens, P. , and Jans, M. , 1997, “Encapsulated Ruffini-like Endings in Human Lumbar Facet Joints,” J. Anat., 191(4), pp. 571–583. 10.1046/j.1469-7580.1997.19140571.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. McLain, R. F. and Pickar, J. G. , 1998, “Mechanoreceptor Endings in Human Thoracic and Lumbar Facet Joints,” Spine, 23(2), pp. 168–173. 10.1097/00007632-199801150-00004 [DOI] [PubMed] [Google Scholar]
- [88]. Chen, C. , Lu, Y. , Kallakuri, S. , Patwardhan, A. , and Cavanaugh, J. M. , 2006, “Distribution of A-Delta and C-Fiber Receptors in the Cervical Facet Joint Capsule and Their Response to Stretch,” J. Bone Jt. Surg Am., 88(8), pp. 1807–1816. 10.2106/JBJS.E.00880 [DOI] [PubMed] [Google Scholar]
- [89]. Vasavada, A. N. , Peterson, B. W. , and Delp, S. L. , 2002, “Three-Dimensional Spatial Tuning of Neck Muscle Activation in Humans,” Exp. Brain Res., 147(4), pp. 437–448. 10.1007/s00221-002-1275-6 [DOI] [PubMed] [Google Scholar]
- [90]. Blouin, J. S. , Siegmund. G. P., Carpenter, M. G. , and Inglis, J. T. , 2007, “Neural Control of Superficial and Deep Neck Muscles in Humans,” J. Neurophysiol., 98(2), pp. 920–928. 10.1152/jn.00183.2007 [DOI] [PubMed] [Google Scholar]
- [91]. Siegmund, G. P. , Blouin, J. S. , Brault, J. R. , Hedenstierna, S. , and Inglis, J. T. , 2007, “Electromyography of Superficial and Deep Neck Muscles During Isometric, Voluntary, and Reflex Contractions,” J. Biomech. Eng., 129(1), pp. 66–77. 10.1115/1.2401185 [DOI] [PubMed] [Google Scholar]
- [92]. Haher, T. R. , O'Brien, M. , Dryer, J. W. , Nucci, R. , Zipnick, R. , and Leone, D. J. , 1994, “The Role of the Lumbar Facet Joints in Spinal Stability. Identification of Alternative Paths of Loading,” Spine, 19(23), pp. 2667–2671. 10.1097/00007632-199412000-00012 [DOI] [PubMed] [Google Scholar]
- [93]. Kim, C. W. , Perry, A. , and Garfin, S. R. , 2005, “Spinal Instability: the Orthopedic Approach,” Semin. Musculoskelet. Radiol., 9(1), pp. 77–87. 10.1055/s-2005-867098 [DOI] [PubMed] [Google Scholar]
- [94]. Panjabi, M. M. , 2003, “Clinical Spinal Instability and Low Back Pain,” J. Electromyogr. Kinesiol., 13(4), pp. 371–379. 10.1016/S1050-6411(03)00044-0 [DOI] [PubMed] [Google Scholar]
- [95]. Reeves, N. P. , Narendra, K. S. , and Cholewicki, J. , 2007, “Spine Stability: The Six Blind Men and the Elephant,” Clin. Biomech. (Bristol, Avon), 22(3), pp. 266–274. 10.1016/j.clinbiomech.2006.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Adams, M. A. and Hutton, W. C. , 1980, “The Effect of Posture on the Role of the Apophysial Joints in Resisting Intervertebral Compressive Forces,” J. Bone Jt. Surg. Br., 62(3), pp. 358–362. [DOI] [PubMed] [Google Scholar]
- [97]. Yoganandan, N. , Kumaresan, S. , and Pintar, F. A. , 2001, “Biomechanics of the Cervical Spine Part 2. Cervical Spine Soft Tissue Responses and Biomechanical Modeling,” Clin. Biomech. (Bristol, Avon), 16(1), pp. 1–27. 10.1016/S0268-0033(00)00074-7 [DOI] [PubMed] [Google Scholar]
- [98]. Raynor, R. B. , Pugh, J. , and Shapiro, I. , 1985, “Cervical Facetectomy and its Effect on Spine Strength,” J. Neurosurg., 63(2), pp. 278–282. 10.3171/jns.1985.63.2.0278 [DOI] [PubMed] [Google Scholar]
- [99]. Raynor, R. B. , Moskovich, R. , Zidel, P. , and Pugh, J. , 1987, “Alterations in Primary and Coupled Neck Motions After Facetectomy,” Neurosurgery, 21(5), pp. 681–687. 10.1227/00006123-198711000-00014 [DOI] [PubMed] [Google Scholar]
- [100]. Cusick, J. F. , Yoganandan, N. , Pintar, F. , Myklebust, J. , and Hussain, H. , 1988, “Biomechanics of Cervical Spine Facetectomy and Fixation Techniques,” Spine, 13(7), pp. 808–812. 10.1097/00007632-198807000-00017 [DOI] [PubMed] [Google Scholar]
- [101]. Abumi, K. , Panjabi, M. M. , Kramer, K. M. , Duranceau, J. , Oxland, T. , and Crisco, J. J. , 1990, “Biomechanical Evaluation of Lumbar Spinal Stability After Graded Facetectomies,” Spine, 15(11), pp. 1142–1147. 10.1097/00007632-199011010-00011 [DOI] [PubMed] [Google Scholar]
- [102]. Zdeblick, T. A. , Zou, D. , Warden, K. E. , McCabe, R. , Kunz, D. , and Vanderby, R. , 1992, “Cervical Stability After Foraminotomy. A Biomechanical in Vitro Analysis,” J. Bone Jt. Surg. Am., 74(1), pp. 22–27. [PubMed] [Google Scholar]
- [103]. Nowinski, G. P. , Visarius, H. , Nolte, L. P. , and Herkowitz, H. N. , 1993, “A Biomechanical Comparison of Cervical Laminaplasty and Cervical Laminectomy With Progressive Facetectomy,” Spine, 18(14), pp. 1995–2004. 10.1097/00007632-199310001-00012 [DOI] [PubMed] [Google Scholar]
- [104]. Tender, G. C. , Kutz. S., Baratta, R. , and Voorhies, R. M. , 2005, “Unilateral Progressive Alterations in the Lumbar Spine: A Biomechanical Study,” J. Neurosurg. Spine., 2(3), pp. 298–302. 10.3171/spi.2005.2.3.0298 [DOI] [PubMed] [Google Scholar]
- [105]. Zdeblick, T.A. , Abitbol, J. J. , Kunz, D. N. , McCabe, R. P. , and Garfin, S. , 1993, “Cervical Stability After Sequential Capsule Resection,” Spine 18, pp. 2005–2008. 10.1097/00007632-199310001-00013 [DOI] [PubMed] [Google Scholar]
- [106]. Jaumard, N. V. , Bauman, J. A. , Welch, W. C. , and Winkelstein, B. A. , 2010, “Biomechanical Comparison of Contact Pressure in the Cervical Facet Joint During Bending Using a Probe and Pressure-Sensitive Paper,” ASME Summer Bioengineering Conference, Naples, FL, June 2010, 19498. [Google Scholar]
- [107]. Panjabi, M. M. , Goel, V. K. , and Takata, K. , 1982, “Physiologic Strains in the Lumbar Spinal Ligaments. An In Vitro Biomechanical Study (1981) Volvo Award in Biomechanics,” Spine, 7(3), pp. 192–203. 10.1097/00007632-198205000-00003 [DOI] [PubMed] [Google Scholar]
- [108]. Ianuzzi, A. , Little, J. S. , Chiu, J. B. , Baitner, A. , Kawchuk, G. , and Khalsa, P. S. , 2004, “Human Lumbar Facet Joint Capsule Strains: I. During Physiological Motions,” Eur. Spine J., 4(2), pp. 141–152. 10.1016/j.spinee.2003.07.008 [DOI] [PubMed] [Google Scholar]
- [109]. Siegmund, G. P. , Myers, B. S. , Davis, M. B. , Bohnet, H. F. , and Winkelstein, B. A. , 2000, “Human Cervical Motion Segment Flexibility and Facet Capsular Ligament Strain Under Combined Posterior Shear, Extension and Axial Compression,” Stapp Car Crash J., 44, pp. 159–170. [DOI] [PubMed] [Google Scholar]
- [110]. Winkelstein, B. A. , McLendon, R. E. , Barbir, A. , and Myers, B. S. , 2001, “An Anatomical Investigation of the Human Cervical Facet Capsule, Quantifying Muscle Insertion Area,” J. Anat., 198(4), pp. 455–461. 10.1017/S0021878201007518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Quinn, K. P. , Lee, K. E. , Ahaghotu, C. C. , and Winkelstein, B. A. , 2007, “Structural Changes in the Cervical Facet Capsular Ligament: Potential Contributions to Pain Following Subfailure Loading,” Stapp Car Crash Journal, 51 , pp. 169–187. [DOI] [PubMed] [Google Scholar]
- [112]. Quinn, K. P. , Bauman, J. A. , Crosby, N. D. , and Winkelstein, B. A. , 2010, “Anomalous Fiber Realignment During Tensile Loading of the Rat Facet Capsular Ligament Identifies Mechanically Induced Damage and Physiological Dysfunction,” J. Biomech., 43(10), pp. 1870–1875. 10.1016/j.jbiomech.2010.03.032 [DOI] [PubMed] [Google Scholar]
- [113]. Lee, K. E. , Davis, M. B. , Mejilla, R. M. , and Winkelstein, B. A. , 2004, “In Vivo Cervical Facet Capsule Distraction: Mechanical Implications for Whiplash and Neck Pain,” Stapp Car Crash J., 48, pp. 373–395. [DOI] [PubMed] [Google Scholar]
- [114]. Lee, K. E. , Franklin, A. N , Davis, M. B. , and Winkelstein, B. A. , 2006, “Tensile Cervical Facet Capsule Ligament Mechanics: Failure and Subfailure Responses in the Rat,” J. Biomech., 39(7), pp. 1256–1264. 10.1016/j.jbiomech.2005.03.018 [DOI] [PubMed] [Google Scholar]
- [115]. Lee, K. E. and Winkelstein, B. A. , 2009, “Joint Distraction Magnitude is Associated With Different Behavioral Outcomes and Substance P Levels for Cervical Facet Joint Loading in the Rat,” J. Pain, 10(4), pp. 436–445. 10.1016/j.jpain.2008.11.009 [DOI] [PubMed] [Google Scholar]
- [116]. Lu, Y. , Chen, C. , Kallakuri, S. , Patwardhan, A. , and Cavanaugh, J. M. , 2005, “Neural Response of Cervical Facet Joint Capsule to Stretch: a Study of Whiplash Pain Mechanism,” Stapp Car Crash J., 49, pp. 49–65. [DOI] [PubMed] [Google Scholar]
- [117]. Lu, Y. , Chen, C. , Kallakuri, S. , Patwardhan, A. , and Cavanaugh, J. M. , 2005, “Development of an in Vivo Method to Investigate Biomechanical and Neurophysiological Properties of Spine Facet Joint Capsules,” Eur. Spine J., 14(6), pp. 565–572. 10.1007/s00586-004-0835-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Chen, C. , Lu, Y. , Cavanaugh, J. M. , Kallakuri, S. , and Patwardhan, A. , 2005, “Recording of Neural Activity From Goat Cervical Facet Joint Capsule Using Custom-Designed Miniature Electrodes,” Spine, 30(12), pp. 1367–1372. 10.1097/01.brs.0000166193.39389.21 [DOI] [PubMed] [Google Scholar]
- [119]. Quinn, K. P. and Winkelstein, B. A. , 2007, “Cervical Facet Capsular Ligament Yield Defines the Threshold for Injury and Persistent Joint-Mediated Neck Pain,” J. Biomech., 40(10), pp. 2299–2306. 10.1016/j.jbiomech.2006.10.015 [DOI] [PubMed] [Google Scholar]
- [120]. Quinn, K. P. and Winkelstein, B. A. , 2008, “Altered Collagen Fiber kinematics define the onset of localized ligament damage during loading,” J. Appl. Physiol., 105(6), pp. 1881–1888. 10.1152/japplphysiol.90792.2008 [DOI] [PubMed] [Google Scholar]
- [121]. Kallakuri, S. , Singh, A. , Lu, Y. , Chen, C. , Patwardhan, A. , and Cavanaugh, J. M. , 2008, “Tensile Stretching of Cervical Facet Joint Capsule and Related Axonal Changes,” Eur. Spine J., 17(4), pp. 556–563. 10.1007/s00586-007-0562-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122]. Azar, N. R. , Kallakuri, S. , Chen, C. , Lu, Y. , and Cavanaugh, J. M. , 2009, “Strain and Load Thresholds for Cervical Muscle Recruitment in Response to Quasi-Static Tensile Stretch of the Caprine C5-C6 Facet Joint Capsule,” J. Electromyogr. Kinesiol., 19(6), pp. E387–E394. 10.1016/j.jelekin.2009.01.002 [DOI] [PubMed] [Google Scholar]
- [123]. Dong, L. and Winkelstein, B. A. , 2010, “Simulated Whiplash Modulates Expression of the Glutamatergic System in the Spinal Cord Suggesting Spinal Plasticity is Associated With Painful Dynamic Cervical Facet Loading,” J. Neurotrauma., 27(1), pp. 163–174. 10.1089/neu.2009.0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Dong, L. , Odeleye, A. O. , Jordan-Sciutto, K. L. , and Winkelstein, B. A. , 2008, “Painful Facet Joint Injury Induces Neuronal Stress Activation in the DRG: Implications for Cellular Mechanisms of Pain,” Neurosci. Lett., 443(2), pp. 90–94. 10.1016/j.neulet.2008.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Bader, D. L. and Kempson, G. E. , 1994, “The Short-Term Compressive Properties of Adult Human Articular Cartilage,” Biomed. Mater. Eng., 4(3), pp. 245–256. [PubMed] [Google Scholar]
- [126]. Lu, X. L. and Mow, V. C. , 2008, “Biomechanics of Articular Cartilage and Determination of Material Properties,” Med. Sci. Sports Exercise, 40(2), pp. 193–199. 10.1249/mss.0b013e31815cb1fc [DOI] [PubMed] [Google Scholar]
- [127]. Setton, L. A. , Zhu, W. , and Mow, V. C. , 1993, “The Biphasic Poroviscoelastic Behavior of Articular Cartilage: Role of the Surface Zone in Governing the Compressive Behavior,” J. Biomech., 26(4-5), pp. 581–592. 10.1016/0021-9290(93)90019-B [DOI] [PubMed] [Google Scholar]
- [128]. Cohen, N. P. , Foster, R. J. , and Mow, C. , 1998, “Composition and Dynamics of Articular Cartilage: Structure, Function, and Maintaining Healthy State,” J. Orthop. Sports Phys. Ther., 28(4), pp. 203–215. [DOI] [PubMed] [Google Scholar]
- [129]. Fortis, A. P. , Kostopoulos, V. , Panagiotopoulos, E. , Tsantzalis, S. , and Kokkinos, A. , 2004, “Viscoelastic Properties of Cartilage-Subchondral Bone Complex in Osteoarthritis,” J. Med. Eng. Technol., 28(5), pp. 223–226. 10.1080/03091900410001676003 [DOI] [PubMed] [Google Scholar]
- [130]. Fulcher, G. R. , Hukins, D. W. , and Shepherd, D. E. , 2009, “Viscoelastic Properties of Bovine Articular Cartilage Attached to Subchondral Bone at High Frequencies,” BMC Musculoskelet. Disord., 10, pp. 61. 10.1186/1471-2474-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Buttermann, G. R. , Kahmann, R. D. , Lewis, J. L. , and Bradford, D. S. , 1991, “An Experimental Method for Measuring Force on the Spinal Facet Joint: Description and Application of the Method,” J. Biomech. Eng., 113(4), pp. 375–386. 10.1115/1.2895415 [DOI] [PubMed] [Google Scholar]
- [132]. Chang, U. K. , Kim, D. H. , Lee, M. C. , Willenberg, R. , Kim, S. H. , and Lim, J. , 2007, “Changes in Adjacent-Level Disc Pressure and Facet Joint Force After Cervical Arthroplasty Compared With Cervical Discectomy and Fusion,” J. Neurosurg. Spine., 7(1), pp. 33–39. 10.3171/SPI-07/07/033 [DOI] [PubMed] [Google Scholar]
- [133]. Zhu, Q. A. , Park, Y. B. , Sjovold, S. G. , Niosi, C. A. , Wilson, D. C. , Cripton, P. A. , and Oxland, T. R. , 2008, “Can Extra-Articular Strains Be Used to Measure Facet Contact Forces in the Lumbar Spine? An In-Vitro Biomechanical Study,” Proc. Inst. Mech. Eng. Part H: J. Eng. Med., 222(2), pp. 171–184. 10.1243/09544119JEIM290 [DOI] [PubMed] [Google Scholar]
- [134]. Rousseau, M. A. , Bradford, D. S. , Bertagnoli, R. , Hu, S. S. , and Lotz, J. C. , 2006, “Disc Arthroplasty Design Influences Intervertebral Kinematics and Facet Forces,” Eur. Spine J., 6(3), pp. 258–266. 10.1016/j.spinee.2005.07.004 [DOI] [PubMed] [Google Scholar]
- [135]. Wilson, D. C. , Niosi, C. A. , Zhu, Q. A. , Oxland, T. R. , and Wilson, D. R. , 2006, “Accuracy and Repeatability of a New Method for Measuring Facet Loads in the Lumbar Spine,” J. Biomech., 39(2), pp. 348–-353. 10.1016/j.jbiomech.2004.12.011 [DOI] [PubMed] [Google Scholar]
- [136]. Niosi, C. A. , Wilson, D. C. , Zhu, Q. , Keynan, O. , Wilson, D. R. , and Oxland, T. R. , 2008, “The Effect of Dynamic Posterior Stabilization on Facet Joint Contact Forces: an In-Vitro Investigation,” Spine, 33(1), pp. 19–26. 10.1097/BRS.0b013e31815e7f76 [DOI] [PubMed] [Google Scholar]
- [137]. Dunlop, R. B. , Adams, M. A. , and Hutton, W. C. , 1984, “Disc Space Narrowing and the Lumbar Facet Joints,” J. Bone Jt. Surg. Br., 66(5), pp. 706–710. [DOI] [PubMed] [Google Scholar]
- [138]. Wiseman, C. M. , Lindsey, D. P. , Fredrick, A. D. , and Yerby, S. A. , 2005, “The Effect of an Interspinous Process Implant on Facet Loading During Extension,” Spine, 30, pp. 903–907. 10.1097/01.brs.0000158876.51771.f8 [DOI] [PubMed] [Google Scholar]
- [139]. Jaumard, N. V. , Bauman, J. A. , Welch, W. C. , and Winkelstein, B. A. , 2011, “Pressure Measurement in the Cervical Spinal Facet Joint: Considerations for Maintaining Joint Anatomy and an Intact Capsule,” Spine, 36(15), pp. 1197–1203 10.1097/BRS.0b013e3181ee7de2. [DOI] [PubMed] [Google Scholar]
- [140]. Oxland, T. R. , Panjabi, M. M. , Southern, E. P. , and Duranceau, J. S. , 1991, “An Anatomic Basis for Spinal Instability: a Porcine Trauma Model,” J. Orthop. Res., 9, pp. 452–462. 10.1002/jor.v9:3 [DOI] [PubMed] [Google Scholar]
- [141]. Cook, D. J. and Cheng, B. C. , 2010, “Development of a Model Based Method for Investigating Facet Articulation,” J. Biomech. Eng., 132(6), pp. 064504. 10.1115/1.4001078 [DOI] [PubMed] [Google Scholar]
- [142]. Fregly, B. J. and Sawyer, W. G. , 2003, “Estimation of Discretization Errors in Contact Pressure Measurements,” J. Biomech., 36, pp. 609–613. 10.1016/S0021-9290(02)00436-0 [DOI] [PubMed] [Google Scholar]
- [143]. Bachus, K. N. , DeMarco, A. L. , Judd, K. T. , Horwitz, D. S. , and Brodke, D. S. , 2006, “Measuring Contact Area, Force, and Pressure for Bioengineering Applications: Using Fuji Film and TekScan Systems,” Med. Eng. Phys., 28, pp. 483–488. 10.1016/j.medengphy.2005.07.022 [DOI] [PubMed] [Google Scholar]
- [144]. Hoffmann, K. and Decker, K. , 2007, “Inaccuracies in Measurement of Contact Pressure Due to the Measuring Grid of a Foil Sensor,” Int. J. Intelligent Systems Technologies and Applications, 3(1–2), pp. 80–94. 10.1504/IJISTA.2007.014128 [DOI] [Google Scholar]
- [145]. el-Bohy, A. A. , Yang, K. H. , and King, A. I. , 1989, “Experimental Verification of Facet Load Transmission by Direct Measurement of Facet Lamina Contact Pressure,” J. Biomech., 22, pp. 931–941. 10.1016/0021-9290(89)90077-8 [DOI] [PubMed] [Google Scholar]
- [146]. Jaumard N. V., Bauman J. A., Weisshaar C. L., Guarino B. B., Welch W. C., and Winkelstein B. A., 2011, “Contact Pressure in the Facet Joint During Sagittal Bending of the Cadaveric Cervical Spine” (in press). [DOI] [PubMed] [Google Scholar]
- [147]. Lee, J. H. , Kisiday, J. , and Grodzinsky, A. J. , 2003, “Tissue-Engineered Versus Native Cartilage: Linkage Between Cellular Mechano-Transduction and Biomechanical Properties,” Novartis Found Symp., 249, pp. 52–64. 10.1002/0470867973 [DOI] [PubMed] [Google Scholar]
- [148]. Papachristou, D. J. , Papachroni, K. K. , Basdra, E. K. , and Papavassiliou, A. G. , 2009, “Signaling Networks and Transcription Factors Regulating Mechanotransduction in Bone,” BioEssays, 31(7), pp. 794–804. 10.1002/bies.v31:7 [DOI] [PubMed] [Google Scholar]
- [149]. Ramage, L. , Nuki, G. , and Salter, D. M. , 2009, “Signalling Cascades in Mechanotransduction: Cell-Matrix Interactions and Mechanical Loading,” Scand. J. Med. Sci. Sports, 19(4), pp. 457–469. 10.1111/j.1600-0838.2009.00912.x [DOI] [PubMed] [Google Scholar]
- [150]. Cavanaugh, J. M. , Ozaktay, A. C. , Yamashita, H. T. , and King, A. I. , 1996, “Lumbar Facet Pain: Biomechanics, Neuroanatomy and Neurophysiology,” J. Biomech., 29(9), pp. 1117–1129. 10.1016/0021-9290(96)00023-1 [DOI] [PubMed] [Google Scholar]
- [151]. Elder, B. D. , Kim, D. H. , and Athanasiou, K. A. , 2010, “Developing an Articular Cartilage Decellularization Process Toward Facet Joint Cartilage Replacement,” Neurosurgery, 66(4), pp. 722–727. 10.1227/01.NEU.0000367616.49291.9F [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152]. Kallakuri, S. , Singh, A. , Chen, C. , and Cavanaugh, J. M. , 2004, “Demonstration of Substance P, Calcitonin Gene-Related Peptide, and Protein Gene Product 9.5 Containing Nerve Fibers in Human Cervical Facet Joint Capsules,” Spine, 29(11), pp. 1182–1186. 10.1097/00007632-200406010-00005 [DOI] [PubMed] [Google Scholar]
- [153]. Provenzano, P. P. , Alejandro-Osorio, A. L. , Valhmu, W. B. , Jensen, K. T. , and Vanderby, R., Jr. , 2005, “Intrinsic Fibroblast-Mediated Remodeling of Damaged Collagenous Matrices in Vivo,” Matrix Biol., 23(8), pp. 543–555. 10.1016/j.matbio.2004.09.008 [DOI] [PubMed] [Google Scholar]
- [154]. Cavanaugh, J. M. , Lu, Y. , Chen, C. , and Kallakuri, S. , 2006, “Pain Generation in Lumbar and Cervical Facet Joints,” J. Bone Jt. Surg Am. Vol., 88(2), pp. 63–67. 10.2106/JBJS.E.01411 [DOI] [PubMed] [Google Scholar]
- [155]. Winkelstein, B. A. and Santos, D. G. , 2008, “An Intact Facet Capsular Ligament Modulates Behavioral Sensitivity and Spinal Glial Activation Produced by Cervical Facet Joint Tension,” Spine, 33(8), pp. 856–862. 10.1097/BRS.0b013e31816b4710 [DOI] [PubMed] [Google Scholar]
- [156]. Devor, M. and Seltzer, Z. , 1999, Textbook of Pain - Pathophysiology of Damaged Nerves in Relation to Chronic Pain 4th ed., Wall P. D. and Melzack R., eds., WB Saunders, Philadelphia, pp. 129–164. [Google Scholar]
- [157]. Winkelstein, B. A. , Dunk, N. , and Weinstein, J. N. , 2011, The Cervical Spine 5th edition - Pain Mechanisms: Relevant Anatomy, Pathogenesis and Clinical Implications Clark C. R., ed. (to be published). [Google Scholar]
- [158]. Ozaktay, A. C. , Cavanaugh, J. M. , Blagoev, D. C. , Getchell, T. V. , and King, A. I. , 1994, “Effects of a Carrageenan-Induced Inflammation in Rabbit Lumbar Facet Joint Capsule and Adjacent Tissues,” J. Neurosci Res., 20(4), pp. 355–364. 10.1016/0168-0102(94)90058-2 [DOI] [PubMed] [Google Scholar]
- [159]. McMahon, S. B. , Cafferty, W. B. J. , and Marchand, F. , 2005, “Immune and Glial Cell Factors as Pain Mediators and Modulators,” Exp. Neurol., 192, pp. 444–462. 10.1016/j.expneurol.2004.11.001 [DOI] [PubMed] [Google Scholar]
- [160]. Igarashi, A. , Kikuchi, S. , and Konno, S. , 2007, “Correlation Between Inflammatory Cytokines Released From the Lumbar Facet Joint Tissue and Symptoms in Degenerative Lumbar Spinal Disorders,” J. Orthop. Sci., 12(2), pp. 154–160. 10.1007/s00776-006-1105-y [DOI] [PubMed] [Google Scholar]
- [161]. Lee, K. E. , Davis, M. B. , and Winkelstein, B. A. , 2008, “Capsular Ligament Involvement in the Development of Mechanical Hyperalgesia After Facet Joint Loading: Behavioral and Inflammatory Outcomes in a Rodent Model of Pain,” J. Neurotrauma, 25(11), pp. 1383–1393. 10.1089/neu.2008.0700 [DOI] [PubMed] [Google Scholar]
- [162]. Bjelle, A. , 1975, “Content and Composition of Glycosaminoglycans in Human Knee Joint Cartilage. Variation With Site and Age in Adults,” Connect Tissue Res., 3(2), pp. 141–147. 10.3109/03008207509152172 [DOI] [PubMed] [Google Scholar]
- [163]. Roberts, S. , Weightman, B. , Urban, J. , and Chappell, D. , 1986, “Mechanical and Biochemical Properties of Human Articular Cartilage in Osteoarthritic Femoral Heads and in Autopsy Specimens,” J. Bone Jt. Surg. Br., 68(2), pp. 278–288. [DOI] [PubMed] [Google Scholar]
- [164]. Slowman, S. D. and Brandt, K. D. , 1986, “Composition and Glycosaminoglycan Metabolism of Articular Cartilage From Habitually Loaded and Habitually Unloaded Sites,” Arthritis Rheum., 29(1), pp. 88–94. 10.1002/art.v29:1 [DOI] [PubMed] [Google Scholar]
- [165]. Huselstein, C. , Netter, P. , de Isla, N. , Wang, Y. , Gillet, P. , Decot, V. , Muller, S. , Bensoussan, D. , and Stoltz, J. F. , 2008, “Mechanobiology, Chondrocyte and Cartilage,” Biomed. Mater. Eng., 18(4–5), pp. 213–220. 10.3233/BME-2008-0527 [DOI] [PubMed] [Google Scholar]
- [166]. Pelletier, J. P. , Martel-Pelletier, J. , and Abramson, S. B. , 2001, “Osteoarthritis, an Inflammatory Disease: Potential Implication for this Selection of New Therapeutic Targets,” Arthritis Rheum., 44, pp. 1237–1247. [DOI] [PubMed] [Google Scholar]
- [167]. Browning, J. A. , Saunders, K. , Urban, J. P. , and Wilkins, R. J. , 2004, “The Influence and Interactions of Hydrostatic and Osmotic Pressures on theIintracellular Milieu of Chondrocytes,” Biorheology, 41(3–4), pp. 299–308. [PubMed] [Google Scholar]
- [168]. Mobasheri, A. , Barrett-Jolley, R. , Carter, S. D. , Martín-Vasallo, P. , Schulze-Tanzil, G. , and Shakibaei, M. , 2005, Mechanosensitivity in Cells and Tissues - Functional Roles of Mechanosensitive Ion Channels, ß1 Integrins and Kinase Cascades in Chondrocyte Mechanotransduction, Kamkin A. and Kiseleva I., eds., Academia, Moscow. [PubMed] [Google Scholar]
- [169]. Blain, E. J. , 2007, “Mechanical Regulation of Matrix Metalloproteinases,” Front. Biosci., 12, pp. 507–527. 10.2741/2078 [DOI] [PubMed] [Google Scholar]
- [170]. Spiteri, C. , Raizman, I. , Pilliar, R. M. , and Kandel, R. A. , 2010, “Matrix Accumulation by Articular Chondrocytes During Mechanical Stimulation is Influenced by Integrin-Mediated Cell Spreading,” J. Biomed. Mater. Res. A., 94(1), pp. 122–129. 10.1002/jbm.a.32706 [DOI] [PubMed] [Google Scholar]
- [171]. De Croos, J. N. , Dhaliwal, S. S. , Grynpas, M. D. , Pilliar, R. M. , and Kandel, R. A. , 2006, “Cyclic Compressive Mechanical Stimulation Induces Sequential Catabolic and Anabolic Gene Changes in Chondrocytes Resulting in Increased Extracellular Matrix Accumulation,” Matrix Biol., 25, pp. 323–331. 10.1016/j.matbio.2006.03.005 [DOI] [PubMed] [Google Scholar]
- [172]. Mow, V. C. , Wang, C. C. , and Hung, C. T. , 1999, “The Extracellular Matrix, Interstitial Fluid and Ions as a Mechanical Signal Transducer in Articular Cartilage,” Osteoarthritis Cartilage, 7(1), pp. 41–58. 10.1053/joca.1998.0161 [DOI] [PubMed] [Google Scholar]
- [173]. Bourret, L. A. and Rodan, G. A. , 1976, “The Role of Calcium in the Inhibition of cAMP Accumulation in Epiphyseal Cartilage Cells Exposed to Physiological Pressure,” J. Cell. Physiol., 88(3), pp. 353–361. 10.1002/jcp.v88:3 [DOI] [PubMed] [Google Scholar]
- [174]. Ueki, M. , Tanaka, N. , Tanimoto, K. , Nishio, C. , Honda, K. , Lin, Y. Y. , Tanne, Y. , Ohkuma, S. , Kamiya, T. , Tanaka, E. , and Tanne, K. , 2008, “The Effect of Mechanical Loading on the Metabolism of Growth Plate Chondrocytes,” Ann. Biomed. Eng., 36(5), pp. 793–800. 10.1007/s10439-008-9462-7 [DOI] [PubMed] [Google Scholar]
- [175]. Hall, A. C. , Urban, J. P. , and Gehl, K. A. , 1991, “The Effects of Hydrostatic Pressure on Matrix Synthesis in Articular Cartilage,” J. Orthop Res., 9(1), pp. 1–10. 10.1002/jor.v9:1 [DOI] [PubMed] [Google Scholar]
- [176]. Grodzinsky, A. J. , Levenston, M. E. , Jin, M. , and Frank, E. H. , 2000, “Cartilage Tissue Remodeling in Response to Mechanical Forces,” Annu. Rev. Biomed. Eng., 2, pp. 691–713. 10.1146/annurev.bioeng.2.1.691 [DOI] [PubMed] [Google Scholar]
- [177]. Kim, Y. J. , Bonassar, L. J. , and Grodzinsky, A. J. , 1995, “The Role of Cartilage Streaming Potential, Fluid Flow and Pressure in the Stimulation of Chondrocyte Biosynthesis During Dynamic Compression,” J. Biomech., 28(9), pp. 1055–1066. 10.1016/0021-9290(94)00159-2 [DOI] [PubMed] [Google Scholar]
- [178]. Neu, C. P. , Khalafi, A. , Komvopoulos, K. , Schmid, T. M. , and Reddi, A. H. , 2007, “Mechanotransduction of Bovine Articular Cartilage Superficial Zone Protein by Transforming Growth Factor Beta Signaling,” Arthritis Rheum., 56(11), pp. 3706–3714. 10.1002/art.v56:11 [DOI] [PubMed] [Google Scholar]
- [179]. Gray, M. L. , Pizzanelli, A. M. , Lee, R. C. , Grodzinsky, A. J. , and Swann, D. A. , 1989, “Kinetics of the Chondrocyte Biosynthetic Response to Compressive Load and Release,” Biochim. Biophys. Acta., 991(3), pp. 415–425. [DOI] [PubMed] [Google Scholar]
- [180]. Smith, R. L. , Lin, J. , Trindade, M. C. , Shida, J. , Kajiyama, G. , Vu, T. , Hoffman, A. R. , van der Meulen, M. C. , Goodman, S. B. , Schurman, D. J. , and Carter, D. R. , 2000, “Time-Dependent Effects of Intermittent Hydrostatic Pressure on Articular Chondrocyte Type II Collagen and Aggrecan mRNA Expression,” J. Rehabil. Res. Dev., 37(2), pp. 153–161. [PubMed] [Google Scholar]
- [181]. Duncan, R. L. and Turner, C. H. , 1995, “Mechanotransduction and the Functional Response of Bone to Mechanical Strain,” Calcif. Tissue Int., 57(5), pp. 344–358. 10.1007/BF00302070 [DOI] [PubMed] [Google Scholar]
- [182]. Fukada, E. and Yasuda I., 1957, “On the Piezoelectric Effect of bone,” J. Phys. Soc. Jpn., 12(10), pp. 1158–1162. 10.1143/JPSJ.12.1158 [DOI] [Google Scholar]
- [183]. Friedenberg, Z. B. and Brighton, C. T. , 1966, “Bioelectric Potentials in Bone,” J. Bone Jt. Surg., 48A(5), pp. 915–923. [PubMed] [Google Scholar]
- [184]. Gross, D. and Williams, W. S. , 1982, “Streaming Potential and the Electromechanical Response of Physiologically-Moist Bone,” J. Biomech., 15(4), pp. 277–295. 10.1016/0021-9290(82)90174-9 [DOI] [PubMed] [Google Scholar]
- [185]. Guzelsu, N. and Walsh, W. R. , 1990, “Streaming Potential of Intact Wet Bone,” J. Biomech., 23(7), pp. 673–685. 10.1016/0021-9290(90)90167-2 [DOI] [PubMed] [Google Scholar]
- [186]. Turner, C. H. , Forwood, M. R. , and Otter, M.W. , 1994, “Mechanotransduction in Bone: Do Bone Cells Act as Sensors of Fluid Flow?” FASEB J., 8(11), pp. 875–878. [DOI] [PubMed] [Google Scholar]
- [187]. Liedert, A. , Kaspar, D. , Blakytny, R. , Claes, L. , and Ignatius, A. , 2006, “Signal Transduction Pathways Involved in Mechanotransduction in Bone Cells,” Biochem. Biophys. Res. Commun., 349(1), pp. 1–5. 10.1016/j.bbrc.2006.07.214 [DOI] [PubMed] [Google Scholar]
- [188]. Benjamin, M. and Hillen, B. , 2003, “Mechanical Influences on Cells, Tissues and Organs - ‘Mechanical Morphogenesis’,” Eur. J. Morphol., 41(1), pp. 3–7. 10.1076/ejom.41.1.3.28102 [DOI] [PubMed] [Google Scholar]
- [189]. Matyas, J. , Edwards, P. , Miniaci, A. , Shrive, N. , Wilson, J. , Bray, R. , and Frank, C. , 1994, “Ligament Tension Affects Nuclear Shape in Situ: an in Vitro Study,” Connect Tissue Res., 31(1), pp. 45–53. 10.3109/03008209409005634 [DOI] [PubMed] [Google Scholar]
- [190]. Ziegler, N. , Alonso, A. , Steinberg, T. , Woodnutt, D. , Kohl, A. , Müssig, E. , Schulz, S. , and Tomakidi, P. , 2010, “Mechano-Transduction in Periodontal Ligament Cells Identifies Activated States of MAP-Kinases p42/44 and p38-Stress Kinase as a Mechanism for MMP-13 Expression,” BMC Cell. Biol., 11, pp. 10. 10.1186/1471-2121-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191]. Kook, S. H. , Hwang, J. M. , Park, J. S. , Kim, E. M. , Heo, J. S. , Jeon, Y. M. , and Lee, J. C. , 2009, “Mechanical Force Induces Type I Collagen Expression in Human Periodontal Ligament Fibroblasts Through Activation of ERK/JNK and AP-1,” J. Cell. Biochem., 106(6), pp. 1060–1067. 10.1002/jcb.22085 [DOI] [PubMed] [Google Scholar]
- [192]. Hadley, M. N. , Fitzpatrick, B. C. , Sonntag, V. K. , and Browner, C. M. , 1992, “Facet Fracture-Dislocation Injuries of the Cervical Spine,” Neurosurgery, 30(5), pp. 661–666. 10.1227/00006123-199205000-00001 [DOI] [PubMed] [Google Scholar]
- [193]. Dvorak, M. F. , Fisher, C. G. , Aarabi, B. , Harris, M. B. , Hurbert, R. J. , Rampersaud, Y. R. , Vaccaro, A. , Harrop, J. S. , Nockels, R. P. , Madrazo, I. N. , Schwartz, D. , Kwon, B. K. , Zhao, Y. , and Fehlings, M. G. , 2007, “Clinical Outcomes of 90 Isolated Unilateral Facet Fractures, Subluxations, and Dislocations Treated Surgically and Nonoperatively,” Spine, 32(26), pp. 3007–3013. 10.1097/BRS.0b013e31815cd439 [DOI] [PubMed] [Google Scholar]
- [194]. Ivancic, P. C. , Ito, S. , Tominaga, Y. , Rubin, W. , Coe, M. P. , Ndu, A. B. , Carlson, E. J. , and Panjabi, M. M. , 2008, “Whiplash Wauses Increased Laxity of Cervical Capsular Ligament,” Clin. Biomech. (Bristol, Avon), 23(2), pp. 159–165. 10.1016/j.clinbiomech.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195]. Lee, S. H. and Sung, J. K. , 2009, “Unilateral Lateral Mass-Facet Fractures With Rotational Instability: New Classification and a Review of 39 Cases Treated Conservatively and With Single Segment Anterior Fusion,” J. Trauma, 66(3), pp. 758–767. 10.1097/TA.0b013e31818cc32a [DOI] [PubMed] [Google Scholar]
- [196]. Sonntag, V. K. and Hadley, M. N. , 1988, “Nonoperative Management of Cervical Spine Injuries,” Clin. Neurosurg., 34, pp. 630–649. [PubMed] [Google Scholar]
- [197]. Cusick, J. F. , Pintar, F. A. , and Yoganandan, N. , 2001, “Whiplash Syndrome: Kinematic Factors Influencing Pain Patterns,” Spine, 26(11), pp. 1252–1258. 10.1097/00007632-200106010-00015 [DOI] [PubMed] [Google Scholar]
- [198]. Pearson, A. M. , Ivancic, P. C. , Ito, S. , and Panjabi, M. M. , 2004, “Facet Joint Kinematics and Injury Mechanisms During Simulated Whiplash,” Spine, 29(4), pp. 390–397. 10.1097/01.BRS.0000090836.50508.F7 [DOI] [PubMed] [Google Scholar]
- [199]. Panjabi, M. M. , Ito, S. , Pearson, A. M. , and Ivancic, P. C. , 2004, “Injury Mechanisms of the Cervical Intervertebral Disc During Simulated Whiplash,” Spine, 29(11), pp. 1217–1225. 10.1097/00007632-200406010-00011 [DOI] [PubMed] [Google Scholar]
- [200]. Chen, H. B. , Yang, K. H. , and Wang, Z. G. , 2009, “Biomechanics of Whiplash Injury,” Chin. J. Traumatol., 12(5), pp. 305–314. [PubMed] [Google Scholar]
- [201]. Beatson, T. R. , 1963, “Fractures and Dislocations of the Cervical Spine,” J. Bone Jt. Surg. Br., 45, pp. 21–35. [Google Scholar]
- [202]. O'Brien, P. J. , Scweigel, J. F. , and Thompson, W. J. , 1982, “Dislocations of the Lower Cervical Spine,” J. Trauma, 22, pp. 710–714. 10.1097/00005373-198208000-00012 [DOI] [PubMed] [Google Scholar]
- [203]. Stauffer, E. S. , 1984, “Neurologic Recovery Following Injuries to the Cervical Spinal Cord and Nerve Roots,” Spine, 9, pp. 532–534. 10.1097/00007632-198407000-00024 [DOI] [PubMed] [Google Scholar]
- [204]. Vaccaro, A. R. , Falatyn, S. P. , Flanders, A. E. , Balderston, R. A. , Northrup, B. E. , and Cotler, J. M. , 1999, “Magnetic Resonance Evaluation of the Intervertebral Disc, Spinal Ligaments, and Spinal Cord Before and After Closed Traction Reduction of Cervical Spine Dislocations,” Spine, 24(12), pp. 1210–1217. 10.1097/00007632-199906150-00007 [DOI] [PubMed] [Google Scholar]
- [205]. Vaccaro, A. R. , Madigan, L. , Schweitzer, M. E. , Flanders, A. E. , Hilibrand, A. S. , and Albert, T. J. , 2001, “Magnetic Resonance Imaging Analysis of Soft Tissue Disruption After Flexion-Distraction Injuries of the Subaxial Cervical Spine,” Spine 26(17), pp. 1866–1872. 10.1097/00007632-200109010-00009 [DOI] [PubMed] [Google Scholar]
- [206]. Crawford, N. R. , Duggal, N. , Chamberlain, R. H. , Park, S. C. , Sonntag, V. K. , and Dickman, C. A. , 2002, “Unilateral Cervical Facet Dislocation: Injury Mechanism and Biomechanical Consequences,” Spine, 7(17), pp. 1858–1864. 10.1097/00007632-200209010-00010 [DOI] [PubMed] [Google Scholar]
- [207]. Sonntag, V. K. , 1981, “Management of Bilateral Locked Facets of the Cervical Spine,” Neurosurgery, 8(2), pp. 150–152. 10.1227/00006123-198102000-00002 [DOI] [PubMed] [Google Scholar]
- [208]. Lifeso, R. M. and Colucci, M. A. , 2000, “Anterior Fusion for Rotationally Unstable Cervical Spine Fractures,” Spine 25(16), pp. 2028–2034. 10.1097/00007632-200008150-00005 [DOI] [PubMed] [Google Scholar]
- [209]. Boden, S. D. , Martin, C. , Rudolph, R. , Kirkpatrick, J. S. , Moeini, S. M. , and Hutton, W. C. , 1994, “Increase of Motion Between Lumbar Vertebrae After Excision of the Capsule and Cartilage of the Facets. A Cadaver Study,” J. Bone Jt. Surg. Am., 76(12), pp. 1847–1853. [DOI] [PubMed] [Google Scholar]
- [210]. Kotani, Y. , Abumi, K. , Ito, M. , and Minami, A. , 2005, “Cervical Spine Injuries Associated With Lateral Mass and Facet Joint Fractures: New Classification and Surgical Treatment With Pedicle Screw Fixation,” Eur. Spine J., 14(1), pp. 69–77. 10.1007/s00586-004-0793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211]. Akamaru, T. , Kawahara, N. , Tim Yoon, S. , Minamide, A. , Su Kim, K. , Tomita, K. , and Hutton, W.C. , 2003, “Adjacent Segment Motion After a Simulated Lumbar Fusion in Different Sagittal Alignments: A Biomechanical Analysis,” Spine, 28(14), pp. 1560–1566. 10.1097/01.BRS.0000076820.44132.99 [DOI] [PubMed] [Google Scholar]
- [212]. Finn, M. A. , Brodke, D. S. , Daubs, M. , Patel, A. , and Bachus, K. N. , 2009, “Local and Global Subaxial Cervical Spine Biomechanics After Single-Level Fusion or Cervical Arthroplasty,” Eur. Spine J., 18(10), pp. 1520–1527. 10.1007/s00586-009-1085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213]. Lee, C. K. and Langrana, N. A. , 1984, “Lumbosacral Spinal Fusion. A Biomechanical Study,” Spine, 9(6), pp. 574–581. 10.1097/00007632-198409000-00007 [DOI] [PubMed] [Google Scholar]
- [214]. Park, P. , Garton, H. J. , Gala, V. C. , Hoff, J. T. , and McGillicuddy, J. E. , 2004, “Adjacent Segment Disease After Lumbar or Lumbosacral Fusion: Review of the Literature,” Spine, 29(17), pp. 1938–1944. 10.1097/01.brs.0000137069.88904.03 [DOI] [PubMed] [Google Scholar]
- [215]. Sears, W. R. , Sergides, I. G. , Kazemi, N. , Smith, M. , White, G. J. , and Osburg, B. , 2011, “Incidence and Prevalence of Surgery at Segments Adjacent to a Previous Posterior Lumbar Arthrodesis,” Eur. Spine J., 11(1), pp. 11–20. 10.1016/j.spinee.2010.09.026 [DOI] [PubMed] [Google Scholar]
- [216]. Terai, T. , Faizan, A. , Sairyo, K. , and Goel, V. K. , 2011, “Operated and Adjacent Segment Motions for Fusion versus Cervical Arthroplasty: A Pilot Study,” Clin. Orthop. Relat. Res., 469(3), pp. 682–687. 10.1007/s11999-010-1646-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217]. Shanmuganathan, K. , Mirvis, S. E. , and Levine, A. M. , 1994, “Rotational Injury of Cervical Facets: CT Analysis of Fracture Patterns With Implications for Management and Neurologic Outcome,” Am. J. Roentgenol., 163(5), pp. 1165–1169. 10.1016/S0009-9260(05)82768-0 [DOI] [PubMed] [Google Scholar]
- [218]. Rabb, C. H. , Lopez, J. , Beauchamp, K. , Witt, P. , Bolles, G. , and Dwyer, A. , 2007, “Unilateral Cervical Facet Fractures With Subluxation: Injury Patterns and Treatment,” J. Spinal Disord., 20(6), pp. 416–422. 10.1097/BSD.0b013e318030d32a [DOI] [PubMed] [Google Scholar]
- [219]. Rodriguez, M. , Tyberghien, A. , and Matgé, G. , 2001, “Asymptomatic Vertebral Artery Injury After Acute Cervical Spine Trauma,” Acta Neurochir. Suppl. (Wien), 143(9), pp. 939–945. 10.1007/s007010170025 [DOI] [PubMed] [Google Scholar]
- [220]. Bosita, R. V. , Hong, T. , and Ohnmeiss, D. D. , 2009, “Avascular Necrosis of Lumbar Facet Joints Associated With Bilateral Facet Fractures,” Eur. Spine J., 9(6), pp. E1–E4. 10.1016/j.spinee.2008.11.007 [DOI] [PubMed] [Google Scholar]
- [221]. Stemper, B. D. , Yoganandan, N. , Gennarelli, T. A. , and Pintar, F. A. , 2005, “Localized Cervical Facet Joint Kinematics Under Physiological and Whiplash Loading,” J. Neurosurg. Spine, 3(6), pp. 471–476. 10.3171/spi.2005.3.6.0471 [DOI] [PubMed] [Google Scholar]
- [222]. Barnsley, L. , Lord, S. , and Bogduk, N. , 1994, “Whiplash Injuries,” Pain, 58, pp. 283–307. 10.1016/0304-3959(94)90123-6 [DOI] [PubMed] [Google Scholar]
- [223]. Anderson, R. W. G. , Gibson, T. J. , Cox, M. , Ryan, G. A. , and Gun, R. T. , (2006), “Whiplash Associated Disorders: A Comprehensive Review,” CASR Road Safety Research Report, http://digital.library.adelaide.edu.au/dspace/bitstream/2440/36410/1/CASR016.pdf
- [224]. Kaneoka, K. , Ono, K. , Inami, S. , and Hayashi, K. , 1999, “Motion Analysis of Cervical Vertebrae During Whiplash Loading,” Spine, 24(8), pp. 763–770. 10.1097/00007632-199904150-00006 [DOI] [PubMed] [Google Scholar]
- [225]. Luan, F. , Yang, K. H. , Deng, B. , Begeman, P. C. , Tashman, S. , and King, A. I. , 2000, “Qualitative Analysis of Neck Kinematics During Low-Speed Rear-End Impact,” Clin. Biomech., 15(9), pp. 649–657. 10.1016/S0268-0033(00)00031-0 [DOI] [PubMed] [Google Scholar]
- [226]. Panjabi, M. M. , Nibu, K. , and Cholewicki, J. , 1998, “Whiplash Injuries and the Potential for Mechanical Instability,” Eur. Spine J., 7(6), pp. 484–492. 10.1007/s005860050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227]. Provenzano, P. P. , Hayashi, K. , Kunz, D. N. , Markel, M. D. , and Vanderby, R., Jr. , 2002, “Healing of Subfailure Ligament Injury: Comparison Between Immature and Mature Ligaments in a Rat Model,” J. Orthop. Res., 20(5), pp. 975–983. 10.1016/S0736-0266(02)00036-0 [DOI] [PubMed] [Google Scholar]
- [228]. Liu, Y. K. , Goel, V. K. , Dejong, A. , Njus, G. , Nishiyama, K. , and Buckwalter, J. , 1985, “Torsional Fatigue of the Lumbar Intervertebral Joints,” Spine, 10(10), pp. 894–900. 10.1097/00007632-198512000-00006 [DOI] [PubMed] [Google Scholar]
- [229]. Mankin, H. J. , 1982, “The Response of Articular Cartilage to Mechanical Injury,” J. Bone Jt. Surg. Am., 64, pp. 460–466. [PubMed] [Google Scholar]
- [230]. Jeffrey, J. E. and Aspden, R. M. , 2006, “The Biophysical Effects of a Single Impact Load on Human and Bovine Articular Cartilage,” Proc. Inst. Mech. Eng., Part H: J. Eng. Med., 220(6), pp. 677–686. 10.1243/09544119JEIM31 [DOI] [PubMed] [Google Scholar]
- [231]. Natoli, R. M. , Scott, C. C. , and Athanasiou, K. A. , 2008, “Temporal Effects of Impact on Articular Cartilage Cell Death, Gene Expression, Matrix Biochemistry, and Biomechanics,” Ann. Biomed. Eng., 36(5), pp. 780–792. 10.1007/s10439-008-9472-5 [DOI] [PubMed] [Google Scholar]
- [232]. Kurz, B. , Jin, M. , Patwari, P. , Cheng, D. M. , Lark, M. W. , and Grodzinsky, A. J. , 2001, “Biosynthetic Response and Mechanical Properties of Articular Cartilage After Injurious Compression,” J. Orthop. Res., 19(6), pp. 1140–1146. 10.1016/S0736-0266(01)00033-X [DOI] [PubMed] [Google Scholar]
- [233]. Li, L. P. , Korhonen, R. K. , Iivarinen, J. , Jurvelin, J. S. , and Herzog, W. , 2008, “Fluid Pressure Driven Fibril Reinforcement in Creep and Relaxation Tests of Aticular Cartilage,” Med. Eng. Phys., 30(2), pp. 182–189. 10.1016/j.medengphy.2007.03.001 [DOI] [PubMed] [Google Scholar]
- [234]. Ewers, B. J. , Weaver, B. T. , Sevensma, E. T. , and Haut, R. C. , 2002, “Chronic Changes in Rabbit Retropatellar Cartilage and Subchondral Bone After Blunt Impact Loading of the Patellofemoral Joint,” J. Orthop. Res., 20, pp. 545–550. 10.1016/S0736-0266(01)00135-8 [DOI] [PubMed] [Google Scholar]
- [235]. Piperno, M. , Reboul, P. , Hellio le Graverand, M. P. , Peschard, M. , Annefeld, M. , Richard, M. , and Vignon, E. , 1998, “Osteoarthritic Cartilage Fibrillation is Associated With a Decrease in Chondrocyte Adhesion to Fibronectin,” Osteoarthritis Cartilage, 6(6), pp. 393–399. 10.1053/joca.1998.0138 [DOI] [PubMed] [Google Scholar]
- [236]. Chen, C. T. , Burton-Wurster, N. , Borden, C. , Hueffer, K. , Bloom, S. E. , and Lust, G. , 2001, “Chondrocyte Necrosis and Apoptosis in Impact Damaged Articular Cartilage,” J. Orthop Res., 19(4), pp. 703–711. 10.1016/S0736-0266(00)00066-8 [DOI] [PubMed] [Google Scholar]
- [237]. Torzilli, P. A. , Deng, X. H. , and Ramcharan, M. , 2006, “Effect of Compressive Strain on Cell Viability in Statically Loaded Articular Cartilage,” Biomech. Model. Mechanobiol., 5(2–3), pp. 123–132. 10.1007/s10237-006-0030-5 [DOI] [PubMed] [Google Scholar]
- [238]. Krane, S. M. and Goldring, M. B. , 1990, “Clinical Implications of Cartilage Metabolism in Arthritis,” Eur. J. Rheumatol. Inflamm., 10(1), pp. 4–9. [PubMed] [Google Scholar]
- [239]. Thompson, R.C., Jr. , Oegema, T. R., Jr. , Lewis, J. L. , and Wallace, L. , 1991, “Osteoarthrotic Changes After Acute Transarticular Load, an Animal Model,” J. Bone Jt. Surg. Am., 73, pp. 990–1001. [PubMed] [Google Scholar]
- [240]. Triantafillopoulos, I. K. , Papagelopoulos, P. J. , Politi, P. K. , and Nikiforidis, P. A. , 2002, “Articular Changes in Experimentally Induced Patellar Trauma,” Knee Surg. Sports Traumatol. Arthrosc., 10, pp. 144–153. 10.1007/s001670100248 [DOI] [PubMed] [Google Scholar]
- [241]. Taylor, J. R. and Twomey, L. T. , 1986, “Age Changes in Lumbar Zygapophyseal Joints. Observations on Structure and Function,” Spine 11(7), pp. 739–745. 10.1097/00007632-198609000-00014 [DOI] [PubMed] [Google Scholar]
- [242]. Fujiwara, A. , Tamai, K. , Yamato, M. , An, H. S. , Yoshida, H. , Saotome, K. , and Kurihashi, A. , 1999, “The Relationship Between Facet Joint Osteoarthritis and Disc Degeneration of the Lumbar Spine: An MRI Study,” Eur. Spine J., 8, pp. 396–401. 10.1007/s005860050193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243]. Tischer, T. , Aktas, T. , Milz, S. , and Putz, R. V. , 2006, “Detailed Pathological Changes of Human Lumbar Facet Joints L1-L5 in Elderly Individuals,” Eur. Spine J., 15, pp. 308–315. 10.1007/s00586-005-0958-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244]. Manchikanti, L. , Manchikanti, K. N. , Cash, K. A. , Singh, V. , and Giordano, J. , 2008, “Age-Related Prevalence of Facet-Joint Involvement in Chronic Neck and Low Back Pain,” Pain Physician, 11(1), pp. 67–75. 10.1111/j.1533-2500.2007.00169.x [DOI] [PubMed] [Google Scholar]
- [245]. Butler, D. , Trafimow, J. H. , Andersson, G. B. , McNeill, T. W. , and Huckman, M. S. , 1990, “Discs Degenerate Before Facets,” Spine, 15(2), pp. 111–113. 10.1097/00007632-199002000-00012 [DOI] [PubMed] [Google Scholar]
- [246]. Kaito, T. , Hosono, N. , Mukai, Y. , Makino, T. , Fuji, T. , and Yonenobu, K. , 2010, “Induction of Early Degeneration of the Adjacent Segment After Posterior Lumbar Interbody Fusion by Excessive Distraction of Lumbar Disc Space,” J. Neurosurg. Spine, 12(6), pp. 671–679. 10.3171/2009.12.SPINE08823 [DOI] [PubMed] [Google Scholar]
- [247]. Michel-Batôt, C. , Dintinger, H. , Blum, A. , Olivier, P. , Laborde, F. , Bettembourg-Brault, I. , Pourel, J. , Loeuille, D. , and Chary-Valckenaere, I. , 2008, “A Particular Form of Septic Arthritis: Septic Arthritis of Facet Joint,” Jt., Bone Spine, 75(1), pp. 78–83. 10.1016/j.jbspin.2007.02.006 [DOI] [PubMed] [Google Scholar]
- [248]. Hauge, T. , Magnaes, B. , and Skullerud, K. , 1980, “Rheumatoid Arthritis of the Lumbar Spine Leading to Anterior Vertebral Subluxation and Compression of the Cauda Equina,” Scand. J. Rheumatol., 9, pp. 241–244. 10.3109/03009748009112356 [DOI] [PubMed] [Google Scholar]
- [249]. Kawaguchi, Y. , Matsuno, H. , Kanamori, M. , Ishihara, H. , Ohmori, K. , and Kimura, T. , 2003, “Radiologic Findings of the Lumbar Spine in Patients With Rheumatoid Arthritis and a Review of Pathologic Mechanisms,” J. Spinal Disord, 16(1), pp. 38–43. 10.1097/00024720-200302000-00007 [DOI] [PubMed] [Google Scholar]
- [250]. Chan, F. L. , Ho, E. K. , Fang, D. , Hsu, L. C. , Leong, J. C. , and Ngan, H. , 1987, “Spinal Pseudoarthrosis in Ankylosing Spondylitis,” Acta Radiol., 28(4), pp. 383–388. 10.3109/02841858709177367 [DOI] [PubMed] [Google Scholar]
- [251]. Pathria, M. , Sartoris, D. J. , and Resnick, D. , 1987, “Osteoarthritis of the Facet Joints: Accuracy of Oblique Radiographic Measurement,” Radiology, 164, pp. 227–230. [DOI] [PubMed] [Google Scholar]
- [252]. Sharma, L. , Kapoor, D. , and Issa, S. , 2006, “Epidemiology of Osteoarthritis: An Update,” Curr. Opin. Rheumatol., 18, pp. 147–156. 10.1097/01.bor.0000209426.84775.f8 [DOI] [PubMed] [Google Scholar]
- [253]. Kettler, A. and Wilke, H. J. , 2006, “Review of Existing Grading Systems for Cervical or Lumbar Disc and Facet Joint Degeneration,” Eur. Spine J., 15, pp. 705–718. 10.1007/s00586-005-0954-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254]. Ziv, I. , Maroudas, C. , Robin, G. , and Maroudas, A. , 1993, “Human Facet Cartilage: Swelling and Some Physicochemical Characteristics as a Function of Age. Part 2: Age Changes in Some Biophysical Parameters of Human Facet Joint Cartilage,” Spine, 18(1), pp. 136–146. 10.1097/00007632-199301000-00020 [DOI] [PubMed] [Google Scholar]
- [255]. Margulies, J. Y. , Payzer, A. , Nyska, M. , Neuwirth, M. G. , Floman, Y. , and Robin, G. C. , 1996, “The Relationship Between Degenerative Changes and Osteoporosis in the Lumbar Spine,” Clin. Orthop. Relat. Res., 324, pp. 145–152. 10.1097/00003086-199603000-00017 [DOI] [PubMed] [Google Scholar]
- [256]. Serhan, H. A. , Varnavas, G. , Dooris, A. P. , Patwadhan, A. , and Tzermiadianos, M. , 2007, “Biomechanics of the Posterior Lumbar Articulating Elements,” Neurosurg. Focus., 22(1), p. E1. 10.3171/foc.2007.22.1.1 [DOI] [PubMed] [Google Scholar]
- [257]. Haefeli, M. , Kalberer, F. , Saegesser, D. , Nerlich, A. G. , Boos, N. , and Paesold, G. , 2006, “The Course of Macroscopic Degeneration in the Human Lumbar Intervertebral Disc,” Spine, 31(14), pp. 1522–1531. 10.1097/01.brs.0000222032.52336.8e [DOI] [PubMed] [Google Scholar]
- [258]. Kettler, A. , Werner, K. , and Wilke, H. J. , 2007, “Morphological Changes of Cervical Facet Joints in Elderly Individuals,” Eur. Spine J., 16(7), pp. 987–992. 10.1007/s00586-006-0275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259]. McAfee, P. C. , Cunningham, B. W. , Hayes, V. , Sidiqi, F. , Dabbah, M. , Sefter, J. C. , Hu, N. , and Beatson, H. , 2006, “Biomechanical Analysis of Rotational Motions After Disc Arthroplasty: Implications for Patients With Adult Deformities,” Spine, 31(19 Suppl), pp. S152–S160. 10.1097/01.brs.0000234782.89031.03 [DOI] [PubMed] [Google Scholar]
- [260]. Lazaro, B. C. , Yucesoy, K. , Yuksel, K. Z. , Kowalczyk, I. , Rabin, D. , Fink, M. , and Duggal, N. , 2010, “Effect of Arthroplasty Design on Cervical Spine Kinematics: Analysis of the Bryan Disc, ProDisc-C, and Synergy Disc,” Neurosurg. Focus, 28(6), p. E6. 10.3171/2010.3.FOCUS1058 [DOI] [PubMed] [Google Scholar]
- [261]. Park, J. J. , Quirno, M. , Cunningham, M. R. , Schwarzkopf, R. , Bendo, J. A. , Spivak, J. M. , and Goldstein, J. A. , 2010, “Analysis of Segmental Cervical Spine Vertebral Motion After Prodisc-C Cervical Disc Replacement,” Spine, 35(8), pp. E285–E289. 10.1097/BRS.0b013e3181c88165 [DOI] [PubMed] [Google Scholar]
- [262]. Duggal, N. , Bertagnoli, R. , Rabin, D. , Wharton, N. , and Kowalczyk, I. , 2010, “ProDisc-C: An in Vivo Kinematic Study,” J. Spinal Disord. (in press). [DOI] [PubMed] [Google Scholar]
- [263]. Gaffey, J. L. , Ghanayem, A. J. , Voronov, M. L. , Havey, R. M. , Carandang, G. , Abjornson, C. , and Patwardhan, A. G. , 2010, “Effect of Increasing Implant Height on Lumbar Spine Kinematics and Foraminal Size Using the ProDisc-L Prosthesis,” Spine, 35(19), pp. 1777–1782. 10.1097/BRS.0b013e3181ebaa4d [DOI] [PubMed] [Google Scholar]
- [264]. Dooris, A. P. , Goel, V. K. , Grosland, N. M. , Gilbertson, L. G. , and Wilder, D. G. , 2001, “Load-Sharing Between Anterior and Posterior Elements in a Lumbar Motion Segment Implanted With an Artificial Disc,” Spine, 26(6), pp. E122–E129. 10.1097/00007632-200103150-00004 [DOI] [PubMed] [Google Scholar]
- [265]. Lee, K. K. , Teo, E. C. , Qiu, T. X. , and Yang, K. , 2004, “Effect of Facetectomy on Lumbar Spinal Stability Under Sagittal Plane Loadings,” Spine, 29(15), pp. 1624–1631. 10.1097/01.BRS.0000132650.24437.15 [DOI] [PubMed] [Google Scholar]
- [266]. Rousseau, M. A. , Bonnet, X. , and Skalli, W. , 2008, “Influence of the Geometry of a Ball-and-Socket Intervertebral Prosthesis at the Cervical Spine: A Finite element Study,” Spine, 33(1), pp. E10–E14. 10.1097/BRS.0b013e31815e62ea [DOI] [PubMed] [Google Scholar]
- [267]. Ahn, H. S. and DiAngelo, D. J. , 2008, “A Biomechanical Study of Artificial Cervical Discs Using Computer Simulation,” Spine, 33(8), pp. 883–892. 10.1097/BRS.0b013e31816b1f5c [DOI] [PubMed] [Google Scholar]
- [268]. Rohlmann, A. , Zander, T. , Rao, M. , and Bergmann, G. , 2009, “Realistic Loading Conditions for Upper Body Bending,” J. Biomech., 42(7), pp. 884–890. 10.1016/j.jbiomech.2009.01.017 [DOI] [PubMed] [Google Scholar]
- [269]. Womack, W. , Ayturk, U. M. , and Puttlitz, C. M. , 2011, “Cartilage Thickness Distribution Affects Computational Model Predictions of Cervical Spine Facet Contact Parameters,” J. Biomech. Eng., 133(1), pp. 011009. 10.1115/1.4002855 [DOI] [PubMed] [Google Scholar]
- [270]. Kang, H. , Park, P. , La Marca, F. , Hollister, S. J. , and Lin, C. Y. , 2010, “Analysis of Load Sharing on Uncovertebral and Facet Joints at the C5-6 Level With Implantation of the Bryan, Prestige LP, or ProDisc-C Cervical Disc Prosthesis: An in Vivo Image-Based Finite Element Study,” Neurosurg. Focus, 28(6), pp. E9. 10.3171/2010.3.FOCUS1046 [DOI] [PubMed] [Google Scholar]
- [271]. Schmidt, H. , Heuer, F. , Claes, L , and Wilke, H. J. , 2008, “The Relation Between the Instantaneous Center of Rotation and Facet Joint Forces – A Finite Element Analysis,” Clin. Biomech., 23(3), pp. 270–278. 10.1016/j.clinbiomech.2007.10.001 [DOI] [PubMed] [Google Scholar]
- [272]. Schmidt, H. , Heuer, F. , and Wilke, H. J. , 2008, “Interaction Between Finite Helical Axes and Facet Joint Forces Under Combined Loading,” Spine, 33(25), pp. 2741–2748. 10.1097/BRS.0b013e31817c4319 [DOI] [PubMed] [Google Scholar]
- [273]. Schmidt, H. , Galbusera, F. , Rohlmann, A. , Zander, T. , and Wilke, H. J. , 2010, “Effect of Multilevel Lumbar Disc Arthroplasty on Spine Kinematics and Facet Joint Loads in Flexion and Extension: A Finite Element Analysis,” Eur. Spine J. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274]. Rundell, S. A. , Auerbach, J. D. , Balderston, R. A. , and Kurtz, S. M. , 2008, “Total Disc Replacement Positioning Affects Facet Contact Forces and Vertebral Body Strains,” Spine, 33(23), pp. 2510–2517. 10.1097/BRS.0b013e318186b258 [DOI] [PubMed] [Google Scholar]
- [275]. Katsimihas, M. , Bailey, C. S. , Issa, K. , Fleming, J. , Rosas-Arellano, P. , Bailey, S. I. , and Gurr, K. R. , 2010, “Prospective Clinical and Radiographic Results of CHARITÉ III Artificial Total Disc Arthroplasty at 2- to 7-Year Follow-up: A Canadian Experience,” Can. J. Surg., 53(6), pp. 408–4145. [PMC free article] [PubMed] [Google Scholar]
- [276]. Zindrick, M. , Harris, M. B. , Humphreys, S. C. , O'Leary, P. T. , Schneiderman, G. , Watters, W. C., III , Turkelson, C. M. , Wies, J. L. , and Raymond, L. , 2010, “Cervical Disc Arthroplasty,” J. Am. Acad. Orthop. Surg., 18(10), pp. 631–637. [DOI] [PubMed] [Google Scholar]
- [277]. Ryu, K. S. , Park, C. K. , Jun, S. C. , and Huh, H. Y. , 2010, “Radiological Changes of the Operated and Adjacent Segments Following Cervical Arthroplasty After a Minimum 24-Month Follow-up: Comparison Between the Bryan and Prodisc-C Devices,” J. Neurosurg. Spine, 13(3), pp. 299–307. 10.3171/2010.3.SPINE09445 [DOI] [PubMed] [Google Scholar]
- [278]. Cardoso, M. J. and Rosner, M. K. , 2010, “Multilevel Cervical Arthroplasty With Artificial Disc Replacement,” Neurosurg. Focus, 28(5), pp. E19. 10.3171/2010.1.FOCUS1031 [DOI] [PubMed] [Google Scholar]
- [279]. Tumialán, L. M. , Ponton, R. P. , Garvin, A. , and Gluf, W. M. , 2010, “Arthroplasty in the Military: A Preliminary Experience With ProDisc-C and ProDisc-L,” Neurosurg. Focus, 28(5), pp. E18. 10.3171/2010.1.FOCUS102 [DOI] [PubMed] [Google Scholar]
- [280]. Kikkawa, J. , Cunningham, B. W. , Shirado, O. , Hu, N. , McAfee, P. C. , and Oda, H. , 2010, “Biomechanical Evaluation of a Posterolateral Lumbar Disc Arthroplasty Device: An in Vitro Human Cadaveric Model,” Spine, 35(19), pp. 1760–1768. 10.1097/BRS.0b013e3181c87692 [DOI] [PubMed] [Google Scholar]
- [281]. Botelho, R. V. , 2010, “Disc Arthroplasty,” J. Neurosurg. Spine, 12(5), pp. 580–581. 10.3171/2009.10.SPINE09836 [DOI] [PubMed] [Google Scholar]
- [282]. Cunningham, B. W. , Hu, N. , Zorn, C. M. , and McAfee, P. C. , 2010, “Biomechanical Comparison of Single- and Two-Level Cervical Arthroplasty Versus Arthrodesis: Effect on Adjacent-Level Spinal Kinematics,” Eur. Spine J., 10(4), pp. 341–349. 10.1016/j.spinee.2010.01.006 [DOI] [PubMed] [Google Scholar]
- [283]. Grogan, J. , Nowicki, B. H. , Schmidt, T. A. , and Haughton, V. M. , 1997, “Lumbar Facet Joint Tropism Does Not Accelerate Degeneration of the Facet Joints,” AJNR Am. J. Neuroradiol., 18, pp. 1325–1329. [PMC free article] [PubMed] [Google Scholar]
- [284]. Berlemann, U. , Jeszenszky, D. J. , Buhler, D. W. , and Harms, J. , 1998, “Facet Joint Remodeling in Degenerative Spondylolisthesis: An Investigation of Joint Orientation and Tropism,” Eur. Spine J., 7, pp. 376–380. 10.1007/s005860050093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285]. Cyron, B. M. and Hutton, W. C. , 1980, “Articular Tropism and Stability of the Lumbar Spine,” Spine, 5(2), pp. 168–172. 10.1097/00007632-198003000-00011 [DOI] [PubMed] [Google Scholar]
- [286]. Gallucci, M. , Puglielli, E. , Splendiani, A. , Pistoia, F. , and Spacca, G. , 2005, “Degenerative Disorders of the Spine,” Eur. Radiol. 15, pp. 591–598. 10.1007/s00330-004-2618-4 [DOI] [PubMed] [Google Scholar]
- [287]. Dai, L. Y. , 2001, “Orientation and Tropism of Lumbar Facet Joints in Degenerative Spondylolisthesis,” Int. Orthop., 25(1), pp. 40–42. 10.1007/s002640000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288]. Kong, M. H. , He, W. , Tsai, Y. D. , Chen, N. F. , Keorochana, G. , Do, D. H. , and Wang, J. C. , 2009, “Relationship of Facet Tropism With Degeneration and Stability of Functional Spinal Unit,” Yonsei Med. J., 50(5), pp. 624–629. 10.3349/ymj.2009.50.5.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289]. Murtagh, F. R. , Paulsen, R. D. , and Rechtine, G. R. , 1991, “The Role and Incidence of Facet Tropism in Lumbar Spine Degenerative Disc Disease,” J. Spinal Disord., 4(1), pp. 86–89. [PubMed] [Google Scholar]
- [290]. Cassidy, J. D. , Loback, D. , Yong-Hing, K. , and Tchang, S. , 1992, “Lumbar Facet Joint Asymmetry. Intervertebral Disc Herniation,” Spine, 17(5), pp. 570–574. 10.1097/00007632-199205000-00016 [DOI] [PubMed] [Google Scholar]
- [291]. Boden, S. D. , Riew, K. D. , Yamaguchi, K. , Branch, T. P. , Schellinger, D. , and Wiesel, S. W. , 1996, “Orientation of the Lumbar Facet Joints: Association With Degenerative Disc Disease,” J. Bone Jt. Surg. Am., 78-A, pp. 403–411. [DOI] [PubMed] [Google Scholar]
- [292]. Kunakornsawat, S. , Ngamlamaidt, K. , Tungsiripat, R. , and Prasartritha, T. , 2007, “The Relationship of Facet Tropism to Lumbar Disc Herniation,” J. Med. Assoc. Thai., 90(7), pp. 1337–1341. [PubMed] [Google Scholar]
- [293]. Wheaton, A. J. , Dodge, G. R. , Elliott, D. M. , Nicoll, S. B. , and Reddy, R. , 2005, “Quantification of Cartilage Biomechanical and Biochemical Properties Via T1rho Magnetic Resonance Imaging,” Magn. Reson. Med., 54(5), pp. 1087–1093. 10.1002/mrm.v54:5 [DOI] [PubMed] [Google Scholar]
- [294]. Fujiwara, A. , Lim, T. H. , An, H. S. , Tanaka, N. , Jeon, C. H. , Andersson, G. B. , and Haughton, V. M. , 2000, “The Effect of Disc Degeneration and Facet Joint Osteoarthritis on the Segmental Flexibility of the Lumbar Spine,” Spine, 25(23), pp. 3036–3044. 10.1097/00007632-200012010-00011 [DOI] [PubMed] [Google Scholar]
- [295]. Boszczyk, B. M. , Boszczyk, A. A. , Korge, A. , Grillhösl, A. , Boos, W. D. , Putz, R. , Milz, S. , and Benjamin, M. , 2003, “Immunohistochemical Analysis of the Extracellular Matrix in thePposterior Capsule of the Zygapophysial Joints in Patients With Degenerative L4-5 Motion Segment Instability,” J. Neurosurg., 99(1 Suppl), pp. 27–33. [DOI] [PubMed] [Google Scholar]
- [296]. Boszczyk, B. M. , Boszczyk, A. A. , Putz, R. , Buttner, A. , Benjamin, M. , and Milz, S. , 2001, “An Immunohistochemical Study of the Dorsal Capsule of the Lumbar and Thoracic Facet Joints,” Spine, 26, pp. E338–E343. 10.1097/00007632-200108010-00006 [DOI] [PubMed] [Google Scholar]
- [297]. Tanno, I. , Oguma, H. , Murakami, G. , Sato, S. , and Yamashita, T. , 2003, “Which Portion in a Facet is Specifically Affected by Articular Cartilage Degeneration With Aging in the Human Lumbar Zygapophysial Joint?” Okajimas Folia Anat. Jpn., 80(1), pp. 29–34. 10.2535/ofaj.80.29 [DOI] [PubMed] [Google Scholar]
- [298]. Igarashi, A. , Kikuchi, S. , Konno, S. , and Olmarker, K. , 2004, “Inflammatory Cytokines Released From the Facet Joint Tissue in Degenerative Lumbar Spinal Disorders,” Spine, 29(19), pp. 2091–2095. 10.1097/01.brs.0000141265.55411.30 [DOI] [PubMed] [Google Scholar]
- [299]. Cartwright, M. J. , Nehls, D. G. , Carrion, C. A. , and Spetzler, R. F. , 1985, “Synovial Cyst of a Cervical Facet Joint: Case Report,” Neurosurgery, 16(6), pp. 850–852. 10.1227/00006123-198506000-00024 [DOI] [PubMed] [Google Scholar]
- [300]. Wang, Y. Y. , McKelvie, P. , Trost, N. , and Murphy, M. A. , 2004, “Trauma as a Precipitant of Haemorrhage in Synovial Cysts,” J. Clin. Neurosci., 11(4), pp. 436–439. 10.1016/j.jocn.2003.04.007 [DOI] [PubMed] [Google Scholar]
- [301]. Alicioglu, B. and Sut, N. , 2009, “Synovial Cysts of the Lumbar Facet Joints: A Retrospective Magnetic Resonance Imaging Study Investigating Their Relation With Degenerative Spondylolisthesis,” Prague Med. Rep., 110(4), pp. 301–309. [PubMed] [Google Scholar]
- [302]. Hubbard, R. D. and Winkelstein, B. A. , 2008, “Dorsal Root Compression Produces Myelinated Axonal Degeneration Near the Biomechanical Thresholds for Mechanical Behavioral Hypersensitivity,” Exp. Neurol., 212(2), pp. 482–489. 10.1016/j.expneurol.2008.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303]. Jagannathan, J. , Sherman, J. H. , Szabo, T. , Shaffrey, C. I. , and Jane, J. A. , 2009, “The Posterior Cervical Foraminotomy in the Treatment of Cervical Disc/Osteophyte Disease: A Single-Surgeon Experience With a Minimum of 5 Years' Clinical and Radiographic Follow-up,” J. Neurosurg. Spine., 10(4), pp. 347–356. 10.3171/2008.12.SPINE08576 [DOI] [PubMed] [Google Scholar]
- [304]. Rothman, S. M. , Huang, Z. H. , Lee, K. E. , Weisshaar, C. L. , and Winkelstein, B. A. , 2009, “Cytokine mRNA Expression in Painful Radiculopathy,” J. Pain, 10(1), pp. 90–99. 10.1016/j.jpain.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305]. Rothman, S. M. , Ma, L. H. , Whiteside, G. T. , and Winkelstein, B. A. , 2011, “Inflammatory Cytokine and Chemokine Expression is Differentially Modulated Acutely in the Dorsal Root Ganglion in Response to Different Nerve Root Compressions,” Spine, 36(3), pp. 197–202. 10.1097/BRS.0b013e3181ce4f4d [DOI] [PubMed] [Google Scholar]
- [306]. Citow, J. S. and Macdonald, R. L. , 1999, “Posterior Decompression of the Vertebral Artery Narrowed by Cervical Osteophyte: Case Report,” Surg. Neurol., 51(5), pp. 495–499. 10.1016/S0090-3019(98)00121-9 [DOI] [PubMed] [Google Scholar]
- [307]. Carrera, G. F. , Haughton, V. M. , Syvertsen, A. , and Williams, A. L. , 1980, “Computed Tomography of the Lumbar Facet Joints,” Radiology, 134(1), pp. 145–148. [DOI] [PubMed] [Google Scholar]
- [308]. Yoganandan, N. , Kumaresan, S. C. , Voo, L. , Pintar, F. A. , and Larson, S. J. , 1996, “Finite Element Modeling of the C4-C6 Cervical Spine Unit,” Med. Eng. Phys., 18(7), pp. 569–574. 10.1016/1350-4533(96)00013-6 [DOI] [PubMed] [Google Scholar]
- [309]. Kumaresan, S. , Yoganandan, N. , Pintar, F. A. , and Maiman, D. J. , 1999, “Finite Element Modeling of the Cervical Spine: Role of Intervertebral Disc Under Axial and Eccentric Loads,” Med. Eng. Phys., 21(10), pp. 689–700. 10.1016/S1350-4533(00)00002-3 [DOI] [PubMed] [Google Scholar]
- [310]. Brolin, K. and Halldin, P. , 2004, “Development of a Finite Element Model of the Upper Cervical Spine and a Parameter Study of Ligament Characteristics,” Spine, 29(4), pp. 376–385. 10.1097/01.BRS.0000090820.99182.2D [DOI] [PubMed] [Google Scholar]
- [311]. Hussain, M. , Natarajan, R. N. , An, H. S. , and Andersson, G. B. , 2010, “Reduction in Segmental Flexibility Because of Disc Degeneration is Accompanied by Higher Changes in Facet Loads Than Changes in Disc Pressure: A Poroelastic C5-C6 Finite Element Investigation,” Eur. Spine J., 10(12), pp. 1069–1077. 10.1016/j.spinee.2010.09.012 [DOI] [PubMed] [Google Scholar]
- [312]. Clausen, J. D. , Goel, V. K. , Traynelis, V. C. , and Scifert, J. , 1997, “Uncinate Processes and Luschka Joints Influence the Biomechanics of the Cervical Spine: Quantification Using a Finite Element Model of the C5-C6 Segment,” J. Orthop. Res., 15(3), pp. 342–347. 10.1002/jor.v15:3 [DOI] [PubMed] [Google Scholar]
- [313]. Natarajan, R. N. , Andersson, G. B. , Patwardhan, A. G. , and Andriacchi, T. P. , 1999, “Study on Effect of Graded Facetectomy on Change in Lumbar Motion Segment Torsional Flexibility Using Three-Dimensional Continuum Contact Representation for Facet Joints,” J. Biomech. Eng., 121(2), pp. 215–221. 10.1115/1.2835106 [DOI] [PubMed] [Google Scholar]
- [314]. Rohlmann, A. , Mann, A. , Zander, T. , and Bergmann, G. , 2009, “Effect of an Artificial Disc on Lumbar Spine Biomechanics: A Probabilistic Finite Element Study,” Eur. Spine J., 18(1), pp. 89–97. 10.1007/s00586-008-0836-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315]. Sharma, M. , Langrana, N. A. , and Rodriguez, J. , 1998, “Modeling of Facet Articulation as a Nonlinear Moving Contact Problem: Sensitivity Study on Lumbar Facet Response,” J. Biomech. Eng., 120(1), pp. 118–125. 10.1115/1.2834291 [DOI] [PubMed] [Google Scholar]
- [316]. Wu, J. S. and Chen, J. H. , 1996, “Clarification of the Mechanical Behaviour of Spinal Motion Segments Through a Three-Dimensional Poroelastic Mixed Finite Element Model,” Med. Eng. Phys., 18(3), pp. 215–224. 10.1016/1350-4533(95)00027-5 [DOI] [PubMed] [Google Scholar]
- [317]. Shea, M. , Edwards, W. T. , White, A. A. , and Hayes, W. C. , 1991, “Variations of Stiffness and Strength Along the Human Cervical Spine,” J. Biomech., 24(2), pp. 95–107. 10.1016/0021-9290(91)90354-P [DOI] [PubMed] [Google Scholar]
- [318]. Ng, H. W. , Teo, E. C. , Lee, K. K. , and Qiu, T. X. , 2003, “Finite Element Analysis of Cervical Spinal Instability Under Physiologic Loading,” J. Spinal Disord., 16(1), pp. 55–65. 10.1097/00024720-200302000-00010 [DOI] [PubMed] [Google Scholar]
- [319]. Zhang, Q. H. , Teo, E. C. , Ng, H. W. , and Lee, V. S. , 2006, “Finite Element Analysis of Moment-Rotation Relationships for Human Cervical Spine,” J. Biomech., 39(1), pp. 189–193. 10.1016/j.jbiomech.2004.10.029 [DOI] [PubMed] [Google Scholar]
- [320]. Wheeldon, J. A. , Stemper, B. D. , Yoganandan, N. , and Pintar, F. A. , 2008, “Validation of a Finite Element Model of the Young Normal Lower Cervical Spine,” Ann. Biomed. Eng., 36(9), pp. 1458–1469. 10.1007/s10439-008-9534-8 [DOI] [PubMed] [Google Scholar]
- [321]. Sharma, M. , Langrana, N. A. , and Rodriguez, J. , 1995, “Role of Ligaments and Facets in Lumbar Spinal Stability,” Spine, 20(8), pp. 887–900. 10.1097/00007632-199504150-00003 [DOI] [PubMed] [Google Scholar]
- [322]. Goel, V. K. and Clausen, J. D. , 1998, “Prediction of Load Sharing Among Spinal Components of a C5-C6 Motion Segment Using the Finite Element Approach,” Spine, 23(6), pp. 684–691. 10.1097/00007632-199803150-00008 [DOI] [PubMed] [Google Scholar]
- [323]. Kumaresan, S. , Yoganandan, N. , Pintar, F. A. , Maiman, D. J. , and Goel, V. K. , 2001, “Contribution of Disc Degeneration to Osteophyte Formation in the Cervical Spine: A Biomechanical Investigation,” J. Orthop. Res., 19(5), pp. 977–984. 10.1016/S0736-0266(01)00010-9 [DOI] [PubMed] [Google Scholar]
- [324]. Panzer, M. B. and Cronin, D. S. , 2009, “C4-C5 Segment Finite Element Model Development, Validation, and Load-Sharing Investigation,” J. Biomech., 42(4), pp. 480–490. 10.1016/j.jbiomech.2008.11.036 [DOI] [PubMed] [Google Scholar]
- [325]. Moroney, S. P. , Schultz, A. B. , Miller, J. A. A. , and Andersson, G. B. J. , 1988, “Load-Displacement Properties of Lower Cervical Spine Motion Segments,” J. Biomech., 21, pp. 767–779. 10.1016/0021-9290(88)90285-0 [DOI] [PubMed] [Google Scholar]
- [326]. Voo, L. M. , Kumaresan, S. , Yoganandan, N. , Pintar, F. A. , and Cusick, J. F. , 1997, “Finite Element Analysis of Cervical Facetectomy,” Spine, 22(9), pp. 964–969. 10.1097/00007632-199705010-00006 [DOI] [PubMed] [Google Scholar]
- [327]. Ng, H. W. , Teo, E. C. , and Zhang, Q. H. , 2004, “Biomechanical Effects of C2-C7 Intersegmental Stability Due to Laminectomy With Unilateral and Bilateral Facetectomy,” Spine, 29(16), pp. 1737–1746. 10.1097/01.BRS.0000134574.36487.EB [DOI] [PubMed] [Google Scholar]
- [328]. Qiu, T. X. , Teo, E. C. , and Zhang, Q. H. , 2006, “Effect of Bilateral Facetectomy of ThoracoLumbar Spine T11-L1 on Spinal Stability,” Med. Biol. Eng. Comput., 44(5), pp. 363–370. 10.1007/s11517-006-0048-y [DOI] [PubMed] [Google Scholar]
- [329]. Rohlmann, A. , Burra, N. K. , Zander, T. , and Bergmann, G. , 2007, “Comparison of the Effects of Bilateral Posterior Dynamic and Rigid Fixation Devices on the Loads in the Lumbar Spine: A Finite Element Analysis,” Eur. Spine J., 16(8), pp. 1223–1231. 10.1007/s00586-006-0292-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330]. Womack, W. , Leahy, P. D. , Patel, V. V. , and Puttlitz, C. M. , 2011, “Finite Element Modeling of Kinematic and Load Transmission Alterations Due to Cervical Intervertebral Disc Replacement,” Spine (in press). [DOI] [PubMed] [Google Scholar]
- [331]. Kumaresan, S. , Yoganandan, N. , and Pintar, F. A. , 1998, “Finite Element Modeling Approaches of Human Cervical Spine Facet Joint Capsule,” J. Biomech., 31(4), pp. 371–376. 10.1016/S0021-9290(98)00008-6 [DOI] [PubMed] [Google Scholar]
- [332]. Kuo, C. S. , Hu, H. T. , Lin, R. M. , Huang, K. Y. , Lin, P. C. , Zhong, Z. C. , and Hseih, M. L. , 2010, “Biomechanical Analysis of the Lumbar Spine on Facet Joint Force and Intradiscal Pressure − a Finite Element Study,” BMC Musculoskelet. Disord., 11, p. 151. 10.1186/1471-2474-11-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333]. Kitagawa, Y. , Yasuki, T. , and Hasegawa, J. , 2006, “A Study of Cervical Spine Kinematics and Joint Capsule Strain in Rear Impacts Using a Human FE Model,” Stapp Car Crash J., 50, pp. 545–566. [DOI] [PubMed] [Google Scholar]
- [334]. El-Rich, M. , Arnoux, P. J. , Wagnac, E. , Brunet, C. , and Aubin, C. E. , 2009, “Finite Element Investigation of the Loading Rate Effect on the Spinal Load-Sharing Changes Under Impact Conditions,” J. Biomech., 42(9), pp. 1252–1262. 10.1016/j.jbiomech.2009.03.036 [DOI] [PubMed] [Google Scholar]