Abstract
Respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD) contribute significantly to neonatal morbidity and mortality. Pulmonary function depends on the interaction between alveolar microvasculature and airspace development. While it has been shown in various animal models that vascular endothelial growth factor (VEGF) and its receptors increase in normal animal lung development, its pathophysiological role in neonatal respiratory failure is not yet entirely clear. Current animal and human studies exhibit controversial results. Though animal models are invaluable tools in the study of human lung disease, inherent differences in physiology mandate clarification of the timing of these studies to ensure that they appropriately correlate with the human stages of lung development. The purpose of this review article is to highlight the importance of considering the temporal relationship of VEGF and lung development in human neonates and developmentally-appropriate animal models with RDS and BPD.
Keywords: VEGF, bronchopulmonary dysplasia, neonatal lung disease, respiratory distress syndrome
INTRODUCTION
Respiratory failure continues to be a common cause of morbidity and mortality among neonates. For normal gas exchange to occur at birth, the complex processes of alveolar microvasculature and airspace development must be coordinated. The formation of normal pulmonary vasculature results from the interplay of many growth factors. This review article will specifically focus on vascular endothelial growth factor (VEGF) as an important cytokine for lung development and injury in the premature neonatal population.
VEGF-A is a member of a family of endothelialspecific, heparin-binding, angiogenic growth factors, which also includes VEGF-B, VEGF-C, VEGF-D, and placental growth factor. VEGF expression is upregulated by various growth factors, including platelet derived growth factor, transforming growth factor-beta (TGF-β), tumor necrosis factor-alpha (TNF-α), and interleukin-1beta (IL-1β), and environmental alterations, including oxidative stress, acidosis, hyperoxia, and hypoxia. Under hypoxic conditions, hypoxia inducible factor 1-alpha (HIF1-α) dimerizes with HIF1-β and this complex binds the VEGF promoter, leading to transcription [1]. Production of VEGF protein and activation of the VEGF-receptor (VEGF-R) pathway lead to multiple signaling networks being triggered including the upregulation of integrin expression, induction of u-plasminogen activator (uPA) and u-plasminogen activator receptor (uPAR), activation of matrix metalloproteinases (MMPs), and the production of nitric oxide (NO). VEGF ultimately stimulates endothelial cell survival, mitogenesis, migration, differentiation, vascular permeability, and mobilization of endothelial precursor cells from the basement membrane [2].
Specifically, the binding of VEGF to one of its receptors, vascular endothelial growth factor receptor – 1 (VEGFR-1 or flt-1), mediates endothelial cell migration and vascular organization; the binding of VEGF to its other receptor, VEGFR-2 (or flk-1), results in differentiation, proliferation, and migration of endothelial cells, as well as angioblast differentiation. Lastly, VEGF-C and VEGF-D bind VEGFR-3 (or flt-4) [1].
In humans, alternative messenger RNA (mRNA) splicing of exons 6 and 7 of VEGF produces 5 isoforms ranging from 121 to 206 amino acids. While the other isoforms show increasing trends in size and heparin-binding ability, VEGF121 lacks a heparin-binding domain and is soluble. VEGF189 is highly bound to the extracellular membrane (ECM) and the predominant isoform in most tissues is VEGF165 [1].
In lung development, VEGF regulates vascularization but its location varies throughout this process. In the embryonic lung, VEGF is found in the primitive airway epithelium and mesenchyme but by the pseudoglandular stage, VEGF is expressed mostly by branching airway epithelial cells. In the mature lung, VEGF is expressed by type II epithelial cells [2].
VEGF and its receptors, -R1 and -R2, increase in normal animal lung development [1, 3–5]. However, D'Angio et al. found in a single study of VEGF protein collected from lung fluid of newborn (NB) infants born at 24–33 weeks’ gestational age (GA) that increasing GA was correlated with decreasing VEGF levels on day 1 of life [6]. The pathophysiological roles of VEGF in the development of respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD) are currently being investigated. The literature is filled with human and animal studies exhibiting controversial results regarding the presumed levels of VEGF in these diseases. For example, comparison of lungs from infants dying with BPD and those from non-pulmonary causes yielded decreased VEGF mRNA and decreased VEGF immunostaining in infants with BPD [7]. VEGF levels in pulmonary epithelial lining fluid obtained by bronchoalveolar lavage (BAL) from intubated premature NBs showed an elevation in VEGF levels [1], while another study showed that lavage VEGF levels were not associated with the development of BPD [6].
In this review, we will examine the gestational and postnatal (PN) changes in VEGF concentrations in humans and developmentally appropriate animal models to:
determine how these levels relate to development of RDS and BPD in humans and developmentally appropriate animal models, and
determine how developmentally appropriate animal models might be used to predict human response to stimuli such as hyperoxia.
The vast majority of the human studies have been done using BAL/tracheal aspirate (TA) fluid. When human lung tissue or TA cellular samples were used, it has so been specified. In contrast, the vast majority of animal studies use lung homogenates. VEGF expression and production is context specific and temporally defined by cell type, and hence, may not be congruent between developmentally matched human and animal samples. While not ideal, this is the best data that is available in the literature, as it is technically challenging to collect BAL/TA samples from fetal animal models (except from the primate models).
Detailed discussion about the molecular biology of VEGF-signaling pathways in pulmonary development is beyond the scope of this focused review.
VEGF LEVELS IN HUMANS
In considering VEGF studies done with human babies, it is important to do so in the context of stages of lung development, as it is known that GA, indicative of maturity, affects VEGF levels.
The embryonic phase of lung development occurs from 3–7 weeks’ gestation, followed by the pseudoglandular phase from 5–17 weeks and the canalicular phase at 16–26 weeks. The saccular stage lasts from 24–38 weeks of gestation. Alveolarization, which consists of elastogenesis and angiogenesis, occurs during the alveolar phase, occurring from 32 weeks’ GA until 18 months after birth [2].
Preterm infants are often at risk for surfactant-deficient RDS within the first minutes of life. Development of the pulmonary surfactant system is not complete until the end of the third trimester but surfactant, which disperses at the air–liquid interface of the alveolus, is necessary to prevent alveolar collapse. It is composed of phospholipid and glycoprotein, produced by type II pneumocytes, and functions by reducing surface tension. Though administration of steroids prenatally and PN surfactant rescue therapy have reduced the incidence of RDS, current research still aims to refine the timing, method, and dosing intervals of therapy [8]. The following studies consider the relationship between VEGF and RDS.
To consider the role of VEGF in RDS, as it may contribute to surfactant secretion and pulmonary maturation, we can only consider human data within the first 24 hours of life in babies born prematurely as these infants are still surfactant deficient during this time. By 24 hours, exogenous surfactant replacement has occurred that could potentially increase the expression of VEGF in the lung, as suggested by animal studies [9, 10].
A study of VEGF protein collected on the first day after birth from infants born at 24–33 weeks’ GA elicited a trend of higher VEGF levels correlating with decreased GA on PN days 1 and 3 [6]. Exogenous surfactant was administered to these infants. Similarly, Lassus et al. showed that the concentration of VEGF in TA fluid was higher at birth and during the first 10 PN days in preterm than in term infants [11]. Exogenous surfactant was also administered to these infants. Lassus et al. looked at VEGF mRNA in TA samples collected at a median of 6 days after birth in both preterm and term infants. This study found that preterm infants with lower VEGF suffered more severe RDS and it identified the presence of VEGF mRNA in the alveolar epithelium of infants developing BPD [11]. Tsao et al.'s study looked at cord blood from infants born at 32 weeks’ GA or earlier and showed that infants who developed RDS had significantly lower cord blood levels of VEGF [12].
Premature infants with RDS are also predisposed to BPD. BPD is a chronic pulmonary disorder causing morbidity and mortality in premature infants, especially those weighing less than 1000 g at birth. Most infants who go on to develop BPD are born during the saccular phase of lung development, which occurs at 24–38 weeks. At this stage, the potential air spaces are larger and the saccular walls are thinner for gas exchange due to loss of mesenchymal cells via apoptosis, in addition to the progression of capillaries into a double loop network.
BPD is commonly defined as having an oxygen requirement at 36 weeks’ postmenstrual age (PMA) and is attributed to a multitude of factors, including hyperoxic injury from mechanical ventilation, genetic predisposition, pre- and PN infections, and cytokine imbalance. A key component of BPD is dysregulated angiogenesis, though its precise mechanism remains unclear. Since VEGF levels at birth as well as surfactant replacement can modify the response to VEGF in the lung, human studies looking at BPD need to go beyond the first 24 hours of life.
Within the first 24 hours, D'Angio et al. found that higher lavage VEGF levels were correlated with lower GA at birth but that levels were not associated with the development of BPD [6]. In contrast, Been et al. considered infants who were ventilated for RDS and found that significantly lower day 0 (within the first 24h of life) VEGF BAL fluid (BALF) levels were predictive of BPD but there was no statistical difference in VEGF levels on day 1 in those who did or did not develop BPD [13].
Regarding studies done using samples taken at 72 hours, D'Angio et al. also found that lower day 3 levels of VEGF correlated with lower birth GA but was still not associated with development of BPD [6]. Been et al. did find that lower day 3 levels of VEGF were associated with the development of BPD [13].
Been et al. continued to find that levels equalized at day 7 [6]. Interestingly, analysis of TA samples from intubated preterm infants resulted in the finding that VEGF levels were increased when compared to days 1–3 but babies who went on to develop BPD actually had lower values than those who did not [14].
In another study [15], VEGF concentrations were undetectable in BALF obtained immediately after birth, but increased thereafter in control infants with RDS who recovered or went on to develop BPD (requirement of supplemental oxygen at 28 days). The VEGF concentration increased in the RDS and BPD infants by 4 days of life, but remained undetectable in the control group. Thereafter, it increased gradually in the BPD and RDS groups, but increased more rapidly in the control group by 10 days of life. There was no relationship between concentration of VEGF in the BALF and GA or birth weight [15].
Finally, Bhatt et al. found that lung samples from infants who died of BPD >7 days after birth had decreased VEGF mRNA and protein [7]. D'Angio et al. identified that VEGF levels in pulmonary epithelial lining fluid obtained by BALF from intubated human premature infants rose from days 1 to 3 and continued to be elevated through day 28 if intubated [1].
SUMMARY OF HUMAN STUDIES
Thus, it appears that in most cases, VEGF levels tend to increase over time in ventilated preterm infants but lower levels at various time points appear to be associated with the development of BPD.
In contrast to the above discussion, Bhandari et al.'s study found that babies with RDS who later developed BPD had higher levels of VEGF in the first 12 hours, a decrease in VEGF in days 3–5, and increased levels in days 21–28 [8].
It thus appears that VEGF levels tend to be lower in neonates with RDS except for Bhandari et al.'s study; however, this study looked at VEGF levels in the first 12 hours PN while Tsao et al.'s study utilized cord blood, which may be influenced by a variety of factors, not necessarily lung related. Additionally, Lassus et al.'s findings may have been affected by surfactant replacement therapy.
All studies done in humans were measurements done on VEGF protein except the one by Bhatt et al. [7], which was done on mRNA. The study described samples taken from infants at average PMA of death of 36 weeks, or 90% of full term GA, placing them in the alveolar stage of lung development. However, the fold change noted in that study was congruent with most of the other studies measuring VEGF protein in BALF samples, as VEGF decreased in babies with BPD.
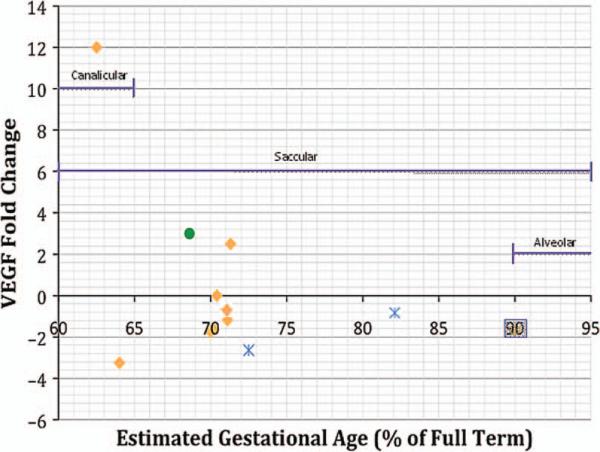
These studies have been summarized in Table 1. For studies conducted on infants between 60% and 70% of GA, there is great disparity among the changes in VEGF levels, but for infants greater than 70% of GA the VEGF level fold changes tend to be more similar and less extreme in value (Figure 1).
TABLE 1.
Human VEGF Levels at Various Postnatal Time Points
| 24 hours PN | 72 hours PN | 4–7 days PN | >7 days PN |
|---|---|---|---|
| Increased TA VEGF on day 1 correlated with lower GA; not correlated with BPD [6] | Decreased TA VEGF correlated with lower birth GA; not associated with BPD [6] | No difference in TA VEGF [13] | Decreased lung VEGF associated with BPD [7] |
| Decreased day 0 TA VEGF predictive of BPD; no difference in day 1 levels [13] | Decreased TA VEGF associated with BPD [13] | Decreased TA VEGF associated with BPD [14] | Increased TA VEGF through day 28 associated with BPD [1] |
| Increased VEGF in first 12 hours associated with BPD [8] | Higher BALF levels in RDS and BPD infants, compared to undetectable levels in controls [15] | Decreased VEGF in days 3–5 associated with BPD [8] | Increased VEGF in days 21–28 associated with BPD [8] |
| No relationship of BALF levels with GA or birth weight [15] | Decreased TA VEGF associated with more severe RDS [11] | Higher BALF levels in controls, versus RDS and BPD by PN10 [15] | |
| Undetectable at birth in BALF [15] | |||
| Decreased cord blood VEGF associated with RDS [12] |
BALF = Broncho-alveolar lavage fluid; BPD = Bronchopulmonary dysplasia; PN = postnatal; RA = respiratory distress syndrome, TA = tracheal aspirate; VEGF = vascular endothelial growth factor.
FIGURE 1.
VEGF levels in human infants according to gestational age and stage of lung development. Data extracted from references [6–8, 12–15]. Key: = Infants who went on to develop BPD;
= Infants who went on to develop BPD; = Infants with RDS;
= Infants with RDS; = Preterm infants without lung disease as compared with control term infants. The data point in the gray box denotes mRNA. BPD = Bronchopulmonary dysplasia; RDS = Respiratory Distress Syndrome; VEGF = Vascular endothelial growth factor.
= Preterm infants without lung disease as compared with control term infants. The data point in the gray box denotes mRNA. BPD = Bronchopulmonary dysplasia; RDS = Respiratory Distress Syndrome; VEGF = Vascular endothelial growth factor.
An important caveat is that BALF/TA has been collected only from neonates who were intubated and mechanically ventilated. VEGF levels that are compared in infants who either develop or don't develop BPD are considered a reflection of the degree of ventilator-induced injury. It would be unethical to intubate and collect BALF/TA from “healthy” neonates, and hence data from such “controls” are unavailable.
Another important confounding variable is the diagnosis of chorioamnionitis (histological confirmation being the “gold standard”) that could potentially influence the VEGF concentrations in the BALF. In the present review, most studies [7, 8, 11, 12, 15] excluded such infants. It is, however, a confounding variable. A previous study [16] has evaluated VEGF levels in TA in patients with maternal histological chorioamnionitis. The TA levels in the group with histological chorioamnionitis (n=18) had increased levels of VEGF (uncorrected for=total protein), compared to controls (n = 11). This did not reach statistical significance (P = 0.06) [16].
Three studies in the present review included infants exposed to chorioamnionitis [6, 13, 14]. In the first and second studies, there was no correlation of maternal chorioamnionitis with VEGF levels at any time [6, 13]. In the third study, significantly increased VEGF in TA were noted in samples collected on days 4–7, but not on days 1–3, from infants exposed to clinical chorioamnionitis [14]. Hence, overall, the data would suggest that while maternal chorioamnionitis does tend to increase VEGF levels in BALF, further confirmation is required.
VEGF LEVELS IN ANIMAL MODELS
Various animal models at different GA time points have been used to look at VEGF levels, including but not limited to: rat, mice, rabbit, and baboon models. For the human equivalent of RDS, it is necessary to evaluate murine studies conducted in the late fetal stages (~embryonic day 16 onwards) when the lung is surfactant-deficient. The correlation of animal models of RDS requires the following: studies done using mouse and rat models must be performed prenatally and those using baboons must look at VEGF levels within the first 24 hours of PN life and specify whether surfactant was exogenously administered. For the human equivalent of BPD, studies should largely aim to correlate animal data obtained with the saccular stage of human development. Hence, for the correlation with human BPD, it is more appropriate for mice and rat studies to be conducted on animals within the first 4 days of PN life. It is important to point out that PN murine models are not surfactant-deficient at birth. However, given the increased use of antenatal steroids and exogenous surfactant replacement therapy in human neonates, the lung maturational level and surfactant-replete status of such a human premature NB may be considered fairly akin to that of an early PN murine lung. Baboon studies conducted on 140-day fetal baboons, approximating 75% of gestation, fulfill both conditions of surfactant-deficiency and appropriate stage of lung development.
Prenatal mouse and rat studies are rare but noteworthy because they allow for equivalency with premature surfactant-deficient human lungs. Akeson et al. showed that conditional activation of VEGF-A in bronchial epithelial cells during late gestation disrupted pulmonary angiogenesis and morphological development, resulting in respiratory failure at birth [17]. However, Compernolle et al. showed that loss of HIF-2α and reduction in VEGF resulted in fatal RDS in neonatal mice. When VEGF was administered by intrauterine or intratracheal route, it prevented the development of RDS [18].
It has been well recognized that maternal diabetes mellitus (DM) leads to an increased risk of RDS in their infants [19]. At the molecular level, lower lecithin/sphingomyelin ratios in the amniotic fluid of pregnancies complicated by DM have been reported. Infants of diabetic mothers (IDM) are frequently hyperinsulinemic or have insulin resistance. It has been shown that insulin inhibits the accumulation of surfactant protein A (SP-A) and SP-B mRNA whereas it has no effect on SP-C mRNA levels in human fetal lung tissue maintained in vitro [19].
Reduced expression of SP has been observed in fetuses of streptozotocin-induced diabetic rats or in insulin-treated human fetal lung explants [20]. Phosphatidylinositol 3-kinase (PI3K) is activated by insulin and is responsible for most of the metabolic actions of insulin. The involvement of the PI3K pathway in RDS was suggested by a previous report showing that bronchoalveolar-specific deletion of Pten in mice led to upregulation of the PI3K pathway in the lung and resulted in RDS [21]. The downstream effectors of PI3K that cause RDS and the mechanistic link between the PI3K pathway and HIF-2-dependent VEGF expression in the lung have been elusive. Downstream of the PI3K is the Akt-mTOR pathway. Interestingly, Ikeda et al. generated a transgenic mouse model for RDS by overexpressing Akt1 in fetal lung. These mice were delivered by cesarean section at embryonic day 18.5 and showed downregulation of HIF-2α and VEGF [20]. This suggests that the PI3K-Akt-mTOR pathway in lung epithelial cells in utero may play a causal role in the pathogenesis of RDS in infants with diabetic mothers. Thus, increased insulin in IDM can activate PI3K, which in turn can increase Akt1 and result in decreased VEGF levels causing reduced expression of SP in the developing lung, predisposing them to increased risk of RDS.
VEGF LEVELS IN ANIMAL MODELS IN RESPONSE TO HYPEROXIA
Few, if any, studies have looked at VEGF levels in rats prior to PN4; however, most studies show decreased VEGF levels associated with hyperoxic exposure. Lopez et al. found that NB rat pups exposed to hyperoxia (>95% O2) exhibited decreased expression of VEGF-R2 mRNA but no change in VEGF mRNA or protein levels from PN0 to PN6 [22]. Lin et al. showed that NB rat exposure to hyperoxia for 6 days resulted in decreased VEGF protein on day 7, which persisted through recovery in room air [23]. Likewise, using intra-amniotic endotoxin exposure and PN hyperoxia, Wang et al. showed that although Flt-1 expression increased with age, the expression of VEGF and VEGF-R mRNA and protein decreased in both rats exposed to endotoxin plus hyperoxia and hyperoxia alone on PN7 and PN14 [24]. Further, Thebaud et al. found decreased lung VEGF expression after continuous hyperoxia exposure from birth till PN14 [25].
In contrast, Hosford et al. found that rat pups exposed to >95% O2 between PN4 and PN14 exhibited increased expression of VEGF protein on PN9 but decreased VEGF mRNA and protein on day 12 [26]. Additionally, VEGF164 and VEGF120 in neonatal rat pups showed steady increases up to 2 weeks PN and declined at 12 weeks, with the highest VEGF188 expression at 12 weeks; expression of VEGF-R1 and VEGF-R2 paralleled these trends [27]. Exposure to 75% O2 at 12 hours after birth for a period of 21 days, however, resulted in decreased VEGF, VEGFR1, and VEGF-R2 expression in neonatal rat lung tissue [28].
The following studies done using NB mice also tended to show decreased VEGF expression. Two- to four-day-old mice mechanically ventilated with 40% O2 for 8 hours showed reduced pulmonary expression of VEGF mRNA after 8 hours while 4–6-day-old mice similarly mechanically ventilated with 40% O2 for 24 hours showed decreased VEGF protein [29]. Mokres et al., however, found that 6-day-old mice mechanically ventilated with 40% O2 for 24 hours exhibited no change in lung VEGF-A or VEGF-R1 protein expression but did show a 50% reduction in lung VEGFR-R2 [30]. Further, Balasubramaniam et al. showed that 1-day-old neonatal mice exposed to 80% O2 for 10 days yielded decreased lung VEGF and VEGF-R2 protein [31]. Zimova et al. also showed that hyperoxic injury in NB mice resulted in decreased VEGF mRNA after 7 days [32].
There have not been many studies conducted using rabbit models but Maniscalco et al. reported NB rabbit exposure to 100% oxygen. While animals exposed for 4 days had no change in VEGF mRNA abundance, those exposed for 9 days had an 80% decrease in lung VEGF expression, which returned to control levels following a 5-day recovery period [3]. In fact, Watkins et al. demonstrated the differential expression of mRNA splice variants in NB rabbits exposed to hyperoxic lung injury. After 9 days, levels of VEGF189 decreased while those of VEGF121 and VEGF165 actually increased. Five days of recovery allowed the return of all 3 splice variant levels to those of controls [33].
Multiple studies have been done using baboon models and looking at VEGF levels after 7 days PN but the results are inconsistent. Maniscalco et al. [34] used fetal baboons delivered at 140 days’ gestation (75% of term) ventilated with 100% oxygen for 6 and 10 days and showed no change in VEGF levels at 6 days but a decrease in VEGF mRNA at 10 days. Likewise, after 14 days of supplemental oxygen, baboons delivered at 125 days’ gestation (67% of term) exhibited decreased VEGF mRNA and protein with no difference in splice variants [4]. VEGF-A and Flt-1 levels were also repressed by premature delivery and mechanical ventilation in 125-days’ baboons treated for 14 days [35]. In contrast, Asikainen et al. [36] showed increased VEGF protein after 10–21 days of either 100% O2 or pro re nata (PRN) exposure in preterm baboons (67% and 75% of term). Interestingly, Tambunting et al. looked at baboons delivered at 125 and 140 days’ gestation and exposed to 100% O2 for 14 and 10 days, respectively. Both groups exhibited increased VEGF121 mRNA but decreased VEGF165 and VEGF189 mRNA [37].
SUMMARY OF ANIMAL STUDIES
Animal studies have reported on VEGF protein [20, 30, 31, 36] or both mRNA and protein [3, 22, 24–26, 28, 29] data. In all the latter group of studies, the VEGF mRNA and protein measurements were congruent. Studies that only reported VEGF mRNA data [4, 32, 33, 35, 37] appeared to parallel the trends in VEGF changes from other papers. However, the data points from both mouse and rat studies don't correlate well with each other, mostly because animals of varying GAs were used. In addition, the baboon studies that showed mRNA data indicate decreased VEGF in BPD models [34, 37], while those showing protein data indicate increased VEGF in similar BPD models [36].
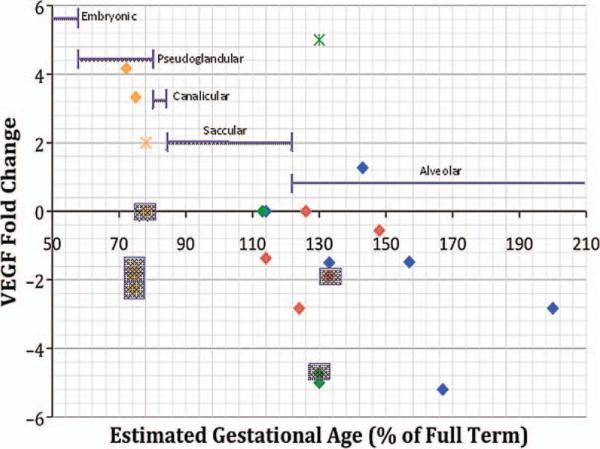
Evidently, there are discrepancies concerning the level of VEGF and its receptors in animal models for neonatal lung disease, which may potentially be explained by considering the relative lung developmental stage and timing of mRNA and protein measurements. These studies have been summarized in Table 2. Studies conducted on animals prior to reaching 100% full GA exhibit marked variation in the direction of VEGF change as compared with controls; however, the graph displays a trend toward decreasing VEGF in those animals exposed to hyperoxia who have reached full term and beyond as compared with control animals of matched GA (Figure 2).
TABLE 2.
Animal VEGF Levels at Various Postnatal Time Points
| Species | Prenatal | 72 hours PN | 4–7 days PN | >7 days PN | Recovery in room air |
|---|---|---|---|---|---|
| Mouse | VEGF-A activation led to respiratory failure [17] | Decreased VEGF associated with 40% O2 MV of 2–4-day-old mice [29] | Decreased VEGF associated with 40% O2 MV of 4–6-day-old mice [29] | Decreased VEGF and VEGF-R2 associated with hyperoxic exposure for 10 days [31] | |
| Decreased VEGF resulted in fatal RDS [18] | Decreased VEGF-R2 but no change in VEGF or VEGF-R1 associated with 40% O2 MV of 6-day-old mice [30] | Decreased VEGF associated with hyperoxic exposure after 7 days [32] | |||
| Decreased VEGF associated with RDS [20] | |||||
| Rat | Decreased VEGF-R2 associated with hyperoxia exposure but no difference in VEGF PN0–6 [22] | Decreased VEGF and VEGF-R associated with endotoxin and hyperoxia exposure on PN14 [24] | Decreased VEGF associated with hyperoxia exposure persisted through recovery in RA [23] | ||
| Decreased VEGF associated with hyperoxia exposure at 6 days [23] | Decreased VEGF on PN14 [25] | ||||
| Decreased VEGF and VEGF-R associated with endotoxin and hyperoxia exposure [24] | Increased VEGF on PN9 but decreased VEGF on PN12 associated with hyperoxia [26] | ||||
| Increasing VEGF and VEGF-R up to 2 weeks PN with peak at 12 weeks [27] | |||||
| Decreased VEGF and VEGF-R associated with hyperoxic exposure at 12 hours PN for 21 days [28] | |||||
| Rabbit | No difference in VEGF after 4 days of hyperoxia exposure [3] | Decreased VEGF associated with 9 days of hyperoxic exposure [3] | Normal VEGF following 5-day recovery after hyperoxic exposure [3] | ||
| Decreased VEGF189 and increased VEGF121 and VEGF165 associated with hyperoxia exposure after 9 days [33] | Normal VEGF levels of all splice variants following 5-day recovery after hyperoxic exposure [33] | ||||
| Baboon | No difference in VEGF levels at 6 days in baboons delivered at 75% of term and ventilated with 100% O2 [4] | Decrease in VEGF levels at 10 days20 | |||
| Decreased VEGF levels at 14 days in baboons delivered at 67% of term and ventilated with O2 [4] | |||||
| Decreased VEGF-A and fit-1 levels at 14 days in baboons delivered at 67% of term and ventilated [35] | |||||
| Increased VEGF after 10–21 days of 100% or PRN O2 in baboons delivered at 75% and 67% of term [36] | |||||
| Increased VEGF121 but decreased VEGF165 and VEGF189 in baboons delivered at 67% of term and exposed to 100% O2 for 14 days and those delivered at 75% of term and exposed for 10 days [37] |
MV = mechanical ventilation; PN = postnatal; RA = room air; VEGF = vascular endothelial growth factor; VEGF-R = vascular endothelial growth factor-receptor.
FIGURE 2.
VEGF levels in various animal models according to gestational age and stage of lung development. Data extracted from references [3, 4, 22, 23, 25, 26, 28–33, 35–37]. Key: = Baboons exposed to hyperoxia;
= Baboons exposed to hyperoxia; = Baboons in room air as compared with control term animals;
= Baboons in room air as compared with control term animals; = Rabbits exposed to hyperoxia;
= Rabbits exposed to hyperoxia; = Rabbits in room air as compared with control term animals;
= Rabbits in room air as compared with control term animals; = Rats exposed to hyperoxia;
= Rats exposed to hyperoxia; = Mice exposed to hyperoxia. Data points in gray boxes denote mRNA. VEGF = Vascular endothelial growth factor.
= Mice exposed to hyperoxia. Data points in gray boxes denote mRNA. VEGF = Vascular endothelial growth factor.
VEGF AS POTENTIAL THERAPY IN HUMANS
Animal data suggests that VEGF may be a potential therapeutic agent to enhance lung maturation [8, 18], as a potential treatment/preventive approach for human RDS. In a similar manner, utilizing the hyperoxia-induced animal models of BPD, VEGF directly [25, 38] or indirectly [39] has been proposed as a potential treatment/preventive approach for human BPD.
Caution must be exercised for a variety of reasons. First, VEGF has myriad effects including some potentially life threatening; for example, pulmonary edema and hemorrhage [8, 40]. Second, one would need to be careful about the timing of administration of such a therapy. Excess VEGF in the early phase of hyperoxia-induced lung injury [8, 41] could potentially exacerbate the significant side effects.
CONCLUSIONS
VEGF levels in humans tend to be low in the early stages of RDS and low to normal in early BPD stages. Though VEGF levels tend to increase with time, multiple studies point toward lower relative levels in infants developing BPD at various chronological points. Bhandari et al.'s study notably contrasts with some of these trends and suggests a bimodal distribution of VEGF levels with proportionately higher levels measured within the first 12 hours of life and by 3–4 weeks PN [8]. To discern the true relative quantity of VEGF right after birth, before the effects of surfactant replacement have occurred, more studies looking at neonates within the first hours of life would be helpful.
VEGF and its receptors increase in normal animal lung development [1, 3–5]. Though animal models are invaluable tools for studying neonatal lung disease, it is important to clarify the timing of the studies, as the models may not always be akin to what is happening in human neonates at the specific lung developmental stage. This is evident from the disparate results obtained, especially from rat and mice studies. Reiteratively, for the human patient population, we need to consider premature babies who are surfactant deficient and in the late canalicular/early saccular stage of lung development. Prenatal mouse and rat studies provide equivalency with premature surfactant-deficient human lungs and these tend to show decreased levels of VEGF associated with RDS. The few studies that have been conducted prior to PN4 also show decreased VEGF levels in BPD models [22, 29]. Most studies go beyond this time point, when the animals are no longer in the saccular stage of lung development but have entered the alveolar stage, and fail to show consistency in the results. It also must be noted that the PN murine lung is not surfactant deficient. The above differences need to be kept in mind when comparing data from animal models to human neonates. The studies conducted on NB rabbit models for hyperoxia-induced BPD exhibit the same down regulation of VEGF demonstrated in human studies; however, the results from baboon studies are less consistent. This may reflect the lack of baboon studies conducted prior to PN7 as well as the specific VEGF mRNA splice variant measured at various time points. Importantly, data from equivalent neonatal baboons should come from those animals delivered around 75% of gestation. Finally, and regarding all animal studies, if the model data does not seem to match the human data, then the modeling system may not be appropriate.
Researchers must be cautious about using animal models to make therapeutic decisions for humans due to existence of some inherent differences in physiology (e.g., surfactant-sufficiency despite a similar immature lung developmental stage). Importantly, the timing of measurement markedly impacts on the results and can explain some discrepant studies. More data is required before VEGF can be considered as potential therapy in human premature infants.
Acknowledgments
Supported in part by grants 0755843T (VB) from the American Heart Association; ATS-07–005 (VB) from the American Thoracic Society; and HL-74195 and HL-085103 (VB) from the NHLBI of the National Institutes of Health, USA. The authors wish to acknowledge the suggestions from Ivan D. Frantz III, MD, for improving earlier drafts of the manuscript.
ABBREVIATIONS
- BALF
Bronchoalveolar Lavage Fluid
- BPD
Bronchopulmonary dysplasia
- ECM
Extracellular membrane
- GA
Gestational age
- HIF
Hypoxia-inducible factor
- IL-1β
Interleukin-1 beta
- MMP
Matrix metalloproteinase
- NB
Newborn
- NO
Nitric oxide
- PMA
Postmenstrual age
- PN
Postnatal
- PRN
Pro re nata
- RDS
Respiratory Distress Syndrome
- SP
Surfactant protein
- TA
Tracheal aspirate
- TGF-β
Transforming growth factor-Beta
- TNF-α
Tumor necrosis factor-Alpha
- uPA
u-Plasminogen activator
- uPAR
u-Plasminogen activator receptor
- VEGF
Vascular endothelial growth factor
- VEGF-R
Vascular endothelial growth factor-receptor
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.D'Angio CT, Maniscalco WM. The role of vascular growth factors in hyperoxia-induced injury to the developing lung. Front Biosci. 2002;7:d1609–d1623. doi: 10.2741/A865. [DOI] [PubMed] [Google Scholar]
- 2.Maniscalco WM, Bhandari V. Disruption of lung microvascular development. In: Abman SH, editor. Bronchopulmonary Dysplasia. Informa Healthcare; New York: 2010. pp. 146–166. [Google Scholar]
- 3.Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyper-oxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol. 1997;16:557–567. doi: 10.1165/ajrcmb.16.5.9160838. [DOI] [PubMed] [Google Scholar]
- 4.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282:L811–L823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt AJ, Amin SB, Chess PR, Watkins RH, Maniscalco WM. Expression of vascular endothelial growth factor and Flk-1 in developing and glucocorticoid-treated mouse lung. Pediatr Res. 2000;47:606–613. doi: 10.1203/00006450-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 6.D'Angio CT, Maniscalco WM, Ryan RM, Avissar NE, Basavegowda K, Sinkin RA. Vascular endothelial growth factor in pulmonary lavage fluid from premature infants: effects of age and postnatal dexamethasone. Biol Neonate. 1999;76:266–273. doi: 10.1159/000014168. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari V, Choo-Wing R, Lee CG, Yusuf K, Nedrelow JH, Ambalavanan N, Malkus H, Homer RJ, Elias JA. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am J Respir Cell Mol Biol. 2008;39:420–430. doi: 10.1165/rcmb.2007-0024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal N, Sanyal SN. Exogenous surfactant protects against endotoxin induced acute respiratory distress syndrome in ro dents via vascular endothelial growth factor. Pathol Res Pract. 2011;207:279–284. doi: 10.1016/j.prp.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Been JV, Zoer B, Kloosterboer N, Kessels CG, Zimmermann LJ, van Iwaarden JF, Villamor E. Pulmonary vascular endothelial growth factor expression and disaturated phospholipid content in a chicken model of hypoxia-induced fetal growth restriction. Neonatology. 2010;97:183–189. doi: 10.1159/000252970. [DOI] [PubMed] [Google Scholar]
- 11.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164:1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 12.Tsao PN, Wei SC, Chou HC, Su YN, Chen CY, Hsieh FJ, Hsieh WS. Vascular endothelial growth factor in preterm infants with respiratory distress syndrome. Pediatr Pulmonol. 2005;39:461–465. doi: 10.1002/ppul.20205. [DOI] [PubMed] [Google Scholar]
- 13.Been JV, Debeer A, van Iwaarden JF, Kloosterboer N, Passos VL, Naulaers G, Zimmermann LJ. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr Res. 2010;67:83–89. doi: 10.1203/PDR.0b013e3181c13276. [DOI] [PubMed] [Google Scholar]
- 14.Lassus P, Ristimaki A, Ylikorkala O, Viinikka L, Andersson S. Vascular endothelial growth factor in human preterm lung. Am J Respir Crit Care Med. 1999;159:1429–1433. doi: 10.1164/ajrccm.159.5.9806073. [DOI] [PubMed] [Google Scholar]
- 15.Currie AE, Vyas JR, MacDonald J, Field D, Kotecha S. Epidermal growth factor in the lungs of infants developing chronic lung disease. Eur Respir J. 2001;18:796–800. doi: 10.1183/09031936.01.00088201. [DOI] [PubMed] [Google Scholar]
- 16.Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res. 2003;53:240–244. doi: 10.1203/01.PDR.0000047656.17766.39. [DOI] [PubMed] [Google Scholar]
- 17.Akeson AL, Cameron JE, Le Cras TD, Whitsett JA, Greenberg JM. Vascular endothelial growth factor-A induces prenatal neovascularization and alters bronchial development in mice. Pediatr Res. 2005;57:82–88. doi: 10.1203/01.PDR.0000148070.89006.3F. [DOI] [PubMed] [Google Scholar]
- 18.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 19.Bental Y, Reichman B, Shiff Y, Weisbrod M, Boyko V, Lerner-Geva L, Mimouni FB. Impact of maternal diabetes mellitus on mortality and morbidity of preterm infants (24–33 weeks’ gestation). Pediatrics. 2011;128:e848–e855. doi: 10.1542/peds.2010-3443. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda H, Shiojima I, Oka T, Yoshida M, Maemura K, Walsh K, Igarashi T, Komuro I. Increased Akt-mTOR signaling in lung epithelium is associated with respiratory distress syndrome in mice. Mol Cell Biol. 2011;31:1054–1065. doi: 10.1128/MCB.00732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, Whitsett JA, Mak TW, Nakano T, Nakazato M, Suzuki A. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez E, Boucherat O, Franco-Montoya ML, Bourbon JR, Delacourt C, Jarreau PH. Nitric oxide donor restores lung growth factor and receptor expression in hyperoxia-exposed rat pups. Am J Respir Cell Mol Biol. 2006;34:738–745. doi: 10.1165/rcmb.2005-0254OC. [DOI] [PubMed] [Google Scholar]
- 23.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res. 2005;58:22–29. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Wei W, Ning Q, Luo XP. Effect of intra-amniotic endotoxin priming plus hyperoxic exposure on the expression of vascular endothelial growth factor and its receptors in lungs of preterm newborn rats. Zhonghua Er Ke Za Zhi. 2007;45:533–538. [PubMed] [Google Scholar]
- 25.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 26.Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L161–L168. doi: 10.1152/ajplung.00285.2002. [DOI] [PubMed] [Google Scholar]
- 27.Mager EM, Renzetti G, Auais A, Piedimonte G. Growth factors gene expression in the developing lung. Acta Paediatr. 2007;96:1015–1020. doi: 10.1111/j.1651-2227.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 28.Feng HY, Lu AZ, Zhang XB, Wang LB, Chen C. Effects of prolonged exposure of high concentration of oxygen on expression of vascular endothelial growth factor and its receptors in neonatal rat lungs. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:927–930. [PubMed] [Google Scholar]
- 29.Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1099–L1110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- 30.Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, Bland RD. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L23–L35. doi: 10.1152/ajplung.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 32.Zimova-Herknerova M, Myslivecek J, Potmesil P. Retinoic acid attenuates the mild hyperoxic lung injury in newborn mice. Physiol Res. 2008;57:33–40. doi: 10.33549/physiolres.930794. [DOI] [PubMed] [Google Scholar]
- 33.Watkins RH, D'Angio CT, Ryan RM, Patel A, Maniscalco WM. Differential expression of VEGF mRNA splice variants in newborn and adult hyperoxic lung injury. Am J Physiol. 1999;276:L858–L867. doi: 10.1152/ajplung.1999.276.5.L858. [DOI] [PubMed] [Google Scholar]
- 34.Maniscalco WM, Watkins RH, Roper JM, Staversky R, O'Reilly MA. Hyperoxic ventilated premature baboons have increased p53, oxidant DNA damage and decreased VEGF expression. Pediatr Res. 2005;58:549–556. doi: 10.1203/01.pdr.0000176923.79584.f7. [DOI] [PubMed] [Google Scholar]
- 35.Pierce RA, Joyce B, Officer S, Heintz C, Moore C, McCurnin D, Johnston C, Maniscalco W. Retinoids increase lung elastin expression but fail to alter morphology or angiogenesis genes in premature ventilated baboons. Pediatr Res. 2007;61:703–709. doi: 10.1203/pdr.0b013e318053661d. [DOI] [PubMed] [Google Scholar]
- 36.Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia-inducible factors and vascular endothelial growth factor in primate lungs. Pediatr Pulmonol. 2005;40:538–546. doi: 10.1002/ppul.20321. [DOI] [PubMed] [Google Scholar]
- 37.Tambunting F, Beharry KD, Waltzman J, Modanlou HD. Impaired lung vascular endothelial growth factor in extremely premature baboons developing bronchopulmonary dysplasia/chronic lung disease. J Investig Med. 2005;53:253–262. doi: 10.2310/6650.2005.53508. [DOI] [PubMed] [Google Scholar]
- 38.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L529–L535. doi: 10.1152/ajplung.00336.2004. [DOI] [PubMed] [Google Scholar]
- 39.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, Yoder BA, Crapo JD, White CW. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J. 2006;20:1698–1700. doi: 10.1096/fj.06-5887fje. [DOI] [PubMed] [Google Scholar]
- 40.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment tran siently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1068–L1078. doi: 10.1152/ajplung.00093.2006. [DOI] [PubMed] [Google Scholar]
- 41.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol. 2004;97:1605–1617. doi: 10.1152/japplphysiol.00202.2004. [DOI] [PubMed] [Google Scholar]