Abstract
In mammals, one of the most pronounced consequences of viral infection is the induction of type I interferons, cytokines with potent antiviral activity. Schlafen (Slfn) genes are a subset of interferon-stimulated early response genes (ISGs) that are also induced directly by pathogens via the interferon regulatory factor 3 (IRF3) pathway1. However, many ISGs are of unknown or incompletely understood function. Here we show that human SLFN11 potently and specifically abrogates the production of retroviruses such as human immunodeficiency virus 1 (HIV-1). Our study revealed that SLFN11 has no effect on the early steps of the retroviral infection cycle, including reverse transcription, integration and transcription. Rather, SLFN11 acts at the late stage of virus production by selectively inhibiting the expression of viral proteins in a codon-usage-dependent manner. We further find that SLFN11 binds transfer RNA, and counteracts changes in the tRNA pool elicited by the presence of HIV. Our studies identified a novel antiviral mechanism within the innate immune response, in which SLFN11 selectively inhibits viral protein synthesis in HIV-infected cells by means of codon-bias discrimination.
SLFN genes encode a family of proteins limited to mammalian organisms. Nine murine and six human SLFN genes share a conserved NH2-terminus containing a putative AAA-domain, and long SLFN genes possess motifs resembling DNA/RNA helicase domains, a trait they share with the nucleic acid sensors RIG-I and MDA-52. Beyond that, SLFN proteins harbour no sequence similarity to other proteins. In vivo, short and long murine SLFN proteins inhibit T-cell development3–5, and levels of murine SLFN proteins are elevated after infection with Brucella or Listeria4. Lipopolysaccharide, poly-inosine-cytosine (poly-IC) or interferon (IFN)-α/β treatment of macrophages results in induction of several murine Slfn genes (our unpublished results). Treatment of human foreskin fibroblasts with IFN-β, poly-IC or poly-dAdT revealed similar induction of SLFN genes (Supplementary Fig. 1a), and human SLFN5 and SLFN11 were consistently the most prominent family members (Supplementary Fig. 1b). Notably, we observed a striking difference in SLFN levels between HEK293 (293) and HEK293T (293T) cells (Supplementary Fig. 1b), and exploited this differential expression to focus on SLFN11 for further studies. We further used SLFN11-targeted short hairpin RNA to generate stable 293 cells that specifically lack SLFN11 expression (293shRNASLFN) (Supplementary Fig. 1c, d).
To test whether lack of SLFN11 in 293shRNASLFN or 293T cells alters their ability to subdue viral infections, we infected these cells with vesicular stomatitis virus (VSV)-G pseudotyped HIV (HIVVSV-G), or amphotropic murine stem cell virus (MSCV), adeno-associated virus (AAV), or herpes simplex virus (HSV). HIVVSV-G-infected cells expressed luciferase after integration of the viral complementary DNAs into the host genome. Regardless of SLFN11 expression, all cell lines had comparable luciferase levels after HIVVSV-G infection (Supplementary Fig. 2). A similar lack of influence of SLFN11 was observed when cells were infected with MSCV, AAV or HSV (not shown).
HEK293T cells are used as packaging cells for production of retroviruses, and we therefore considered the possibility that virus production rather than the response towards them is afflicted by SLFN11. Indeed, 293T cells produced markedly higher HIVVSV-G (Fig. 1b) or MCSV (Supplementary Fig. 3a) titres than 293 cells from the viral vectors pNL4-3.Luc.R+E− or MSCV-IRES-GFP, respectively. Most importantly, this increase in viral titre was also clearly evident in 293shRNASLFN cells, whereas 293 and 293shRNACtl cells produced the same low levels of virus (Fig. 1b and Supplementary Fig. 3a). Notably, the modulation of virus production is limited to particular viruses, as fabrication of retroviruses (Fig. 1b and Supplementary Fig. 3a), but not of AAV (Supplementary Fig. 3c), was affected by SLFN11. We also did not observe any modulation of ISGs such as ISG15, ISG54 or APOBEC3G (not shown) as a consequence of SLFN11 expression, supporting the notion that SLFN11 does not create a general virus-resistant phenotype.
Figure 1. SLFN11 inhibits retrovirus production without affecting intracellular vRNA levels.
a–h, 293T cells were transfected with pNL4-3.Luc.R+E−/pCMV-VSV-G together with SLFN5, SLFN11, SLFN11-N, SLFN11-C or CAT (a, c, e, g), or 293, 293shRNACtl, 293shRNASLFN and 293T cells were transfected with pNL4-3.Luc.R+E− and pCMV-VSV-G (b, d, f, h). a, b, VSV-G-pseudotyped HIV production was assayed by titrated infection and luciferase assay. c, d, Viral particle content in supernatants was analysed by p24 ELISA. e, f, extracellular vRNA concentration was analysed by qPCR of p24.
g, h, intracellular vRNA was determined by qPCR of p24. (average±s.d.; n = 3).
To corroborate that the observed differences are attributable to dissimilar SLFN11 expression, we expressed full-length SLFN11 (amino acids 1–901) in 293T cells and analysed their ability to produce HIVVSV-G. Indeed, SLFN11 strongly inhibited HIVVSV-G (Fig. 1a) or MSCV (Supplementary Fig. 3b) production from 293T cells, with the inhibitory activity residing in the AAA-domain-containing, amino-terminal region (SLFN11-N; amino acids 1–579). No effect of the isolated carboxy-terminal region (SLFN11-C; amino acids 523–901) harbouring the putative helicase sequence was observed (Fig. 1a and Supplementary Fig. 3b). Intriguingly, SLFN5 failed to inhibit retrovirus production but yielded slightly elevated viral titres, illustrating specificity among SLFN proteins in their antiviral activity.
To discern whether SLFN11 reduced the number or the viability of released virus, we measured p24 capsid and viral RNA (vRNA) levels in supernatants of pNL4-3.Luc.R+E−-transfected, HIVVSV-G-producing cells. Extracellular p24 (Fig. 1c and d) or vRNA (Fig. 1e and f) concentration patterns closely matched the titre results from infection assays, demonstrating that SLFN11 diminishes the number of viral particles released from the cells.
To assess a possible reduction of intracellular vRNA, we determined its levels in 293T cells expressing chloramphenicol acetyl transferase (CAT), SLFN5, SLFN11, SLFN11-N or SLFN11-C (Fig. 1g), as well as in 293, 293shRNACtl and 293shRNASLFN cells (Fig. 1h). In contrast to the pronounced variations in extracellular vRNA, only insignificant differences in intracellular vRNA were evident among those cells. We also analysed vRNA in the cytoplasmic fraction specifically, as nuclear export of unspliced vRNA is a hallmark of retroviral RNA processing6–8, but again found no significant alterations attributable to SLFN11 (Supplementary Fig. 4a).
Unlike in BST2-expressing cells9,10, electron-microscopic analysis failed to document accumulation of virions inside or on the surface of virus-producing cells in the presence of SLFN11 (Supplementary Fig. 4b). As such, SLFN11 greatly diminishes the formation of viral particles inside the cell, despite the fact that vRNA is equally available. When the two cell sets were analysed for the expression of viral proteins encoded by pNL4-3.Luc.R+E−, we noted a marked effect of SLFN11 on p55 Gag and p24 capsid proteins11 (Fig. 2a, b). To create a clearer picture of the effect of SLFN11 on p55, we abolished expression of the viral protease by introducing a stop codon into pro in pNL4-3.Luc.R+E−. As anticipated, p24 could no longer be detected; however, modulation of p55 expression by SLFN11 was clearly present (Supplementary Fig. 5a). As with gag-derived proteins, SLFN11 had a notable effect on the protein levels of RT, Vif, Vpu and Vpr (Fig. 2a, b). Intriguingly, we did not observe any reduction of enhanced green fluorescent protein (EGFP) derived from a co-transfected vector, or of GAPDH (Fig. 2a, b, bottom), indicating that the limited viral protein production is not due to a global shutdown of protein synthesis.
Figure 2. SLFN11 selectively inhibits viral protein expression on the basis of codon usage.
a, 293T cells were transfected with pNL4-3.Luc.R+E− and pcDNA5-EGFP together with SLFN5, SLFN11 or CAT, and cell lysates immunoblotted for HIV proteins, EGFP and GAPDH. b, 293, 293shRNACtl and 293shRNASLFN cells were transfected with pNL4-3.Luc.R+E− and pcDNA5-EGFP, and lysates analysed as in a. c, Top: 293T cells were co-transfected with pNL4-3.Luc.R+E− and SLFN5, SLFN11 or CAT (left), or 293, 293shRNACtl and 293shRNASLFN cells were transfected with pNL4-3.Luc.R+E− (right). Lysates were probed for luciferase and GAPDH. Bottom: as above, except pNL4-3-ΔEnv-EGFP was used instead of pNL4-3.Luc.R+E−, and lysates were immunoblotted for Nef and GAPDH; d, Cells were transfected with viral codon-usage-based gag (Gagvir, top) or synonymous human codon usage-optimized gag (Gagopt, bottom). Expression of Gag in cell lysates was determined by anti-V5 immunoblotting.
Even more surprising was that luciferase expression, coded in pNL4-3.Luc.R+E− in place of nef, was mostly impervious to SLFN11 (Fig. 2c, top), contrasting the SLFN11-mediated diminishment of other proteins encoded on this vector. Notably, Nef expression was inhibited by SLFN11 in the context of pNL4-3-ΔEnv-EGFP, which has part of env replaced by EGFP, but retains nef in its original position. (Fig. 2c, bottom). We thus conclude that SLFN11 selectively suppresses viral protein expression via transcript-intrinsic properties rather than external factors or positional elements. This notion is further corroborated by the fact that SLFN11 had no effect on Rev response element (RRE)-mediated events such as nuclear export of unspliced vRNA (Supplementary Fig. 5b).
Viral genomes have biased nucleotide compositions different from human genes12–18. Extremely high frequencies of A nucleotides are found in the RNA genomes of lentiviruses and influenza virus13,17,19,20. Wild isolates of HIV-1, particularly gag and pol sequences, are characterized by low GC content and suboptimal codon usage compared to the host cell preference14,21–24. The unusual rare codon bias favours A/U in the third position, which induces ribosome pausing and inefficient translation. As the inhibitory effect of SLFN11 on viral protein expression is intrinsic to the transcripts, we proposed that SLFN11 exploits viral codon preferences to specifically attenuate viral protein synthesis. We therefore generated vectors containing only the open reading frame of HIV-1 gag with either viral codon-bias (Gagvir), or synonymous substitutions optimizing for human cell expression (Gagopt). As shown in Fig. 2d, SLFN11 strongly affected expression of Gagvir, but was without consequence for Gagopt expression. Differences in translation initiation are not likely, as both Gagvir and Gagopt contain the same translation start sequences. This finding strongly indicates that SLFN11 is exploiting the distinct viral codon bias to selectively attenuate the expression of viral proteins.
Previous reports indicated changes in cellular tRNA levels after HIV infection20, prompting us to investigate whether SLFN11 alters the tRNA composition in the absence or presence of HIV. Using tRNA arrays25,26, we observed little to no changes in tRNA levels as a consequence of SLFN11 expression (Fig. 3a, left column). However, whereas HIV triggered substantial changes in tRNA concentrations in SLFN11-knockdown cells, no changes were observed in the presence of SLFN11 (Fig. 3a, middle and right). Thus, SLFN11 counteracts HIV-induced changes in tRNA composition, presumably initiated to promote viral protein synthesis. To test whether SLFN11 interacts directly with tRNA, we used human tRNA as electrophoretic mobility shift assay (EMSA) probe with fast protein liquid chromatography (FPLC)-purified His-conjugated SLFN11-N. As shown in Fig. 3b, SLFN11-N produced a clear shift of the tRNA in a dose-dependent manner. The shifted band was competed with unlabelled tRNA, but not in-vitro-transcribed vRNA (Fig. 3b), and was disrupted by an antibody against SLFN11 (Fig. 3c). To determine whether SLFN11 has binding preference for particular tRNAs, we performed EMSAs with increasing amounts of SLFN11-N to obtain approximately 50% shifting efficiency (Supplementary Fig. 6). When the shifted (S), unshifted (U) and total tRNA (T) bands were isolated and recovered tRNAs hybridized to tRNA arrays, no enrichment of particular tRNAs in the shifted band was noticeable (Supplementary Fig. 6), indicating a lack of discerning binding preference of SLFN11-N.
Figure 3. SLFN11 binds tRNAs and selectively inhibits protein expression based on codon usage.
a, 293shRNACtl and 293shRNASLFN cells were transfected with pNL4-3.Luc.R+E− or control vector (indicated as +/− HIV). Relative abundances of mature tRNA species were analysed by microarray as described25,26. b, Left: increasing amounts of SLFN11-N or GFP were incubated with 32P-labelled tRNA and subjected to EMSA. Right: 2 × or 10 × unlabelled tRNA or in-vitro-transcribed vRNA corresponding to the gag-pol frame-shifting sequence (120 bases) were added to the binding reaction. c, As in b, except non-specific (NS) or anti-SLFN11 (Anti-S11) monoclonal antibody was added to the binding reaction. d, 293T cells were transfected with V5-tagged GFP, Myc-tagged EGFP and pNL4-3.Luc.R+E− together with either CAT, SLFN5 or SLFN11 (left). Lysates were probed for V5–GFP, Myc–EGFP and GAPDH. Similarly, V5-tagged GFP, Myc-tagged EGFP and pNL4-3.Luc.R+E− were co-transfected into 293, 293shRNACtl and 293shRNASLFN cells (right), and V5–GFP, Myc–EGFP and GAPDH expression determined by immunoblotting. e, V5–GFP and Myc–EGFP protein levels determined from d were quantified and normalized to V5–GFP and Myc–EGFP mRNA levels, respectively (average ± s.d.; n = 4).
The results shown lead to the premise that any protein that uses similar codon usage as HIV would be modulated by HIV and/or SLFN11. To demonstrate unequivocally that codon-bias rather than cryptic regulatory elements in gag accounted for the influence of SLFN11, we tested this hypothesis using non-viral proteins. Natural green fluorescent protein (GFP) harbours a similar codon-bias as that of HIV, whereas EGFP has been optimized for mammalian expression by substituting synonymous codons of highly expressed human genes throughout the GFP open reading frame22. As anticipated, co-transfection of pNL4-3.Luc.R+E− with V5-tagged GFP and Myc-tagged EGFP in the absence of SLFN11 resulted in increased GFP, but not EGFP, expression (not shown). Most importantly, SLFN11 inhibited the expression of GFP in a manner identical to viral proteins, whereas EGFP was largely refractory to suppression by SLFN11 (Fig. 3d, e). As (E)GFP protein levels have been normalized to their respective messenger RNA levels (Fig. 3e), we conclude that any differences in GFP expression are the consequence of altered protein synthesis rather than variations in transcription or mRNA stability.
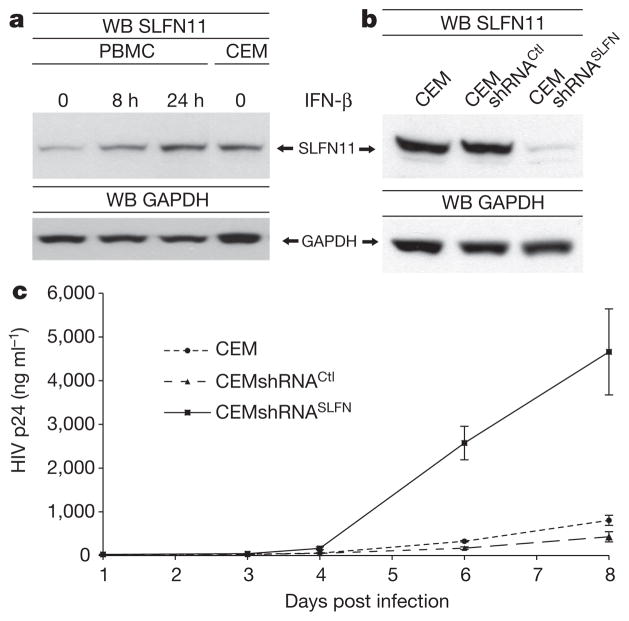
Finally, to demonstrate that the anti-retroviral effects of SLFN11 are not limited to a system using HIVVSV-G and 293 cells, we used CEM cells, a T-cell line widely used to assess HIV replication kinetics. Human peripheral blood mono-nuclear cells (PBMCs) display similar levels of SLFN11 after IFN-β stimulation as CEM cells (Fig. 4a). Control or SLFN11-directed shRNAs were used to generate stable CEM variants (Fig. 4b), and the resulting CEMshRNACtl and CEMshRNASLFN cells and parental CEM cells were infected with wild-type X4-HIV-1LAI, and viral replication was assessed via p24 enzyme-linked immunosorbent assays (ELISA) of the supernatants. As anticipated, CEM and CEMshRNACtl cells produced comparable viral titres at all time points. In contrast, CEMshRNASLFN cells facilitated significantly enhanced HIV-1 replication, yielding an exponentially increasing difference in viral titres (Fig. 4c). A second CEMshRNASLFN cell line established by means of a distinct SLFN11 shRNA further corroborated these results (Supplementary Fig. 7).
Figure 4. SLFN11 inhibits replication of wild-type HIV-1LAI in CEM cells.
a, Human PBMCs were stimulated with 6,000 U ml−1 IFN-β. SLFN11 expression in the derived lysates and in CEM cell lysates was analysed by immunoblotting. b, SLFN11 expression in CEM, CEMshRNACtl and CEMshRNASLFN cells as analysed by immunoblotting. c, CEM, CEMshRNACtl and CEMshRNASLFN cells were infected with HIV-1LAI at a multiplicity of infection (m.o.i.) of 0.01, and viral replication was assayed by p24 ELISA of culture supernatants (average ± s.d.; n = 4).
In summary, systematic analysis of the HIV replication cycle revealed that SLFN11 does not inhibit reverse transcription, integration or production and nuclear export of viral RNA, nor did we observe a block in budding or release of viral particles. However, we discovered a selective inhibition in the synthesis of virally encoded proteins. Intriguingly, a specific inhibition of viral protein synthesis in HIV-infected cells in response to interferon was previously observed, but the factors responsible for the effect were not identified27. We postulate that SLFN11 acts at the point of virus protein synthesis by exploiting the unique viral codon bias towards A/T nucleotides. This model supports the notion that the antiviral activity of SLFN11 extends to other viruses with rare codon bias such as influenza, but apparently not to AAV or HSV. The exact mechanism by which HIV alters tRNA function in its favour and how SLFN11 counteracts this process will require considerable further analysis. Evidently, SLFN11 interacts with all tRNAs in vitro. Direct binding of SLFN11 to tRNA offers the possibility that SLFN11 either sequesters tRNAs, prevents their maturation via post-transcriptional processing or accelerates their deacylation. In either case, if already rare tRNAs are further reduced, tRNA availability might manifest as the rate-limiting step in the synthesis of proteins involving those tRNAs. In contrast, a lesser or no impact would be expected on proteins synthesized via plentiful tRNAs, as even if a fraction of those tRNAs is ‘eliminated’, translation initiation will likely remain the rate-limiting event. In conclusion, our results establish SLFN11 as a potent, interferon-inducible restriction factor against retroviruses such as HIV, mediating its antiviral effects on the basis of codon usage discrimination.
METHODS
Cell culture
HEK293, HEK293T, NIH3T3, HeLa, HFFs (human foreskin fibroblasts) and their derivative cells were maintained in high glucose DMEM. CEM and their derivative cells were maintained in RPMI 1640 medium. Both media were supplemented with 10% heat-inactivated fetal bovine serum, 100 U ml−1 penicillin, 100 μg ml −1 streptomycin, 2 mM L-glutamine, MEM non-essential amino acids, 1 mM sodium pyruvate and 55 μM 2-mercaptoethanol. The Phoenix amphotropic retrovirus packaging cell line (293T) originates from the laboratory of G. Nolan. Human PBMCs were obtained from the San Diego Blood Bank.
Plasmids
The template vectors encoding SLFN5 and SLFN11 sequences were acquired from Open Biosystems (Human MGC Verified FL cDNA (IRAU), clone ID 40123369 and 6258140). The coding sequences were amplified by PCR with PfuUltra (Stratagene) and subcloned into the pcDNA6/V5-His vector (Invitrogen) for mammalian cell expression. Partials of the human SLFN11 coding sequence (GenBank accession no. NM_001104587) were amplified by PCR and subcloned into the pcDNA6/V5-His vector to generate the SLFN11-N (amino acids 1–579) and SLFN11-C (amino acids 523–901) truncation mutants. The pcDNA6/V5-His CAT (chloramphenicol acetyl transferase) vector was generated the same way using pcDNA5/FRT/TO CAT (Invitrogen) as the PCR template for the CAT sequence. The following plasmids were obtained from the Addgene plasmid repository: MSCV-IRES-GFP (Addgene plasmid 20672, source D. Baltimore); pCL-Eco retrovirus packaging vector (Addgene plasmid 12371, source I. Verma); psPAX2 lentivirus packaging vector (Addgene plasmid 12260, source D. Trono); pMD2.G helper vector (Addgene plasmid 12259, source D. Trono) and pCMV-VSV-G vector (Addgene plasmid 8454, source R. M. Weinberg). The following plasmids were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pNL-GFP-RRE(SA) (catalogue no. 11466) from J. Marsh and Y. Wu, pNL4-3-deltaE-EGFP (catalogue no. 11100) from H. Zhang, Y. Zhou and R. Siliciano. The pNL4-3.Luc.R+E− HIV-1 vector has been described previously28. The pNL4-3.Luc.R+E−PRstop construct was created by deletion of thymidine 2478 in pNL4-3.Luc.R+E− HIV-1 to put the following ‘TAGTAG’ into frame such that no pol-encoded viral enzyme proteins would be expressed. Gagvir and Gagopt constructs: gag sequences were cloned into pcDNA6/V5/His between AflII and XhoI sites. Nine base pairs of the wild-type viral sequence preceding the initiation codon were present in both final constructs to assure identical translation initiation sites. Gagvir was generated through PCR amplification of the gag sequence from pGag-EGFP (NIH AIDS Research & Reference Reagent Program, no. 11468) in which only the inhibitory RNA sequence (INS) was eliminated through introduction of silent mutations while otherwise retaining the original viral codon usage. Gagopt was generated through PCR amplification of the gag sequence from p96ZM651gag-opt (NIH AIDS Research & Reference Reagent Program, no. 8675) in which the entire gag sequence was converted to use codon usage in line with highly expressed human genes. GFP and EGFP sequences were cloned into pcDNA6.2/V5 using the pcDNA6.2/GW/D-TOPO kit (Invitrogen). The GFP sequence was tagged with a V5 epitope whereas the EGFP sequence was tagged with a Myc tag (5′-GAGCAGAAGCTGATCAGCGAGGAGGACCTG-3′). The GFP sequence used retains most of the original wild-type protein sequence. The EGFP sequence contains more than 190 silent base changes following human genes codon-usage preferences. Sequences flanking both GFP and EGFP translation initiation site have been converted to a Kozak consensus translation initiation site (5′-CACCATGGTGAGC…) to ensure identical translation initiation efficiency in eukaryotic cells.
shRNA constructs
The pLKO.1 shRNA lentivirus constructs (TRCN0000148990, TRCN0000152230, TRCN0000155578, TRCN0000157747, TRCN0000152057, TRCN0000155564) against SLFN11 were obtained from The RNAi Consortium via Open Biosystems. Construct TRCN0000148990 (5′-CCGGGCTCAGAATTTCCGTACTGAACTCGAGTTCAGTACGGAAATTCTGAGCTTTTTTG-3′) produced maximum SLFN11 knockdown at the protein level and was thus designated as SLFN11 shRNA in this study. pLKO.1-Blasticidin construct was created by replacing puromycin resistance gene of the original pLKO.1 construct with blasticidin resistance gene using BamHI and KpnI sites. The control shRNA construct in the same pLKO.1 vector system (5′-CCGGTGAAGAACTAACCCGGGACTTCTCGAGAAGTCCCGGGTTAGTTCTTCATTTTTG-3′) was also obtained from The RNAi Consortium via Open Biosystems and does not match any human genes.
Antibodies
Anti-SLFN11 antibody for immunoblotting was purchased from Sigma Life Sciences (Atlas Antibodies). Monoclonal anti-SLFN11 antibody for supershift experiments was custom-generated by Abmart. Murine anti-V5 antibody (E10) antibodies were from eBiosciences and Santa Cruz Biotechnology, respectively. Goat polyclonal anti-luciferase antibody was obtained from Promega, rabbit anti-GFP (D5.1) and rabbit anti-GAPDH monoclonal antibodies (14C10) were acquired from Cell Signaling Technology. The following antibodies were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: monoclonal antibody against HIV-1 Gag/p24 (AG3.0) from J. Allan; anti-HIV-1 RT monoclonal antibody (8C4) from D. E. Helland; anti-HIV-1 Vif monoclonal antibody (no. 319) from M. H. Malim; anti-HIV-1 Vpr (1-50) anti-body from J. Kopp; rabbit anti-HIV-1NL4-3 Vpu antiserum from K. Strebel, and anti-HIV-1 Nef (Ag11) monoclonal antibody from J. Hoxie.
Quantitative PCR and primers
Total cellular RNA was extracted with QIAGEN RNeasy Mini kits, and virion RNA in culture supernatants was extracted with QIAGEN QIAamp Viral RNA Mini kits. Cytoplasmic RNA was prepared with QIAGEN RNeasy Mini kits following the manufacturer’s protocol. Briefly, freshly harvested cells were gently lysed on ice for 5 min in buffer RLN (50 mM Tris·Cl, pH 8.0, 140 mM NaCl, 1.5 mM MgCl2, 0.5% (v/v) IGEPAL CA-630, 1,000 U ml−1 RNasin Plus RNase Inhibitor (Promega) and 1 mM DTT). Intact cell nuclei were removed by centrifugation at 4 °C for 2 min at 300g, and cytoplasmic RNA was extracted from the remaining supernatant. RNA was cleaned using Ambion DNA-free kits and reverse transcribed with Applied Biosystems’ High Capacity cDNA Reverse Transcription kit. qPCR was performed on Applied Biosystems 7000 Sequence Detection System or Applied Biosystems StepOnePlus Real-Time PCR System using Power SYBR Green PCR Master Mix. Relative RNA level were calculated after normalization to 18S rRNA unless specified otherwise. The following primer sequences were used in these assays: SLFN5 forward 5′-CAAGCCTGTGTGCATTCATAA-3′, reverse 5′-TCTGGAGTATATACCACTCTGTCTGAA-3′; SLFN11 forward 5′-AAGGCCTGGAACATAAAAAGG-3′, reverse 5′-GGAGTATATCGCAAATATCCTGGT-3′; SLFN12 forward 5′-CTTTGTTCAACACGCCAAGA-3′, reverse 5′-ATGCAGTGTCCAAGCAGAAA-3′; SLFN13 forward 5′-GAGAAAATGATGGACGCAGAT-3′, reverse 5′-AGACTCAAAGGCCTCAGCAA-3′; SLFN12L forward 5′-GAAAGTCAGTTTCTGAGGAATTTCA-3′, reverse 5′-CCAGCTCAGCATAGTTTGTGTC-3′; SLFN14 forward 5′-GGTGGTCATGATGCTGGATA-3′, reverse 5′-TGATGAAATCAGGCAAGAGTTG-3′; ISG54 forward 5′-TGGTGGCAGAAGAGGAAGAT-3′, reverse 5′-CCAAGGAATTCTTATTGTTCTCACT-3′; TBP forward 5′-GCTGGCCCATAGTGATCTTT-3′, reverse 5′-CTTCACACGCCAAGAAACAGT-3′; 18S rRNA forward 5′-GGATGCGTGCATTTATCAGA-3′, reverse 5′-GTTGATAGGGCAGACGTTCG-3′; GFP forward 5′-CTGGAGTTGTCCCAATTCTTG-3′, reverse 5′-TCACCCTCTCCACTGACAGA-3′; EGFP forward 5′-CAGCAGAACACCCCCATC-3′, reverse 5′-TGGGTGCTCAGGTAGTGGTT-3′; luciferase forward 5′-AGGTCTTCCCGACGATGA-3′, reverse 5′-GTCTTTCCGTGCTCCAAAAC-3′; HIV p24 forward 5′-TGCATGGGTAAAAGTAGTAGAAGAGA-3′, reverse 5′-TGATAATGCTGAAAACATGGGTA-3′.
Generation of stable SLFN11 knockdown HEK293 and CEM cell lines
HEK293T cells were transfected with the SLFN11 shRNA or control lentivirus vector and the lentivirus packaging vectors psPAX2 and pMD2.G using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. The cell culture medium was collected and replaced with fresh medium 48, 72 and 96 h after the transfection. The combined supernatants were cleared by centrifugation at 1,000g at 4 °C for 15 min. To generate stable SLFN11 knockdown HEK293 cell lines, cleared supernatants were added to pre-plated HEK293 cells in the presence of 8 μg ml−1 polybrene and centrifuged at 600g for 90 min at room temperature. After 48 h, infected cells were subject to resistance selection with 2 μg ml−1 puromycin. The efficiency of SLFN11 knockdown in the selected cells was assayed by qPCR and immunoblotting. To generate stable SLFN11 knockdown CEM cell lines, cells were first infected and selected as outlined above. Although the same shRNA construct was used, knockdown in CEM cells was less efficient when compared to HEK293 cells. To improve the SLFN11 knockdown in CEM cells, puromycin-selected CEM cells were re-infected with the pLKO.1-Blasticidin construct carrying the same shRNA and subject to resistance selection with 15 μg ml−1 blasticidin and 2 μg ml−1 puromycin. Such double-selected cells displayed a > 90% knockdown of SLFN11 (see Fig. 4b).
Assay for MSCV, HIV and AAV production
To analyse MSCV retrovirus production, MSCV-IRES-GFP (MIG) plasmid and pCL Eco packaging vector were transfected into the cells of interest using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 24 h the cells were moved to 32 °C, and supernatants were collected after 24 h and cleared by centrifugation. Transfection efficiency was determined by analysis of GFP expression in the cells and was subsequently incorporated into the virus titre calculations. The amount of (infectious) virus particles in the supernatants was determined by both infection assays and qPCR analysis of viral RNA. For infection assays, pre-plated NIH3T3 cells were spin-infected with tenfold serially diluted supernatants in the presence of 8 μg ml−1 polybrene at 600g for 90 min at room temperature. 48 h after the spin infection, the NIH3T3 cells were examined for GFP expression by flow cytometry, and the number of GFP+ cells was used to calculate relative viral titres. For viral RNA qPCR assays, viral RNA was extracted from the virus supernatant and quantified as described above with EGFP-specific primers. The production of HIV was tested similarly after pNL4-3.Luc.R+E− HIV vector, pCMV-VSV-G vector and pcDNA5 GFP vector were transfected into cells of interest using Lipofectamine 2000. The transfection efficiency was established by flow cytometric determination of the number of GFP+ cells and used to adjust relative viral titres. Viral titres were determined via infection assays, qPCR of viral RNA and HIV p24 ELISA. The infection assay was similar to the MSCV virus infection assay except HEK293T cells were used for analysis. Expression of luciferase was measured by using the Bright-Glo Luciferase Assay System (Promega) and a microplate luminometer (LUMIstar, BMG-LABTECH) 24 h after the spin infection. HIV viral RNA was quantified by qPCR using luciferase- or HIV-p24-specific primers. The HIV p24 ELISA was performed by the Viral Pathogenesis Core at the UCSD Center for AIDS Research using Alliance HIV1 p24 ELISA kits (Perkin Elmer). For recombinant AAV production assays, cells were transfected with pXX6 (0.6 μg), pXX2 (0.2 μg) and pACLALuc (0.2 μg) using lipofectamine 2000 (Invitrogen), and collected 72 h after transfection. rAAVLuc-containing lysates were generated by freeze/thaw cycles followed by centrifugation and were used to infect HEK293T cells. Luciferase activity was quantified 48 h post-transduction using Steady-Glo luciferase substrate reagent (Promega) in 96-well Lumiplates (Greiner Bio-One) in a TopCount NXT scintillation and luminescence counter (PerkinElmer).
Assays for MSCV and HIV infection
Amphotropic MSCV-IRES-GFP (MIG) retrovirus was prepared by transfecting Phoenix amphotropic retrovirus packaging cells with MSCV-IRES-GFP (MIG) vector using Lipofectamine 2000. The cell culture medium was collected and replaced with fresh medium 48, 72 and 96 h after the transfection. The combined supernatants were cleared by centrifugation at 1,000g at 4 °C for 15 min. Cells to be tested were spin-infected with the indicated dilutions in the presence of 8 μg ml−1 polybrene at 600g for 90 min at room temperature. 48 h after the spin infection, cells were analysed for GFP expression by flow cytometry. Infection efficiency was established by both the number of GFP+ cells and the level of GFP expression. VSV-G pseudotyped HIV was prepared by transfecting HEK293T cells with pNL4-3.Luc.R+E− HIV and pCMV-VSV-G vectors using Lipofectamine 2000. The cell culture medium was collected and cleared as described above. Cells to be tested were spin-infected with the indicated dilutions in the presence of 8 μg ml−1 polybrene at 600g for 90 min at room temperature. 24 h after the spin infection, luciferase expression was measured to determine the relative infection efficiencies.
Transmission electron microscopy
HEK293T cells were transfected with pNL4-3.Luc.R+E− HIV vector together with either pcDNA6/V5-His CAT or pcDNA6/V5-His SLFN11 vectors using Lipofectamine 2000. 48 h after the transfection, cells were fixed and processed by the Microscopy Core at the Scripps Research Institute. Images were acquired with a Philips CM100 transmission electron microscope.
Whole-cell lysis and western blotting
Cells were lysed directly in 1 × NuPAGE LDS Sample Buffer (Invitrogen) containing 2.5% 2-mercaptoethanol and heated at 90 °C for 5 min. Samples were homogenized by QIAshredder (Qiagen), total protein resolved by NuPAGE (Invitrogen) and transferred to PVDF (polyvinylidene difluoride) membranes followed by western blotting with the specified antibodies. Corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies and Amersham ECL Plus Western Blotting Detection Reagent (GE Healthcare) were used to visualize the signals followed by quantification using densitometer and NIH ImageJ64 software. Alternatively, fluorochrome-conjugated secondary antibodies were used and signals were acquired and quantified using Typhoon Trio Variable Mode Imager and ImageQuant software.
Wild-type HIV virus replication assay in CEM cells
HIV-1LAI was used to infect the CEM stable cell lines (CEM, CEMshRNACtl and CEMshRNASLFN) at a m.o.i. of 0.01 in 5 ml of culture at 0.5 × 106 cells per ml using RPMI/poly medium containing 3 μg ml−1 polybrene, 1% glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 10% FBS. The cultures were sampled at the indicated time points and the viral titres were measured by HIV p24 ELISA.
tRNA arrays
mirVana miRNA Isolation kit (Ambion) was used to prepare total RNA from freshly collected cells following the total RNA isolation protocol. RNA samples were labelled and the relative abundances of individual tRNAs were measured using microarrays as previously described25,26.
tRNA electrophoretic mobility shift assay (EMSA)
The partial coding sequence of human SLFN11 (amino acids 1–579; SLFN11-N) and the coding sequence of GFP were cloned into the bacterial expression vector pET101/D-TOPO (Invitrogen) with a 6×His epitope tag. 6×His-tagged SLFN11-N and GFP proteins were expressed in Origami B Escherichia coli cells and purified over Ni-NTA and FPLC columns. The purity of the proteins was >95% as estimated by Coomassie blue staining. Total human tRNA was extracted with the mirVana miRNA Isolation kit (Ambion) following the small RNA isolation protocol, and further purified on a 15% denaturing polyacrylamide TBE-urea gel (Invitrogen). To generate EMSA probes, purified human tRNA was deacylated to remove charging amino acid, dephosphorylated and 5′-labelled using [32P]γ-ATP. Total yeast tRNA was purchased from Invitrogen. To prepare the HIV viral RNA, the sequence corresponding to the gag-pol frame-shifting region (2041–2161, pNL4-3.Luc.R+.E− vector) was amplified by PCR with T7 promoter-containing primers and in vitro transcribed using MEGAscript T7 kit (Ambion). Purified proteins, unlabelled competitor RNA or anti-SLFN11 antibody (Abmart), and probe were combined as specified and incubated on ice for 30 min in EMSA buffer (5% glycerol, 10 mM pH 7.4 Tris-HCl, 5 mM MgCl2, 100 mM KCl, 1% Triton X-100, 1 mM DTT and 1 U μl−1 RNasin Plus RNase Inhibitor). After samples were resolved on a 6% DNA retardation gel (Invitrogen), the gel was dried and visualized with Typhoon Trio Variable Mode Imager (storage phosphor mode).
Data analysis and presentation
Unless indicated otherwise, results are presented in graphs as average ± s.d. of at least three independent transfections or infections. For western blots, a representative of at least three independent transfections or infections is shown. For the experiments shown in Fig. 3d, e, GFP and EGFP protein and mRNA levels were quantified and adjusted to the corresponding GAPDH protein or mRNA concentrations, respectively. Expression levels of the proteins were then normalized to their corresponding standardized mRNA concentrations to compensate for minor variations in mRNA concentrations between the samples.
Supplementary Material
Supplementary Figure 1 – Relative expression and inducibility of huSlfns.
a) Human HFF cells were stimulated with IFNβ (5000 U/ml), pdAdT (1 μg/ml) or pIC (1 μg/ml) for 6 hours, and levels of the indicated mRNAs and TBP were determined by qPCR. The expression of the Slfn and ISG54 mRNAs are presented as ΔCt between TBP and the Slfn mRNA of interest.
b) Total cellular RNA was prepared from the indicated cell lines, and mRNA levels of the huSlfns and of TBP were quantitated by qPCR and presented as in a).
c) huSlfn11 mRNA levels in 293, 293shRNACtl, 293shRNASlfn and 293T cells as determined by qPCR;
d) Same as c), except huSlfn11 protein was visualized by immunoblotting of whole cell lysates.
Supplementary Figure 2 - huSlfn11 does not inhibit early viral infection steps.
293, 293shRNACtl, 293shRNASlfn cells were infected with pseudotyped HIV-1VSV-G virus at the indicated dilutions, and infection efficiency was determined by the expression levels of the transduced luciferase in the infected cells.
Supplementary Figure 3 – huSlfn11 inhibits production of MSCV but not AAV.
a) 293, 293shRNACtl, 293shRNASlfn and 293T cells were transfected with MSCV-IRES-GFP retrovirus vector and pCL-Eco ecotropic retrovirus packaging vector. Viral titers in the supernatants were determined by infection and subsequent flow-cytometric analysis of GFP expression of NIH3T3 cells.
b) 293T cells were transfected with MSCV-IRES-GFP retrovirus vector/pCL-Eco packaging vector and plasmids encoding either huSlfn5, huSlfn11, huSlfn11N, huSlfn11C or CAT as indicated. Virus production was assayed by titrated infection of NIH3T3 cells as in a).
c) 293, 293shRNACtl, 293shRNASlfn and 293T cells were transfected with pXX6, pXX2 and pACLALuc, and rAAVLuc production determined by luciferase assay following titrated infection.
Supplementary Figure 4 – huSlfn11 does not alter cytoplasmic vRNA levels or cause accumulation of viral particles.
a) 293T cells were transfected with pNL4-3.Luc.R+E− together with either huSlfn5, huSlfn11 or CAT. After cellular fractionation, cytoplasmic vRNA concentration was analyzed by qPCR;
b) 293T cells were transfected with HIV-1 pNL4-3.Luc.R+E− vector together with pcDNA6-CAT or pcDNA6-Slfn11. 48 hours later, the cells were fixed and images were acquired by electronic microscopy.
Supplementary Figure 5 – huSlfn11 inhibition is independent of viral enzymes or nuclear export of unspliced RNA.
a) 293T cells were co-transfected with HIV-1 pNL4-3.Luc.R+E−PRstop vector and either huSlfn5, huSlfn11 or CAT as indicated (left panels), or 293, 293shRNACtl and 293shRNASlfn cells were transfected with pNL4-3.Luc.R+E−PRstop vector (right panels). Whole cell lysates derived 48 hours after transfection were immunoblotted with anti-Gag and anti-GAPDH antibodies.
b) 293T cells were transfected with psPAX2 (encoding Gag, Pol, Tat & Rev without any LTR elements) and pNL-GFP-RRE(SA) (providing splicable RNA yielding GFP only if exported as unspliced RNA via Rev/RRE interaction) together with plasmids encoding either huSlfn5, huSlfn11, huSlfn11N, huSlfn11C or CAT as indicated. Whole cell lysates were immunoblotted with anti-GFP, anti-Gag and anti-GAPDH antibodies.
Supplementary Figure 6 – Non-discriminating tRNA-binding of huSlfn11.
Gel purified 32P-labeled total tRNA was incubated with increasing amounts of huSlfn11N to obtain approximate 50% shifting on a native gel. 32P-labeled tRNA from shifted and unshifted bands was recovered and samples hybridized on microarrays containing 96 probes (probes are repeated 8 times each and are complementary to human/mouse nuclear-encoded and mitochondrial-encoded tRNAs).
Supplementary Figure 7 – huSlfn11 inhibits HIV replication.
The pLKO.1 shRNA vector against Slfn11 (TRCN0000152057, CCGGCAGTCTTTGAGAGAGCTTATTCTCGAGAATAAGCTCTCTCAAAGACTGTTTTTTG) was used to independently create a second huSlfn11-deficient CEM cell line (CEMshRNASlfn in this figure). CEM, CEMshRNACtl and the new CEMshRNASlfn cells were infected with HIV-1LAI at an MOI of 0.01. The extent of viral replication was determined by HIV p24 ELISA of culture supernatants at indicated time points.
Acknowledgments
We thank D. Smith for help with the HIV replication studies; M. Wood for performing the electron microscope analysis; D. Xu and D.-Y. Song for technical assistance; and J. Young, J. Guatelli, D. Smith, M. Kaul and S. Chanda for discussion. This work was supported in part by NIH AI81019 and AI074967 to M.D.W., NIH P01AI090935, R01GM101982 and R21AI088490 to M.D., and by resources from the UCSD Center for AIDS Research, NIH P30AI36214, and the HINT Program, NIH P01AI090935. The authors declare no competing financial interests.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions M.L., M.D.W., T.P. and M.D. planned the experiments; M.L., E.K., X.G., M.P.-E., K.L., H.S., T.E.J. and S.L. conducted the experiments; M.L., T.P., M.D.W. and M.D. analysed the data; and M.L. and M.D. wrote the manuscript.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
References
- 1.Sohn WJ, et al. Novel transcriptional regulation of the schlafen-2 gene in macrophages in response to TLR-triggered stimulation. Mol Immunol. 2007;44:3273–3282. doi: 10.1016/j.molimm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Yoneyama M, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9:657–668. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- 4.Geserick P, Kaiser F, Klemm U, Kaufmann SH, Zerrahn J. Modulation of T cell development and activation by novel members of the Schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol. 2004;16:1535–1548. doi: 10.1093/intimm/dxh155. [DOI] [PubMed] [Google Scholar]
- 5.Berger M, et al. An Slfn2 mutation causes lymphoid and myeloid immunodeficiency due to loss of immune cell quiescence. Nature Immunol. 2010;11:335–343. doi: 10.1038/ni.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritz CC, Green MR. HIV Rev uses a conserved cellular protein export pathway for the nucleocytoplasmic transport of viral RNAs. Curr Biol. 1996;6:848–854. doi: 10.1016/s0960-9822(02)00608-5. [DOI] [PubMed] [Google Scholar]
- 7.Fischer U, Huber J, Boelens WC, Mattaj IW, Luehrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 8.Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 9.Van Damme N, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 11.Ganser-Pornillos BK, Yeager M, Sundquist WI. The structural biology of HIV assembly. Curr Opin Struct Biol. 2008;18:203–217. doi: 10.1016/j.sbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman JR, et al. Virus attenuation by genome-scale changes in codon pair bias. Science. 2008;320:1784–1787. doi: 10.1126/science.1155761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou T, Gu W, Ma J, Sun X, Lu Z. Analysis of synonymous codon usage in H5N1 virus and other influenza A viruses. Biosystems. 2005;81:77–86. doi: 10.1016/j.biosystems.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Meintjes PL, Rodrigo AG. Evolution of relative synonymous codon usage in human immunodeficiency virus type-1. J Bioinform Comput Biol. 2005;3:157–168. doi: 10.1142/s0219720005000953. [DOI] [PubMed] [Google Scholar]
- 15.Frelin L, et al. Codon optimization and mRNA amplification effectively enhances the immunogenicity of the hepatitis C virus nonstructural 3/4A gene. Gene Ther. 2004;11:522–533. doi: 10.1038/sj.gt.3302184. [DOI] [PubMed] [Google Scholar]
- 16.Forsberg R, Christiansen FB. A codon-based model of host-specific selection in parasites, with an application to the influenza A virus. Mol Biol Evol. 2003;20:1252–1259. doi: 10.1093/molbev/msg149. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin JB, Dushoff J. Codon bias and frequency-dependent selection on the hemagglutinin epitopes of influenza A virus. Proc Natl Acad Sci USA. 2003;100:7152–7157. doi: 10.1073/pnas.1132114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grantham P, Perrin P. AIDS virus and HTLV-I differ in codon choices. Nature. 1986;319:727–728. doi: 10.1038/319727b0. [DOI] [PubMed] [Google Scholar]
- 19.Wong EHM, Smith DK, Rabadan R, Peiris M, Poon LLM. Codon usage bias and the evolution of influenza A viruses. Codon usage biases of influenza virus. BMC Evol Biol. 2010;10:253. doi: 10.1186/1471-2148-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Weringh A, et al. HIV-1 modulates the tRNA pool to improve translation efficiency. Mol Biol Evol. 2011;28:1827–1834. doi: 10.1093/molbev/msr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kofman A, et al. HIV-1 gag expression is quantitatively dependent on the ratio of native and optimized codons. Tsitologiia. 2003;45:86–93. [PubMed] [Google Scholar]
- 22.Haas J, Park EC, Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996;6:315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- 23.Berkhout B, van Hemert FJ. The unusual nucleotide content of the HIV RNA genome results in a biased amino acid composition of HIV proteins. Nucleic Acids Res. 1994;22:1705–1711. doi: 10.1093/nar/22.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kypr J, Mrazek J. Unusual codon usage of HIV. Nature. 1987;327:20. doi: 10.1038/327020a0. [DOI] [PubMed] [Google Scholar]
- 25.Pavon-Eternod M, et al. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res. 2009;37:7268–7280. doi: 10.1093/nar/gkp787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dittmar KA, Mobley EM, Radek AJ, Pan T. Exploring the regulation of tRNA distribution on the genomic scale. J Mol Biol. 2004;337:31–47. doi: 10.1016/j.jmb.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Coccia EM, Krust B, Hovanessian AG. Specific inhibition of viral protein synthesis in HIV-infected cells in response to interferon treatment. J Biol Chem. 1994;269:23087–23094. [PubMed] [Google Scholar]
- 28.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 – Relative expression and inducibility of huSlfns.
a) Human HFF cells were stimulated with IFNβ (5000 U/ml), pdAdT (1 μg/ml) or pIC (1 μg/ml) for 6 hours, and levels of the indicated mRNAs and TBP were determined by qPCR. The expression of the Slfn and ISG54 mRNAs are presented as ΔCt between TBP and the Slfn mRNA of interest.
b) Total cellular RNA was prepared from the indicated cell lines, and mRNA levels of the huSlfns and of TBP were quantitated by qPCR and presented as in a).
c) huSlfn11 mRNA levels in 293, 293shRNACtl, 293shRNASlfn and 293T cells as determined by qPCR;
d) Same as c), except huSlfn11 protein was visualized by immunoblotting of whole cell lysates.
Supplementary Figure 2 - huSlfn11 does not inhibit early viral infection steps.
293, 293shRNACtl, 293shRNASlfn cells were infected with pseudotyped HIV-1VSV-G virus at the indicated dilutions, and infection efficiency was determined by the expression levels of the transduced luciferase in the infected cells.
Supplementary Figure 3 – huSlfn11 inhibits production of MSCV but not AAV.
a) 293, 293shRNACtl, 293shRNASlfn and 293T cells were transfected with MSCV-IRES-GFP retrovirus vector and pCL-Eco ecotropic retrovirus packaging vector. Viral titers in the supernatants were determined by infection and subsequent flow-cytometric analysis of GFP expression of NIH3T3 cells.
b) 293T cells were transfected with MSCV-IRES-GFP retrovirus vector/pCL-Eco packaging vector and plasmids encoding either huSlfn5, huSlfn11, huSlfn11N, huSlfn11C or CAT as indicated. Virus production was assayed by titrated infection of NIH3T3 cells as in a).
c) 293, 293shRNACtl, 293shRNASlfn and 293T cells were transfected with pXX6, pXX2 and pACLALuc, and rAAVLuc production determined by luciferase assay following titrated infection.
Supplementary Figure 4 – huSlfn11 does not alter cytoplasmic vRNA levels or cause accumulation of viral particles.
a) 293T cells were transfected with pNL4-3.Luc.R+E− together with either huSlfn5, huSlfn11 or CAT. After cellular fractionation, cytoplasmic vRNA concentration was analyzed by qPCR;
b) 293T cells were transfected with HIV-1 pNL4-3.Luc.R+E− vector together with pcDNA6-CAT or pcDNA6-Slfn11. 48 hours later, the cells were fixed and images were acquired by electronic microscopy.
Supplementary Figure 5 – huSlfn11 inhibition is independent of viral enzymes or nuclear export of unspliced RNA.
a) 293T cells were co-transfected with HIV-1 pNL4-3.Luc.R+E−PRstop vector and either huSlfn5, huSlfn11 or CAT as indicated (left panels), or 293, 293shRNACtl and 293shRNASlfn cells were transfected with pNL4-3.Luc.R+E−PRstop vector (right panels). Whole cell lysates derived 48 hours after transfection were immunoblotted with anti-Gag and anti-GAPDH antibodies.
b) 293T cells were transfected with psPAX2 (encoding Gag, Pol, Tat & Rev without any LTR elements) and pNL-GFP-RRE(SA) (providing splicable RNA yielding GFP only if exported as unspliced RNA via Rev/RRE interaction) together with plasmids encoding either huSlfn5, huSlfn11, huSlfn11N, huSlfn11C or CAT as indicated. Whole cell lysates were immunoblotted with anti-GFP, anti-Gag and anti-GAPDH antibodies.
Supplementary Figure 6 – Non-discriminating tRNA-binding of huSlfn11.
Gel purified 32P-labeled total tRNA was incubated with increasing amounts of huSlfn11N to obtain approximate 50% shifting on a native gel. 32P-labeled tRNA from shifted and unshifted bands was recovered and samples hybridized on microarrays containing 96 probes (probes are repeated 8 times each and are complementary to human/mouse nuclear-encoded and mitochondrial-encoded tRNAs).
Supplementary Figure 7 – huSlfn11 inhibits HIV replication.
The pLKO.1 shRNA vector against Slfn11 (TRCN0000152057, CCGGCAGTCTTTGAGAGAGCTTATTCTCGAGAATAAGCTCTCTCAAAGACTGTTTTTTG) was used to independently create a second huSlfn11-deficient CEM cell line (CEMshRNASlfn in this figure). CEM, CEMshRNACtl and the new CEMshRNASlfn cells were infected with HIV-1LAI at an MOI of 0.01. The extent of viral replication was determined by HIV p24 ELISA of culture supernatants at indicated time points.