Abstract
Elucidating interrelations between prior affective experience, current affective state, and acute urge to smoke could inform affective models of addiction motivation and smoking cessation treatment development. This study tested the hypothesis that prior levels of positive (PA) and negative (NA) affect predict current smoking urge via a mediational pathway involving current state affect. We also explored if tobacco deprivation moderated affect-urge relations and compared the effects of PA and NA on smoking urge to one another. At a baseline session, smokers reported affect experienced over the preceding few weeks. At a subsequent experimental session, participants were randomly assigned to 12-hr tobacco deprived (n = 51) or nondeprived (n = 69) conditions and reported state affect and current urge. Results revealed a mediational pathway whereby prior NA reported at baseline predicted state NA at the experimental session, which in turn predicted current urge. This mediational pathway was found primarily for an urge subtype indicative of urgent need to smoke and desire to smoke for NA relief, was stronger in the deprived (vs. nondeprived) condition, and remained significant after controlling for PA. Prior PA and current state PA were inversely associated with current urge; however, these associations were eliminated after controlling for NA. These results cohere with negative reinforcement models of addiction and with prior research and suggest that: (a) NA plays a stronger role in smoking motivation than PA; (b) state affect is an important mechanism linking prior affective experience to current urge; and (c) affect management interventions may attenuate smoking urge in individuals with a history of affective disturbance.
Keywords: positive affect, negative affect, urge, smoking, craving
Negative reinforcement models of addiction purport that affective disturbance is a key precipitant of smoking in tobacco dependent individuals (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). This notion has often been explored by focusing on negative affect and its impact on urge to smoke (Brandon, 1994).1 However, multifactorial models of affect purport that positive (PA) and negative (NA) affect are distinguishable constructs (Watson & Tellegen, 1985) rather than elements of a common dimension. PA involves the experience of positive emotions (e.g., joy, interest, and alertness), whereas NA involves aversive emotions (e.g., sadness, irritability, and anxiety). Thus, affect disturbance may reflect low PA, high NA, or their combination. An emerging literature illustrates that low PA and high NA are associated with higher smoking urge (Brandon, Wetter, & Baker, 1996; Cook, Spring, Mcchargue, & Hedeker, 2004; Leventhal, 2010; Robinson et al., 2011; Sherman, Morse, & Baker, 1986; Zinser, Baker, Sherman, & Cannon, 1992; though there are some reports of positive associations between PA and urge, e.g., nondeprived smokers in Zinser et al., 1992). To further isolate the role of affect in smoking urge, several aspects of this relation require further attention.
First, it is known that prior affective disturbance (e.g., history of depression) can predict current acute smoking urge (e.g., Covey, Glassman, & Stetner, 1990); yet whether momentary affective processes mediate this relation is unclear. It is possible that measures of prior affective disturbance may be a proxy for other nonaffective social, environmental, or intrapersonal factors that directly influence smoking motivation and operate independently of state affect (Chuang, Cubbin, Ahn, & Winkleby, 2005; Minnix, Blalock, Marani, Prokhorov, & Cinciripini, 2011). Alternatively, there may be a functional relation between prior affect and current urge mediated by state affective processes. That is, smokers who experienced higher affective disturbance in the past may be more likely to experience future states of high NA or low PA, and these acute affective states may give rise to momentary smoking urges.
Second, extant urge research has examined PA and NA in isolation from one another, hence ignoring their overlapping variance. This is an important oversight because if only one form of affect predicts smoking urge after partialing out the covariance between PA and NA, that form of affect may comparatively be a more influential precipitant of smoking motivation.
Third, the effect of abstinence on affect-urge relations is not entirely clear. Tiffany’s (1990) cognitive model of urge purports that, in established users, substance use motivation remains unconscious in circumstances when typical use patterns are uninterrupted. Yet, when interrupted by abstinence, the user becomes conscious of their drug use motivation, which manifests as a subjective urge state. Thus, the relation between affect and urge may become stronger in states of abstinence when smokers may be more aware of their motivation to smoke.
Lastly, prior work has focused on unidimensional measures of smoking urge. Multidimensional measures that distinguish between subtypes of smoking urge (e.g., desire to smoke and anticipation of pleasure from smoking vs. urgent need to smoke and anticipation of negative affect relief from smoking; Cox, Tiffany, & Christen, 2001), are useful for distilling phenomenological aspects of the subjective urge experience and may further elucidate the motivational basis of affective influences on smoking (Baker, Morse, & Sherman, 1986).
This study tested the hypothesis that affect over the preceding few weeks reported at a baseline visit predicts acute smoking urge reported at an ensuing experimental visit and that this relation is mediated by state affect during the experimental visit. We predicted that this mediation pathway is stronger when state affect and urge are intensified by tobacco deprivation.
Method
Participants were current smokers attending a southwestern university recruited to participate in a study of the effects of tobacco deprivation (Leventhal et al., 2008). Institutional Review Boards at the investigator-affiliated universities approved the protocol. Participants were included in the study only if they were aged ≥ 18 and reported smoking 8 + cigarettes per day on average for the past 2 years to enhance generalization to target population of moderate-to-heavy smokers sensitive to deprivation (Leventhal et al., 2008). Breath carbon monoxide (CO) was not used to determine study eligibility. Those who planned to quit in the next 30 days, were cutting down substantially, or were using nicotine replacement therapy were excluded.
Following an initial eligibility screen, participants were invited to attend a baseline session at which they provided informed consent, were explained the study procedures, and completed baseline questionnaires. Participants were then randomized to be either nondeprived or deprived for a subsequent experimental session. Nondeprived participants were asked to smoke normally prior to their experimental session and to smoke one cigarette within 30 minutes of the session. Deprived participants were asked to abstain from smoking for at least 12 hours prior to the experimental session. Experimental sessions were conducted within two weeks of the baseline session and took place during the afternoons (12:00 –2:00 p.m.). Breath CO levels were measured at the beginning of the experimental session to verify if participants complied with smoking instructions. Subjects then completed 30 minutes of cognitive tasks measuring processing of smoking-related and affective cues using novel subliminal priming methods (reported elsewhere, Leventhal et al., 2008), followed by state affect and current urge questionnaires. Upon study completion, subjects received course credit and a $15 gift card.
A subset (n = 50) of the 212 smokers who completed a baseline session was lost to follow up prior to the experimental session. Participants at the experimental session whose biochemical confirmation indicated noncompliance (n = 42) of either smoking in the nondeprived group (CO ≥ 9 ppm) or abstinence in the deprived group (CO < 9 ppm) were excluded from analyses.2 The final sample for analyses included 69 nondeprived and 51 deprived participants. Tests of differences by compliance status indicated deprived participants not meeting biochemical abstinence criteria were more likely to be male, heavier, and more dependent smokers than those who met biochemical abstinence criteria. Baseline NA and PA were not significantly different between groups. The significance of each analytic test of our key hypotheses did not change when both compliant and noncompliant participants were included in the analyses. Therefore, the analyses herein utilize the sample of compliant study completers.
Measures
Baseline session
Prior affect was measured with a version of the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) asking participants to rate emotional experience during the “past few weeks” on a 5-point scale. The PANAS includes two 10-item subscales used to measure PA (e.g., “excited,” “interested,” “proud”; Cronbach’s α in this sample = .92) and NA (e.g., “irritable,” “afraid,” “distressed”; α = .86). The PANAS has shown excellent psychometric properties (Watson et al., 1988). An author-constructed questionnaire was used to collect information on years of smoking, number of cigarettes smoked per day, and other characteristics. Nicotine dependence was measured using the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), a widely used, well-validated, 6-item measure of gradations in nicotine dependence severity.
Experimental session
State affect was measured using a version of the 20-item PANAS, which asks participants to report affect “so far today” in order to capture the experience on the day of the experimental session leading up to urge assessment. Current urge was measured using the Questionnaire on Smoking Urges-Brief (QSU; Cox et al., 2001), a 10-item measure asking participants to respond to statements reflecting urge to smoke according to how they feel “right now” on a 6-point scale (0 = strongly disagree to 6 = strongly agree). The QSU provides two distinct 5-item factor scores. Factor 1 (F1) evaluates intention and desire to smoke and anticipation of pleasure from smoking (e.g., “A cigarette would taste good”; “I have a desire to smoke a cigarette”). Factor 2 (F2) assesses urgent need to smoke and anticipation of relief from NA (e.g., “I would do almost anything for a cigarette”; “Smoking would make me less depressed”). Previous data supports the QSU’s two-factor structure (Cox et al., 2001). The internal consistency of both factor scores in this sample was high (F1: α = .94; F2: α = .90). State affect and current urge were measured at a single time point after the tasks.
Data Analysis Plan
Initial models
To examine the link between prior affect and current urge, we calculated linear regression models testing the relation of baseline prior affect (“over the past few weeks”) to experimental session current urge (“right now”; Path “c,” Figure 1). We first tested prior NA and PA in separate models that included only deprivation and a single affect scale as the sole predictors, with an urge scale as the sole outcome. Then, additional models examined the unique versus overlapping roles of prior PA and NA by including deprivation, PA, and NA as concomitant predictors. Finally, we tested a model that added the PA × deprivation interaction term and a separate model that added the NA × deprivation interaction term (Path “d,” Figure 1). Separate analyses were calculated for QSU-F1 and -F2 scales. To examine the link between state affect and current urge, we repeated each of the analyses described above with the only difference being that we substituted the experimental session state PANAS measures (“so far today”) for the baseline session prior PANAS measures (Paths “b” and “e,” Figure 1).
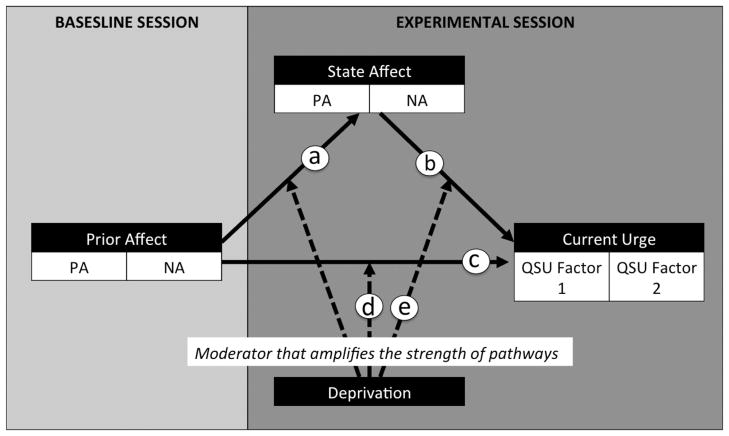
Figure 1.
Visual schematic of the focal pathways in this study. Path “c” represents the effect of baseline prior affect on experimental session current urge. Path “a” represents the effect of baseline prior affect on experimental session state affect (not analyzed). Path “b” represents the effect of experimental session state affect on experimental session current urge. Paths “a” and “b” combined to comprise a mediational pathway to explain the indirect effect by which in path “c” (the effect of baseline prior affect on experimental session current urge) operates. The arrows from the deprivation variable (deprived vs. nondeprived) to each of the pathways (depicted by broken lines; “d” and “e”) illustrate that deprivation is considered a moderator that potentially amplifies the strength of each affect-urge path. PA = Positive Affect; NA = Negative Affect; QSU = Questionnaire of Smoking Urge-Brief.
Mediational models
Cases in which paths “c” and “b” were both significant, we tested a mediational model (prior affect → state affect → current urge). Mediational paths were analyzed by computing the product of the coefficients from the “a” path (prior affect → state affect) and “b” paths (state affect → current urge), which served as the indicator of the strength of the indirect effect. Significance was determined using the PRODCLIN approach involving estimation of asymmetric confidence intervals (CIs) around the mediational effect (MacKinnon, Fritz, Williams, & Lockwood, 2007). The relation between prior affect and state urge after adjusting for state affect indicated the remaining direct effect. In cases of both significant mediation and moderation by deprivation status, we tested for moderated mediation. Here, we examined mediation in sub-samples stratified by deprivation group. If the 95% confidence intervals (CIs) of the mediational effect estimates do not overlap, the strength of the mediational effect differs by deprivation status, providing evidenced for moderated mediation.
All results are reported as standardized regression coefficients (β). Because of the multiple tests performed, significance was set to p < .01 in all analyses.
Results
Preliminary Analyses
The descriptive statistics of key variables and their intercorrelations are reported in Table 1. Additional analyses that controlled for demographic variables and FTND score did not change the significance of results; thus, we report unadjusted results for the primary analyses. Between-group ANOVAs for experimental session CO levels, state affect, and current urge indicated expected significant group differences by deprivation status [deprived versus nondeprived, M(SD); CO: 4.8 (2.0) versus 21.0 (8.7); State PA: 2.4 (1.0) versus 3.1 (0.9); State NA: 2.1 (0.8) versus 1.6 (0.6); QSU-F1: 4.0 (1.2) versus 2.9 (1.3); QSU-F2: 2.3 (1.5) versus 1.2 (1.0); all ps < .01].
Table 1.
Descriptive Statistics and Intercorrelation of Study Variables
| Variable | M (SD) or % | Range | Intercorrelations (r)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| 1. Prior PAa,c | 3.2 (0.9) | 1–5 | — | ||||||||||
| 2. Prior NAa,c | 2.3 (0.8) | 1–4.5 | −.44*** | — | |||||||||
| 3. State PAa,d | 2.8 (1.0) | 1–4.9 | .65*** | −.35*** | — | ||||||||
| 4. State NAa,d | 1.8 (7.2) | 1–4.6 | −.33*** | .70*** | −.39*** | — | |||||||
| 5. QSU-F1b,d | 3.4 (1.4) | 0–5 | −.15 | .26** | −.25** | .30*** | — | ||||||
| 6. QSU-F2b,d | 1.7 (1.4) | 0–5 | −.26** | .37*** | −.36*** | .52*** | .70*** | — | |||||
| 7. FTNDc | 3.7 (2.0) | 0–9 | −.14 | .13 | −.05 | .05 | .20* | .22* | — | ||||
| 8. Cig/dayc | 14.7 (4.9) | 8–40 | .05 | −.06 | −.06 | −.13 | .02 | −.10 | .43*** | — | |||
| 9. Caucasianc | 66% | — | .09e | .07e | .07e | −.01e | −.02e | −.14e | −.06e | .15e | — | ||
| 10. Femalec | 63% | — | −.02 | .33***e | −.08e | .30***e | .06e | .04e | .01e | −.10e | .19*f | — | |
| 11. Agec | 24.5 (6.6) | 18–47 | .18* | −.18* | .25** | −.15 | −.12 | −.16 | .05 | .15 | −.09e | −.01e | — |
Note. Sample size varies across analyses (Ns 117–120 due to missing data). PA = Positive Affect; NA = Negative Affect; QSU = Questionnaire of Smoking Urge-Brief; FTND = Fagerström Test of Nicotine Dependence.
Average score per item (possible range 1–5).
Average score per item (possible range 0–5).
Measured at baseline session.
Measured at experimental session.
Point-biserial correlation.
Spearman’s rho correlation.
p < .05.
p <.01.
p <.001.
Baseline Prior Affect and Experimental Session Current Urge (Paths “c” and “d” Figure 1)
In contrast with unadjusted bivariate correlations (see Table 1), prior PA and NA were not significantly associated with QSU-F1 after accounting for variance due to deprivation status. For QSU-F2, prior PA was related to lower urge (β = −.23, p < .01) and prior NA was associated with higher urge (β =.32, p < .0001). After entering both predictors into a combined model, NA retained a significant effect (β = .27, p < .01) but PA did not. Deprivation did not significantly moderate the association between prior NA and PA to QSU-F1 or the relation between PA and QSU-F2. Yet, deprivation significantly moderated the relation between NA and QSU-F2, with and without controlling for prior PA (βs ≥ .20, ps ≤ .01). The interaction signified that higher NA was associated with higher urge in deprived participants (β-unadjusted = .51, p = .0001; β-adjusted for prior PA = .45, p = .002) but not in nondeprived participants (βs ≤ .12, ps ≥ .19).
Experimental Session State Affect and Current Urge (Paths “b” and “e” Figure 1)
State PA and NA were not significantly associated with QSU-F1. Lower state PA (β = −.27, p < .01) and higher state NA (β = .44, p < .0001) significantly predicted higher urge on the QSU-F2. After entering both predictors simultaneously into a combined model, NA retained a significant effect (β = .44, p < .0001), but PA did not. Deprivation did not significantly moderate the relation of state NA or PA to either measure of urge.
Baseline Prior NA → Experimental Session State NA → Experimental Session Urge
The indirect (“mediational”) effect from prior NA → state NA → QSU-F2 was significant (β = .29, p < .0001). The remaining direct effect of prior NA on QSU-F2 over and above the indirect effect was nonsignificant (β = .06, p = .56), indicating full mediation. Effects were similar when adjusting for prior and state PA (β-indirect = .25, p < .0001, β-direct = .03, p = .79).
We also tested for moderated mediation to identify whether the mediation pathway from prior NA → state NA → QSU-F2 differed as a function of deprivation status. The mediational effect in deprived participants was significant (β-indirect [95% CI] = .47 [.26−.71], p < .0001). The mediational effect in nondeprived participants was also significant but smaller in magnitude (β-indirect [95% CI] = .13 [.03 - .24], p = .007). Because the two 95% CIs do not overlap, we can conclude that there was statistically significant moderated mediation.
Discussion
This study explored the interrelations between prior affect, state affect, and current smoking urge and yielded different findings for PA and NA. PA was inversely associated only with a subtype of urge tapping urgent need to smoke and anticipation of relief from NA. However, these results did not remain after controlling for variation in NA. These results extend past research showing a PA-urge link (Robinson et al., 2011; Sherman et al., 1986; Zinser, Baker, Sherman, & Cannon, 1992) by suggesting that PA does not have incremental predictive validity over and above NA.
Regarding NA, the data supported a mediational pathway whereby higher baseline levels of NA experienced over the preceding few weeks predicted higher levels of state NA experienced the day of the experimental session, which in turn predicted higher levels of acute urges at the experimental session.3 There was no evidence of a direct pathway from prior NA to urge that was separable from the intermediate pathway involving state NA (i.e., full mediation). These results suggest that people who have a history of high NA may be prone to experiencing momentary episodes of smoking urge primarily because they are more likely to experience more frequent states of high NA that quickly give rise to acute smoking motivation (Gilbert, 1997). The NA-urge association was only found for the subtype of urge reflecting urgent need to smoke and desire to smoke to alleviate NA, and remained significant after controlling for PA. This pattern is consistent with the notion that NA per se, as opposed to nonspecific affective disturbance, underlies motivation to smoke and prior NA impacts smoking via affect-mediated negative reinforcement processes (Baker et al., 2004).
The prior NA → state NA → current urge mediational pathway was moderated by deprivation, such that this mediational effect was more robust when state NA and urge were assessed under nicotine deprived (vs. nondeprived) conditions. One interpretation of this finding is that smoking motivation processes that may normally be preconscious for some smokers when satiated become conscious upon abstinence and manifest as subjective urge (Tiffany, 1990). As a result, relations between NA and self-report indices of drug use motivation, such as subjective urge, may be more robust during acute abstinence (Baker et al., 2004). Another interpretation of this finding is that individuals with a high NA tend to experience more acute NA in response to stressors. Thus, when faced with the stress of tobacco deprivation these individuals may experience acute states of increased NA and will have stronger urges to smoke to counteract NA when abstinent (Gilbert, 1997). Alternatively, smokers who have been experiencing more NA in the preceding weeks may have biological diatheses that make them more sensitive to nicotine-induced neuroadaptations in emotional processing pathways. Thus, these smokers might experience elevated levels of acute NA during nicotine withdrawal, which in turn could enhance urge to smoke (Gilbert & Gilbert, 1998).
This study should be interpreted within the context of its limitations. First, the sample lacked CO testing during eligibility, which leaves open the possibility that some individuals overreported their smoking. Second, the sample was mostly restricted to young adult smokers with only moderate levels of smoking severity. It is, therefore, unclear whether these findings would extend to older, more severely dependent adults. Third, data from some participants were excluded because these individuals exhibited high (deprived group) or low (nondeprived group) CO levels at the experimental session, respectively. This procedure increases the likelihood that the smokers in the final sample had indeed smoked normally (nondeprived) or remained abstinent (deprived); however, it likely caused the nondeprived group to be comprised of more severely dependent and older smokers than the deprived group, presumably because more dependent smokers in the latter group failed to maintain abstinence. Concern whether this factor influenced the primary findings is offset because including all participants did not alter primary analyses and CO verification status did not associate with affect. Fourth, power may have been comparatively lower for the moderated mediation analyses in comparison to the other analyses performed; yet, this concern is somewhat lessened given that a statistically significant moderated mediation effect was found for NA. Finally, none of the participants were interested in quitting. Thus, it is unclear whether these findings will generalize to smokers attempting to quit.
In sum, results from this study point toward the possibility that NA is more important to the etiology of smoking urge than PA. These findings also clarify that state affect may be a key mechanism linking affect disturbance over a preceding time period to current smoking urges and suggest that affect-urge relations are intensified during acute tobacco abstinence. If extended to clinical settings, these findings could have important implications for smoking cessation treatment. For instance, it may behoove clinicians to assess prior levels of NA before quit day to identify patients who may experience extreme states of NA and more severe smoking urges early in a quit attempt. Additionally, smokers with a history of high NA may require intensive intervention to counteract acute NA and smoking urges early in the cessation process in order to prevent rapid relapse. Hence, continued investigation of temporal and mediational factors underpinning the affect-smoking urge link could yield significant scientific and clinical benefits.
Acknowledgments
This research was supported by NIDA grants DA026831 and DA025041 and NCI grant CA57730, and the funding source had no other role other than financial support. All authors contributed in a significant way to the manuscript and have read and approved the final manuscript. The authors would like to acknowledge Elizabeth Miller, Evelina Tapia, and Dr. Andrew Waters who were instrumental to collecting these data.
Footnotes
Although the terms urge and craving have sometimes been used to refer to different concepts (Kozlowski & Wilkinson, 1987), we use the term urge to refer to any subjective urge, craving, or desire to use a drug in the current article.
The CO cutoff of 9 ppm was based on Society for Nicotine and Tobacco Research Subcommittee on Biochemical Verification of Tobacco Use published guidelines. A previous similarly designed study utilized this CO cutoff (Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010; SRNT, 2002). However, this particular cutoff has not been empirically validated to specifically distinguish between overnight abstinence vs. smoking within the past 30 minutes, which leaves open the possibility of classification error for some participants.
Both the QSU-F2 and PANAS-NA scales contain items with NA phrases, which raises the possibility that content overlap between the scale could account for their relations. However, upon inspection of the QSU-F2, the only affect-related item is “Smoking would make me less depressed,” yet there is no item on the PANAS that includes the adjective “depressed.” Thus, although there may be construct overlap as one of the items on the QSU-F2 measures smoking to alleviate NA, and the PANAS-NA scale measures NA per se, there is no direct content overlap in terms of text or specific type of NA emotion on the two scales. In addition, we conducted supplemental analyses, which omitted the item “Smoking would make me less depressed,” from the QSU-F2 scale resulting in a 4-item scale with no NA terminology. The statistical significance of all the analyses involving state and trait PANAS-NA in relation to QSU-F2 were identical across models that utilized the full 5-item or the abbreviated 4-item QSU-F2.
None of the authors report a conflict of interest related to the submission of this article.
Contributor Information
Adam M. Leventhal, Departments of Preventive Medicine and Psychology, University of Southern California Keck School of Medicine
Jodie B. Greenberg, Department of Preventive Medicine, University of Southern California Keck School of Medicine
Michael A. Trujillo, Department of Preventive Medicine, University of Southern California Keck School of Medicine
Katherine J. Ameringer, Department of Preventive Medicine, University of Southern California Keck School of Medicine
Nadra E. Lisha, Department of Psychiatry, University of California, San Francisco
Raina D. Pang, Department of Preventive Medicine, University of Southern California Keck School of Medicine
John Monterosso, Department of Psychology, University of Southern California.
References
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. Nebraska Symposium on Motivation. 1986;34:257–323. [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.332004-10332-002[pii]. [DOI] [PubMed] [Google Scholar]
- Brandon T. Negative affect as motivation to smoke. Current Directions in Psychological Science. 1994;3:33–37. doi: 10.1111/1467-8721.ep10769919. [DOI] [Google Scholar]
- Brandon TH, Wetter DW, Baker TB. Affect, expectancies, urges, and smoking: Do they conform to models of drug motivation and relapse? Experimental and Clinical Psychopharmacology. 1996;4:29–36. doi: 10.1037/1064-1297.4.1.29. [DOI] [Google Scholar]
- Chuang YC, Cubbin C, Ahn D, Winkleby MA. Effects of neighbourhood socioeconomic status and convenience store concentration on individual level smoking. Journal of Epidemiology and Community Health. 2005;59:568–573. doi: 10.1136/jech.2004.029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, Mcchargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990;31:350–354. doi: 10.1016/0010-440X(90)90042-Q. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Gilbert D. The situation x trait adaptive response model of drug use, effects, and craving. Human Psychopharmacology: Clinical and Experimental. 1997;12:S89–S102. doi: 10.1002/(SICI)1099-1077(199706)12. 2+<S89::AID-HUP906>3.0.CO;2-P. [DOI] [Google Scholar]
- Gilbert D, Gilbert B. Nicotine and the situation by trait adaptive response (STAR) model: Emotional states and information processing. In: Snel J, Loirst M, editors. Nicotine, caffeine and social drinking: Behaviour and brain function. Amsterdam, The Netherlands: Harwood Academic Publishers; 1998. pp. 131–149. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Wilkinson DA. Use and misuse of the concept of craving by alcohol, tobacco, and drug researchers. British Journal of Addiction. 1987;82:31–36. doi: 10.1111/j.1360-0443.1987.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Leventhal AM. Do individual differences in reinforcement smoking moderate the relationship between affect and urge to smoke? Behavioral Medicine. 2010;36:1–6. doi: 10.1080/08964280903521347. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Breitmeyer BG, Miller EK, Tapia E, Li Y. Subliminal processing of smoking-related and affective stimuli in tobacco addiction. Experimental and Clinical Psychopharmacology. 2008;16:301–312. doi: 10.1037/a0012640. 2008-10619-004[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors. 2010;35:1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39:384–389. doi: 10.3758/BF03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnix JA, Blalock JA, Marani S, Prokhorov AV, Cinciripini PM. Self-efficacy mediates the effect of depression on smoking susceptibility in adolescents. Nicotine & Tobacco Research. 2011;13:699–705. doi: 10.1093/ntr/ntr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Lam CY, Carter BL, Minnix JA, Cui Y, Versace F, Cinciripini PM. A multimodal approach to assessing the impact of nicotine dependence, nicotine abstinence, and craving on negative affect in smokers. Experimental and Clinical Psychopharmacology. 2011;19:40–52. doi: 10.1037/a0022114. 2011-03057-005[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JE, Morse M, Baker TB. Urges/craving to smoke: Preliminary results from withdrawing and continuing smokers. Advances in Behaviour Research & Therapy. 1986;8:253–269. doi: 10.1016/0146-6402(86)90008-1. [DOI] [Google Scholar]
- Society for Research on Nicotine, Tobacco Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295X.97.2.147. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–235. doi: 10.1037/0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of Abnormal Psychology. 1992;101:617–629. doi: 10.1037/0021-843X.101.4.617. [DOI] [PubMed] [Google Scholar]