Abstract
Ethnopharmacological relevance
Our previous work has demonstrated that several plants in the Piperaceae family are commonly used by the Q’eqchi Maya of Livingston, Guatemala to treat amenorrhea, dysmenorrhea, and pain. Extracts of Piper hispidum Swingle (Piperaceae), bound to the estrogen (ER) and serotonin (5-HT7) receptors.
Aim of the study
To investigate the estrogenic and serotonergic activities of P. hispidum extracts in functionalized assays, identify the active chemical constituents in the leaf extract, and test these compounds as agonists or antagonists of ER and 5-HT7.
Materials and methods
The effects of the P. hispidum leaf extracts were investigated in estrogen reporter gene and endogenous gene assays in MCF-7 cells to determine if the extracts acted as an estrogen agonist or antagonist. In addition, the active compounds were isolated using ER- and 5-HT7 receptor bioassay-guided fractionation. The structures of the purified compounds were identified using high-resolution LC-MS and NMR spectroscopic methods. The ER- and 5-HT7-agonist effects of the purified chemical constituents were tested in a 2ERE-reporter gene assay in MCF-7 cells and in serotonin binding and functionalized assays.
Results
Three butenolides including one new compound (1) were isolated from the leaves of P. hispidum, and their structures were determined. Compound 1 bound to the serotonin receptor 5-HT7 with IC50 values of 16.1 and 8.3 μM, respectively, and using GTP shift assays, compound 1 was found to be a partial agonist of the 5-HT7 receptor. The P. hispidum leaf extracts, as well as compounds 2 and 3 enhanced the expression of estrogen responsive reporter and endogenous genes in MCF-7 cells, demonstrating estrogen agonist effects.
Conclusions
Extracts of P. hispidum act as agonists of the ER and 5-HT7 receptors. Compound 1, a new natural product, identified as 9, 10-methylenedioxy-5,6-Z-fadyenolide, was isolated as the 5-HT7 agonist. Compounds 2 and 3 are reported for the first time in P. hispidum, and identified as the estrogen agonists. No inhibition of CYP450 was observed for any of these compounds in concentrations up to 1 μM. These activities are consistent with the Q’eqchi traditional use of the plant for the treatment of disorders associated with the female reproductive cycle.
Keywords: Piper hispidum; Butenolides; Estrogen agonist; Maya; 9, 10-Methylenedioxy-5, 6-Z-fadyenolide; Serotonin agonist
1. Introduction
The genus Piper belongs to the family Piperaceae and consists of approximately 1,300 species in the Neotropics and an estimated 700 species in the Old World tropics (Quijano-Abril et al., 2006). Throughout the tropics, various Piper species are used for many purposes such as foods, spices, perfumes, oils, fish poisons, insecticides, hallucinogens and medicines (Barrett, 1994; Sengupta and Ray, 1987). In Latin America, Piper species have been used to treat a variety of gynecological ailments as well as gastrointestinal problems, depression, anxiety, pain and inflammation, as well as bacterial and fungal infections (Michel et al., 2007).
Piper hispidum Swingle, a shrub native to the lowlands of Mexico, is a species of pan-tropical distribution, commonly found throughout both disturbed and forest sites (Michel et al., 2007; Mooney et al., 1983; Wadt et al., 2004). Also commonly known as “cordoncillo”, previous research has reported the use of P. hispidum to treat aches and pains in Nicaragua (Coe and Anderson, 1996), to regulate menstruation in Peru, and to treat urinary infections in the Amazon (Duke and Vásquez, 1994). In Peru, the leaves of the plant are also traditionally used by the Chayahuitas, an Amazonian Peruvian ethnic group, to heal wounds and to treat symptoms of cutaneous leishmaniasis (Estevez et al., 2007). In terms of chemical constituents, the plant is reported to contain amides, benzenes, benzoic acids, flavonoids and volatile oils, of which had significant antifungal, antimicrobial, antiplasmodial, leishmanicidal and insecticidal activities (Alecio et al., 1998; Burke and Nair, 1986; Friedrich et al., 2005; Jenett-Siems et al, 1999; Machado et al., 1994; Nair et al., 1986; Nair and Burke, 1990; Navickiene et al., 2000; Vieira et al., 1980).
In our previous works, we reported that Q’eqchi Maya healers, midwives, and community members in Livingston, Guatemala use the leaves of P. hispidum to prepare a tea for the treatment of female reproductive disorders including amenorrhea, dysmenorrhea, menopause and pain (Michel et al., 2007). Furthermore, extracts of the leaves was found to bind in vitro to both the estrogen receptors (ERα and ERβ) and the serotonin receptors 5-HT1A and 5-HT7 (Michel et al., 2007). Continuing with this international collaboration, the aim of the present study was to isolate and identify the chemical constituents of the extract that bind to the ER and serotonin receptors 5-HT1A and 5-HT7, and determine if the extract and pure compounds act as an agonist or antagonist of the ER- and 5-HT7 receptor in functionalized assays, thereby providing support data for the traditional ethnomedical uses of P. hispidum. In this work, we have isolated three pure compounds including a new butenolide from P. hispidum from using bioassay-guided fractionation. Compound 1, a new natural product, identified as 9, 10-methylenedioxy-5,6-Z-fadyenolide, was isolated as the 5-HT7 agonist. Compounds 2 and 3 are reported for the first time in P. hispidum, and identified as the estrogen agonists.
2. Materials and methods
2.1 International Agreements
This research was part of a collaborative project between The University of Illinois at Chicago (UIC), USA and the University of San Carlos (Universidad de San Carlos), Guatemala City. The work was performed under a Memorandum of Understanding (MOU), set down by the University of Illinois at Chicago (UIC) as previously described (Michel et al., 2007). Prior to initiating research, Institutional Review Board approval was granted by the University of Illinois at Chicago and plant collection permits were obtained from the Guatemalan government.
2.2. Plant materials
The leaves of Piper hispidum were collected in June, 2005 from Livingston, Izabal, Guatemala. The plant materials were identified by Mario Veliz and Djaja D Soejarto, and the voucher specimen (No. JM32) was deposited at both the BIGUA Herbarium in Guatemala City and the John G. Searle Herbarium at Chicago, Illinois, USA as previously described (Michel et al., 2007).
2.3. General
Melting points were determined on a Bristoline hot-stage apparatus and are uncorrected. IR spectra were recorded on a Perkin-Elmer infrared spectrometer. UV spectra were measured on a HP 8452 A diode array spectrophotometer with MeOH as a solvent. HRESI spectra were obtained on a ThermoFinnigan LTQFT at 4 kv. The NMR spectra were recorded in a Bruker DRX-400 spectrometer operating at 400 MHz (1H) and at 100 MHz (13C) using CDCl3 as a solvent. Column chromatography was carried out using silica gel reverse-phase C-18 (Fisher, Hanover Park, IL, USA) as stationary phase and TLC was conducted on silica gel aluminum sheets (Merck, Whitehouse Station, NJ, USA). High-resolution LC-MS of the compounds was performed as previously described (Huang et al., 2009).
2.4. Extraction and isolation of compounds 1–3
An aqueous infusion of the dried plant material was prepared as an infusion from 100 g of dried leaves in 500 ml of boiling water and the resulting infusion was filtered and lyophilized. For organic extraction, the dried leaves of P. hispidum (600 g) were extracted with 95% EtOH (5 L × 3 times) at room temperature, and the resulting extract was concentrated under vacuum to yield a dark green gummy residue (80 g, yield 13.3%). The residue was then defatted with a 1:1 mixture of hexane and water (4 L × 3 times) and the resulting aqueous extract was partitioned with ethyl acetate (4 L × 3 times). Anhydrous sodium sulfate (7 g) was then added to the EtOAc partition to remove any remaining water and this fraction was concentrated under reduced pressure to afford the dried EtOAc partition (17 g, 2.8 %), which was loaded onto a 2 L silica gel 60 reverse-phase C-18 glass column and eluted with a methanol-water gradient (50 % to 100 % MeOH) to give six fractions. Filtration of fractions 2–5 (combined after TLC analysis) yielded a colorless precipitate, that was re-crystallized from methanol/water (70:30) to afford purified 9, 10-methylenedioxy-5, 6-Z-fadyenolide (1, 48 mg). The filtrate was further subjected to column chromatography over 500 ml reversed phase C-18 silica gel column and eluted with methanol/water (70:30) to afford 5, 6-Z-fadyenolide (2, 54 mg) (Lago et al., 2005) and piperolide (3, 12 mg) (Hänsel and Pelter, 1971), as determined by LR-LCMS, NMR and comparison to authenticated compounds.
2.5. 9, 10-Methylenedioxy-5, 6-Z-fadyenolide (1)
Colorless solid; mp 218 °C; UV (MeOH) λmax 260, 342 nm (log ε 2.01 and 2.89, respectively); IR (film) νmax 1775, 1500, 1575, 1625, 1250, 1040, 900, and 730 cm−1; 1H NMR and 13C NMR, see Table 1. HRESI m/z 277.07075 (C14H13O6 [M + H]+; calc. 277.07066).
Table 1.
1H (400 MHz) and 13C (100 MHz) NMR signals assignments of 1 (in CDCl3).
| No. carbon | δH, J in Hz | δCa |
|---|---|---|
| 2 | 167.9 s | |
| 3 | 5.14 (1H, s) | 87.8 d |
| 4 | 170.9 s | |
| 5 | 129.1 s | |
| 6 | 143.9 s | |
| 7 | 124.4 s | |
| 8 | 6.85 (1H, d, 1.6) | 110.2 d |
| 9 | 147.6 s | |
| 10 | 149.4 s | |
| 11 | 6.82 (1H, d, 8.0) | 108.1 d |
| 12 | 6.92 (1H, dd, 8.0, 1.6) | 125.1 d |
| 13 | 6.04 (2H, s) | 101.7 t |
| 4-OCH3b | 3.76 (3H, s) | 59.1 q |
| 6-OCH3b | 3.67 (3H, s) | 59.0 q |
multiplicitiy obtained from DEPT-135 and DEPT-90 spectra, s: C; d: CH; t: CH2; q: CH3;
Signals interchangeable
2.6. 5, 6-Z-Fadyenolide (2)
Colorless crystals; mp 130–132°C; UV λmax MeOH nm (log ε): 245 (2.72), 313 (3.15); IR (film) νmax 2941, 1745, 1592, 1493, 1443, 1385, 1218, 1135 cm−1; LREIMS m/z (rel. int.): 232 (67) [M]+, 217 (7), 202 (4), 189 (14), 161 (13), 145 (4), 131 (3), 115 (8), 105 (100), 91 (5), 77 (53), 69 (27), 51 (Lago et al., 2005).
2.7. Piperolide (3)
Colorless crystals; mp 110–112°C; LREIMS m/z (rel. int.): 258 (69) [M]+, [C15H14O4] (Hänsel and Pelter, 1971).
2.8. Serotonin receptor binding assays and GTP shift experiments
Radioligand binding studies for the serotonin subtypes 5-HT1A and 5-HT5A were performed according to procedures previously described (Michel et al., 2007). To summarize, 2–4 mg/ml of plant extract in DMSO was diluted with sterile water for a final concentration of 50 μg/ml. Cos M6 cell membrane in a cell homogenate preparations (750 μl), [3H]5-carboxamidotryptamine (5-CT) (1.0 nM final concentration, 200 μl), assay buffer (100 mM Tris-HCl, 1mM EDTA and 1% ascorbic acid, pH 7.7) and plant extracts were then incubated at 37°C for 30 min. Incubations were terminated by rapid filtration (Tomtec, 48 well harvester) through Printed Filtermat B filters and washed four times with 1 ml ice cold assay buffer. Filters were then dried and melted onto scintillation wax. [3H] 5-CT was used to determine nonspecific binding that accounted for <10% of total binding (defined by inclusion of 10 μM 5-CT). Competition and ligand binding data was then analyzed using non-linear curve fitting techniques (RSI, Radlig). Hydroxytryptamine (serotonin, 5-HT) (250 nM) was used to define nonspecific binding, which accounts for <10% of total binding. The data represents the average standard deviation of at least triplicate determinations.
GTP Shift Experiments
Inhibition of binding of [3H]LSD (68.2 Ci/mmol, Perkin Elmer) by the petroleum ether extract was measured in the presence and in the absence of 100 μM GTP. 5-HT (500 μM) was used to define nonspecific binding. The assay was carried out under the same conditions as previously described (Dietz et al., 2005).
2.9. Estrogen receptor binding assays using ERα and ERβ
Radioligand binding studies of the extracts and pure compounds to the ER was performed as described in our previous work (Michel et al., 2007). The median inhibitory concentration was determined by testing the binding affinity of the extracts to the estrogen receptors in concentrations of 20 to 100 μg/ml. This assay is sensitive to extracts in the range of 1–200 μg/ml. In previous work the plant extracts were tested and found to be active in the range of 50–100 μg/ml. Thus, these concentrations were used as the start for compounds concentrations and thus, the compounds were tested in the 1–100 μg/ml range. All experiments were performed in triplicate.
2.10. Cell culture and RNA extraction
To determine if the aqueous infusion, ethanol extract and pure isolated compounds were estrogen agonists or antagonists, we used 2ERE-reporter gene assay in transiently transfected MCF-7 cells. MCF-7 human breast cancer cells were routinely maintained in MEM (Gibco) supplemented with 5% calf serum (Hyclone, Logan, UT). Four days prior to treatment the cells were sub-cultured on to phenol red-free MEM containing 5% charcoal dextran-treated calf-serum. Media were changed on day 2 and day 4 of culture. Cells were treated with 10 nM E2 alone or in combination with 20 μg/ml of the plant extract (Doyle et al., 2009; Frasor et al., 2008). Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. RNA was further purified using RN-easy columns (Qiagen, Valencia, CA) and treatment with ribonuclease-free deoxyribonuclease 1 (Qiagen, Valencia, CA).
2.11. Transfection assays
Transient transfection and luciferase assay: MCF-7 were co-transfected with a plasmid containing a 2ERE-luciferase reporter construct and the control plasmid, Renilla luciferase (Promega Corp., Madison, WI), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) diluted in serum-free, antibiotic-free OptiMEM (Invitrogen, Carlsbad, CA) (Doyle et al., 2009). Six hours after transfection, media were changed to phenol red-free media containing charcoal-dextran stripped serum and the cells were treated with one of the following: vehicle solvent (ethanol); vehicle solvent plus ICI; E2 (10 nM) dissolved in ethanol; E2 plus ICI; plant extract or infusion (20 μg/ml) plus vehicle solvent; or plant extract or infusion (20 μg/ml) plus ICI for 16 h. Compounds 1–3 were tested in the same manner using 1 μg/ml. Reporter activity was measured using dual-luciferase assay according to the manufacturer’s directions (Promega Corp., Madison, WI). Luciferase data shown are the mean ± sem from at least three independent determinations, variation was less than 10% between runs.
2.12. pS2, PR, PTGES
Effect on the expression of the estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells was assayed according to the previously published methods (Doyle et al., 2009). Four days prior to E2 treatment, MCF-7 cells were sub-cultured onto phenol red-free MEM containing 5% charcoal-dextran-treated calf serum and the medium was changed on d 2 of culture. Cells were treated with 10 nM E2 (Sigma-Aldrich Corp., St. Louis, MO) for 4h. Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed in a total volume of 20 μl using 200 U reverse transcriptase, 50 pmol random hexamer, and 1 mM deoxy-NTP (New England Biolabs, Beverly, MA). The resulting cDNA was then diluted to a total volume of 100 μl with sterile H2O.
2.13. Real-time PCR analysis
Each real-time PCR reaction consisted of 1 μl diluted RT product, 1X SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA), and 50 nM forward and reverse primers: 5′-CTTCCTTTTCCTGGGCTTCG-3′ (PTGES forward), 5′-GAAGACCAGGAAGTGCATCCA-3′ (PTGES reverse), 5′-ATGGCCACCATGGAGAACAAGG-3′ (pS2 forward) and 5′-CATAAATTCACACTCCTCTTCTGG-3′ (pS2 reverse), 5′-GAGCTCATCAAGGCAATT-3′ (PR forward) and 5′ CCATCCCTGCCAATATCT-3′ (PR reverse) (Doyle et al., 2009). Reactions were carried out on an ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems) for 40 cycles (95 °C for 15 sec, 60 °C for 1 min) after an initial 10-min incubation at 95 °C. The fold change in expression of each gene was calculated using the comparative Ct method, with the ribosomal protein 36B4 mRNA as an internal control.
2.14. CYP-450 3A4 inhibition assay
As a preliminary test for toxicity, we tested the infusion and ethanol extract, and compounds 1–3 for their effects on the CYP 3A4 isozyme. This enzyme is responsible for metabolizing over 50% of prescription drugs and has been involved in liver toxicities associated with herbal products (Mahady et al., 2005; Huang et al., 2010). The assay was performed using described protocols, with extracts and compound tested at 1–20 μg/ml (Huang et al., 2010).
2.15. Statistics
Statistical analyses were performed using the SAS® statistical package (SAS Institute, Cary, NC). One-way ANOVA was used, followed by Dunnett test for pair-wise comparison between the DMSO control and each of the other extracts. A general linear model with two main effects and their interaction was used to analyze the real time RT-PCR data, followed by post hoc comparison between the control and each of the other extracts. Statistical significance was ascribed to the data when p < 0.05.
3. Results
3.1. Structure identification of compounds 1–3
For isolation of the compounds, a 95% ethanol extract of the leaves of P. hispidum was defatted with hexane, and then partitioned between water and ethyl acetate. Bioassay-guided 5-HT1A and 5-HT7 binding data showed that the EtOAc partition was the most active, with displacement of [3H]-LSD and [3H]-OH-DPAT binding from the 5-HT7 and 5-HTIA of 63–96 % at 50 μg/ml. Reversed phase C18 column chromatography of the EtOAc partition led to the isolation of three butenolides, 9, 10-methylenedioxy-5, 6-Z-fadyenolide (1), 5, 6-Z-fadyenolide (2), and piperolide (3). To our knowledge, compound 1 is a new natural product, and compounds 2 and 3 were isolated for the first time from P. hispidum.
Compound 1, mp 218 °C, was obtained as a colorless amorphous powder. The molecular formula C14H12O6 was determined by HR-ESI-MS at m/z 277.0708 [M+H]+ (calcd for C14H13O6 277.0707). The UV spectrum exhibited maxima absorption at 260 and 342 nm (log ε 2.01 and 2.89). The IR spectrum contained absorptions due to conjugated lactone carbonyl group (1775 cm−1) and aromatic moiety (1575, 1625, 1250 and 1040 cm−1). The 1H and 13C NMR (Table 1) spectra of 1 were very similar to those of 2, except for a 1, 2, 4-trisubstituted aromatic ring at δH 6.92 (1H, dd, J= 8.0, 1.6 Hz, H-12), 6.82 (1H, d, J= 8.0 Hz, H-11) and 6.85 (1H, d, J= 1.6 Hz, H-8) instead of a mono-substituted benzene ring in 2, and an additional methylenedioxyl signals at δH 6.04 (2H, s, H-13) and δC 101.7 (C-13). The 1H NMR spectrum also exhibited two methoxyl signals at δH 3.67 and 3.76 (each 3H, s) and an olefinic proton singlet at 5.14 (1H, s, H-3). These information, associated to the 13C NMR resonances at δC 170.9 (C-4), 167.9 (C-2), 129.1 (C-5) and 87.8 (C-3) indicated a piperolide type structure containing one methoxyl group at C-4 (Lago et al., 2005). The resonance signals at δC 129.1 (C-5) and 143.9 (C-6) are indicative of a tetra-substituted double bond, and thus the second methoxyl group was placed at C-6, which was confirmed by the presence of the correlation peaks from methoxyls to C-4 and C-6, H-8 to C-6, and H-3 to C-4 in HMBC spectrum (Fig. 1). The location of methlyenedioxyl was deduced to be at C-9 and C-10 by the coupling constants of protons in the aromatic ring and verified by the presence of the cross peaks from H-13 to C-9 and C-10. The Z configuration of 1 was supported by comparison of the 13C-NMR data with those of 2 and its E isomer (Pelter et al., 1981). Finally, the structure of 1 was identified as 9, 10-methylenedioxy-5, 6-Z-fadyenolide (Fig. 1).
Fig. 1. Chemical structures of Piper hispidum isolates and key HMBC correlations of 1.
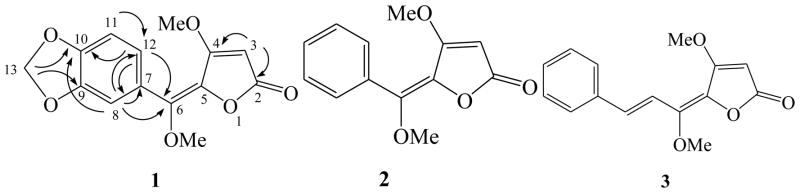
Chemical structures of Piper hispidum isolates and key HMBC correlations (1H to 13C, curved arrows) of 1.
3.2. Binding of compounds 1–3 to serotonin receptors 5-HT1A and 5-HT7 and GTP shift
Previously, we have shown that extracts of P. hispidum bound to the serotonin receptor (Michel et al., 2007). In this work, three butenolides were isolated using bioassay-guided fractionation and the purified compounds were tested against two subtypes 5-HT1A and 5-HT7 of the serotonin receptor because of their association with the hypothalamus, which has been implicated in the generation of menopausal hot flashes. Compound 1 showed strongest binding to serotonin receptors 5-HT1A and 5-HT7, with IC50 values of 16.1 and 8.3 μM respectively, whereas compounds 2 and 3 exhibited binding affinity to 5-HT7, with IC50 values of 23.2 and 34.7 μM respectively.
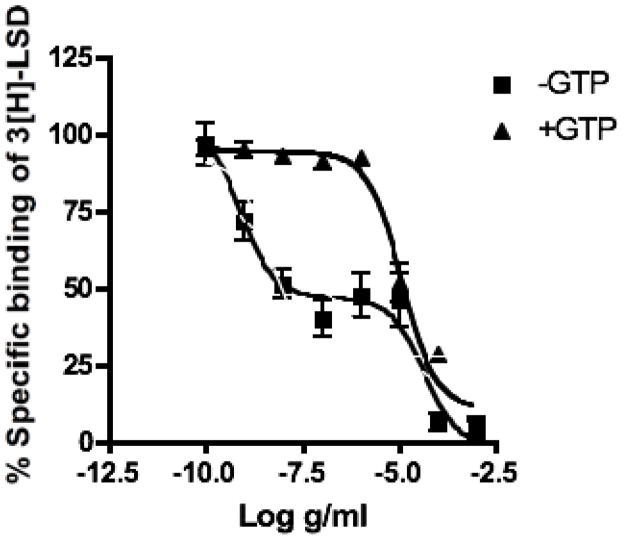
Considering that compound 1 had significant binding affinity for both the 5-HT1A and 5-HT7 receptors, its agonist/antagonist effects were assessed in GTP shift experiments in the 5-HT7-receptor (5-HT7-R). Compound 1 displaced the [3H]-LSD from the 5-HT7-R with an IC50 curve that showed a biphasic shape and best fit a two-site competition equation using the Akaike’s Information Criteria (GraphPad Prism 4.01 for Windows; Figure 2). Compound 1 displaced radioligands from the 5-HT7-R with an IC50 of 14.0 ng/ml (Ki: 8.48 ng/ml) for the high-affinity state and 37.7 μg/ml (Ki: 26.7 ng/ml) for the low affinity state (Figure 2). The addition of GTP resulted in a rightward shift of the Compound 1 binding curve to a low-affinity state or monphasic with an IC50 of 11.5 μg/ml (Ki = 6.88 mg/ml). These data suggest that Compound 1 acts as a partial agonist at the 5-HT7 receptor. Both 5-HT1A and 5-HT7 are located in the hypothalamus, where they are thought to participate in the thermoregulatory dysfunction that is associated with menopause.
Fig. 2. GTP shift of Compound 1 binding to 5-HT7 receptors.
Binding of Compound 1 to the human 5-HT7 receptor in the presence of [3H]LSD and in the presence or absence of GTP (100 μM). The addition of GTP resulted in a right-hand shift of the IC50 curve, indicating that the compound acts as an agonist of the 5-HT7 receptor.
3.3. Effects of P. hispidum extracts and compounds 1–3 on reporter gene expression in MCF-7 cells
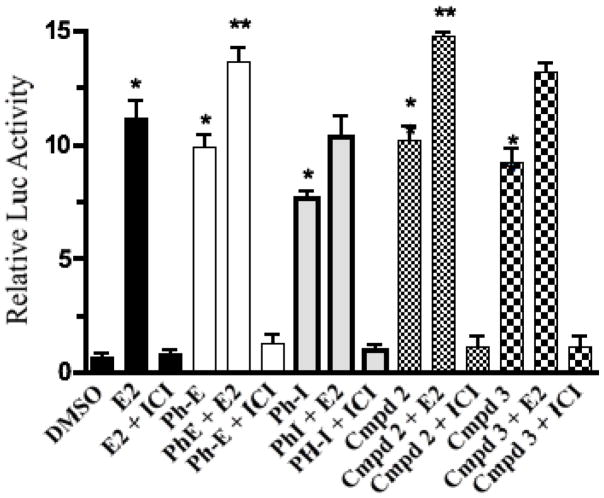
The infusion and 95% EtOH extract of P. hispidum and compounds 1–3 strongly displaced [3H]-17β-estradiol from both ERα and ERβ receptor subtypes and showed significant activity (p < 0.05; 50% or greater ligand displacement at 50μg/ml) in the binding assay (Table 2). Since the infusion, extract and isolated compounds bound to the estrogen receptors, they were tested in a functionalized reporter gene assay in transiently transfected MCF-7 cells to determine if they were estrogen agonists or antagonists. MCF-7 cells were transfected with a 2ERE-luciferase reporter construct and the control plasmid, Renilla luciferase. The infusion (Ph-I), EtOH extract (Ph-E), compounds 2 and 3, and E2 all increased the ERE-transcriptional activation, as measured by the fold increase in luciferase activity in MCF-7 cells (Fig. 3). Compound 1 was not active in this assay (data not shown) and did not enhance ERE-transcription in the presence or absence of E2 (1 nM). E2 significantly increased luciferase activity in transiently transfected MCF-7 cells by approximately 11. 5 fold (p < 0.001), Ph-I (20 μg/ml) by ~ 7.8 fold (p < 0.05); Ph-E (20 μg/ml) by ~ 9.6 fold (p < 0.05), compound 2 (1 μg/ml) by 9.8 fold (p < 0.05) and compound 3 (1 μg/ml) by 8.4 fold (p < 0.05). The induction of ERE-dependent luciferase activity by the extract, compounds 2 and 3 and E2- was inhibited by the co-treatment of the cells with the ER antagonist ICI 182,780 (1 μM), indicating that the activities of these extracts and compounds is mediated via the activation of the ER. The estimated EC50 of the EtOH extract of P. hispidum in activation of ERE-dependent luciferase activity was 10.5 μg/ml, and the concentration of extract required for maximal stimulation of luciferase activity was 20 μg/ml. The estimated maximal stimulation of the infusion of P. hispidum for the activation of ERE-dependent luciferase activity was 20.0 μg/ml.
Table 2.
Estrogen receptor binding data for Piper hispidum extracts and compounds.
| Compound/extract (Plant part) | IC50 a (M) | RBAb (%) |
|---|---|---|
| estradiol | 4.27 × 10−9 | 100 |
| Piper hispidum elhanol extract (Ph-E) | 5.54 × 10−6 | 0.14 |
| Piper hispidum infusion (Ph-I) | 25.2 × 10−5 | 0.008 |
| Compound 1 | 11.7 × 10−5 | 0.017 |
| Compound 2 | 29.4 × 10−5 | 0.003 |
| Compound 3 | 5.14 × 10−5 | 0.003 |
IC50 , concentration giving 50% inhibitory competition.
Relative binding affinity = IC50 [estradiol]/lC50 [competitor (extracts)] × 100.
LF = leaves; E = ethanol
FIG. 3.
The ER antagonist ICI 182780 specifically blocks the induction of luciferase activity by E2, PH, Cmpd 2 and 3 through ER in MCF-7 cells transiently transfected with a pERE-luciferase plasmid. Cells were treated with 1 nM E2, or 20 g/ml Ph-E or PhI; or 1 g/ml Cmpd 2 or 3 or 1 nM E2 + 1 M ICI 182780 or 20 g/ml PH or 1 g/ml Cmpd 2 or 3 + 1 M ICI 182780 to assess induction. Cells were treated for 16 h before they were lysed and reacted with substrate luciferin. Relative luciferase units were measured by luminometer. The PH extract, as well as Compounds 2 and 3 and E2 treatments significantly increased estrogen dependent activation of luciferase gene transcription in MCF-7 cells. ICI 182780 specifically blocked the actions of all in MCF-7 cells. Results are expressed as fold induction above control (DMSO vehicle solvent). Data represent the mean SD of three separate experiments performed in triplicate. * P < 0.01 versus control solvent (none) or **p < 0.05 versus E2. Abbreviation: Ph-E = Piper hispidum ethanol extract; Ph-I = Piper hispidum infusion.
3.4. P. hispidum infusion and extract induces up-regulation of endogenous genes pS2, PTGES and PR in MCF-7 cells
Real-time-PCR analysis was used to detect changes in levels of mRNA of the estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells in the presence of each of the EtOH extract (Ph-E) and the infusion (Ph-I) (Figure 4–6). The 95% EtOH extract of P. hispidium significantly induced transcription of the endogenous estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells via ERα (Figures 4–6). Ph-E up-regulated pS2 mRNA by 22.5 fold (p < 0.01), PR mRNA by 7.8 fold (p < 0.05), and PTGES mRNA by 6.0 fold (p < 0.05) above DMSO control (Figures 4–6). While the infusion of P. hispidum (Ph-I) up-regulated only pS2 mRNA expression by 12.8 fold (p < 0.05) and had no effect on the mRNA of PR or PTGES (Figure 4–6). The potential for synergistic or antagonistic effects of the extract in the presence of E2 in the MCF cells was also investigated (Figure 4). Ph-E in combination with E2 significantly (p < 0.01) enhanced pS2 mRNA by 24 fold as compared with E2 alone (15 fold), suggesting an additive, or possibly synergistic effect. Co-treatment of the MCF cells with Ph-E and E2 resulted in significant (p < 0.05) increases in PTGES (12.5 fold) and PR mRNA expression (13.5 fold), as compared with E2 alone (11.5 fold), however these results were not statistically significant (Figures 4–6).
Fig. 4–6.
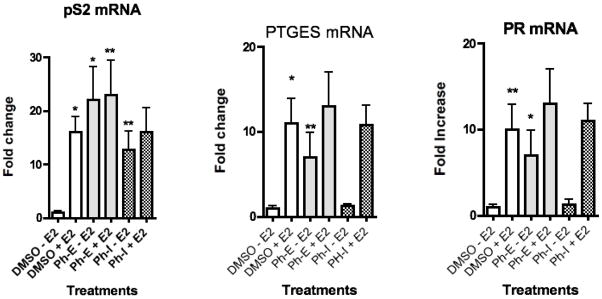
Results of RT-PCR analysis of endogenous estrogen responsive genes in MCF-7 breast cancer cells after treatment with the P. hispidium ethanol extract (Ph-E) or aqueous infusion (Ph-I) at a concentration of 20 μg/ml. Data is represented as fold increase in mRNA above control for (Figure 4) pS2, (Figure 5) PR, (Figure 6) PTGES. Data represent the mean SD of three separate experiments performed in triplicate. *p<0.05 and **p<0.01 versus control. Data is represented as fold increase in mRNA above control.
3.5. CYP-450 3A4 inhibition assay
The infusion and ethanol extract of P. hispidum, as well as compounds 1–3 were tested for their inhibitory effects of the CYP 3A4 isozyme. None of the extracts or compounds inhibited the CYP 3A4 isozyme in the concentration range tested (1–20 μg/ml).
4. Discussion
4.1 Infusions and extracts of Piper hispidium have estrogen and serotonin agonist effects
As part of ongoing research to investigate the pharmacological activities of traditional plant medicines from Central America used for reproductive and gynecological disorders for women’s health, we have been testing plant collected in Guatemala and Costa Rica (Doyle et al., 2009; Michel et al., 2007).
In Guatemala, the Q’eqchi Maya of Livingston commonly use plants within the Piperaceae as traditional medicines for the management of female reproductive disorders such as amenorrhea, dysmenorrhea, regulation of menstruation, and menopause. Piper hispidum is one species that is commonly used and our initial work indicated that extracts of the leaves of P. hispidum bound to both estrogen receptors (ERα and ERβ) and the serotonin receptors, suggesting possible estrogen and serotonin agonist or antagonist effects (Michel et al., 2007). In this work we demonstrate that an infusion of the leaves, as well as a 95% EtOH extract exhibit estrogen agonist activities, both in reporter gene assays and endogenous gene assays in MCF-7 breast cancer cells. The 95% EtOH extract also had serotonergic effects in 5-HT1A and 5-HT7 receptors (R).
4.2 Butenolides isolated from Piper hispidium are estrogen and serotonin agonists
Bioassay-guided fractionation of the 95% EtOH extract using ER, 5-HT1A-R and 5-HT7-R binding data showed that the EtOAc partition was the most active, with displacement of 3H-estradiol of 90% at 50 μg/ml [3H]-OH-DPAT and [3H]-LSD binding from 5-HTIA and 5-HT7 of 63–96 % at 50 μg/ml. Isolation and purification of the active compounds from the ethyl acetate fraction afforded three butenolides 1–3. One of these compounds, 9, 10-methylenedioxy-5, 6-Z-fadyenolide (1) is a new compound, while 5, 6-Z-fadyenolide (2) (Lago et al., 2005) and piperolide (3) are known compounds (Hänsel and Pelter, 1971) but have never been reported in Piper hispidium. There are few reports of butenolides in Piperaceae species and to date they have been only been isolated from P. fadyenii (Pelter et al., 1981; Nair et al., 1986), P. malacophyllum (Lago et al., 2005) and P. sanctum (Dharmaratne et al., 2002; Ganzera and Khan, 1999) collected in Jamaica, Brazil and Mexico, respectively.
The three isolated butenolides 1–3 were tested in the 5-HT1A-R and 5-HT7-R binding assays. Only compound 1 had binding affinity for the two subtypes of receptors, with an IC50 of 16.1 and 8.3 μM to 5-HT1A and 5-HT7, respectively. Due to the association of G-proteins with the 5-HT7-R receptor, GTP shift experiments were performed to determine if Compound 1 exhibited 5-HT7 agonist or antagonist activities. While agonists stimulate the binding of GTP to the G-protein, neutral antagonists have no effect, and antagonists with inverse agonistic activity reduce GTP binding. Thus, the addition of GTP induces an uncoupling of the receptor from the G-protein leading to a shift of the receptor from the high-to the low-affinity state for agonists (Dietz et al., 2005). In this work, the binding curve for the Compound 1 was biphasic in the absence of GTP, and addition of GTP resulted in a rightward shift of the Compound 1 binding curve to a low-affinity state, indicating that Compound 1 is a partial agonist of the 5-HT7-R.
Recent distribution studies have shown a high abundance of 5-HT7 receptors in the brain, particularly in the hippocampus, thalamus and hypothalamus that are associated with the symptoms of PMS and menopause (Medina et al., 2007). In the hypothalamus, 5-HT7-R is similar to 5-HT5A in that it is expressed mainly in the suprachiasmatic nucleus, which is the clock of the brain that orchestrates circadian and circannual biological rhythms, such as the rhythms of hormones, body temperature, sleep and mood (Dietz et al 2005; Medina et al., 2007). Its presence in the hypothalamus also correlates with its involvement in thermoregulation and endocrine function (Medina et al., 2007). These rhythms are frequently disturbed in menopause and PMS and may be restored by selective serotonin reuptake inhibitors or sex hormone replacement therapy. Thus, along with estrogen agonists, agonists of 5-HT7 may be useful for the treatment of menopause and other women’s health disorders such as PMS.
4.3 Infusions, extracts and isolated compounds from Piper hispidium are estrogen agonists
Up-regulation of the expression of ERE-reporter genes and endogenous genes pS2, PR and PTGES in MCF-7 cells are indicative of estrogenic effects via ERα (Doyle et al., 2009; Frasor et al., 2008). It is well known that pS2 expression is regulated in MCF-7 cells by estrogens, and that pS2 is predominantly, but not exclusively, expressed in ER-dependent cancers. In addition to pS2, mRNA expression of the PR and PTGES genes in MCF-7 cells are also regulated in a characteristic manner by estrogenic compounds (Doyle et al., 2009). In this work, RT-PCR was performed to determine the change of pS2, PR and PTGES mRNA production in MCF-7 cells treated with the infusion or the extract. The 95% EtOH extract of P. hispidum up-regulated mRNA expression of all three ER-responsive genes, suggesting that in MCF-7 cells they act as an estrogen agonist. The aqueous infusion however, up-regulated the expression of only pS2 mRNA suggesting a partial response. The EtOH extract, plus compounds 2 and 3 were active in up-regulating an estrogen-responsive 2ERE-reporter gene in transiently transfected MCF-7 cells further supporting the estrogen agonist effects. The estrogen antagonist ICI 182,780 inhibited the activity of the extract and compounds 2 and 3 indicating that gene transcription was mediated through the estrogen receptor (ERα). Compound 1 was not active in these assays. The data presented in this work demonstrate that infusions and extracts of the leaves of P. hispidum, as well as the two of the isolated butenolides, act as estrogen agonists. The EtOH extract also acts as a 5-HT7 receptor agonist, with compound 1 being the active constituent.
These data appear to explain some of the traditional ethnopharmacological uses of the leaves of P. hispidum by the Q’eqchi Maya of Livingston, Guatemala to for the treatment of disorders associated with the reproductive cycle.
Acknowledgments
This work was made possible by Grant Number R21-AT02381 from the National Center for Complementary and Alternative Medicine, NIH (GBM) and a U.S. Fulbright Fellowship (JLM). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or NIH. The authors would like to thank the Q’eqchi Maya and Asociacion Ak’Tenamit in Livingston, Guatemala for their collaboration, friendship, and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alecio AC, Bolzani SV, Young MCM, Kato MJ, Furlan M. Antifungal amides from leaves of Piper hispidum. Journal of Natural Products. 1998;61:637–639. doi: 10.1021/np9703656. [DOI] [PubMed] [Google Scholar]
- Barrett B. Medicinal Plants of Nicaragua’s Atlantic Coast. Economic Botany. 1994;48:8–20. [Google Scholar]
- Burke B, Nair M. Phenylpropene, benzoic acid and flavonoid derivatives from fruits of Jamaican Piper species. Phytochemistry. 1986;25:1427–1430. [Google Scholar]
- Coe FG, Anderson GJ. Screening of medicinal plants used by the Garífuna of eastern Nicaragua for bioactive compounds. Journal of Ethnopharmacology. 1996;53:29–50. doi: 10.1016/0378-8741(96)01424-9. [DOI] [PubMed] [Google Scholar]
- Dharmaratne HRW, Nanayakkara NPD, Khan IA. Kavalactones from Piper methysticum, and their 13C NMR spectroscopic analyses. Phytochemistry. 2002;59:429–433. doi: 10.1016/s0031-9422(01)00443-5. [DOI] [PubMed] [Google Scholar]
- Dietz B, Mahady GB, Pauli G, Farnsworth NR. Valerian extracts and valerenic acid are partial agonists of the 5HT5a receptor. Molecular Brain Research. 2005;138:191–197. doi: 10.1016/j.molbrainres.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle BJ, Frasor J, Perez A, Locklear TD, Mahady GB. Estrogenic and antiestrogenic activities of plant extracts from Costa Rica, used for the treatment of menopausal symptoms. Menopause. 2009;16:748–755. doi: 10.1097/gme.0b013e3181a4c76a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke JA, Vásquez R. Amazonian ethnobotanical dictionary. CRC Press; Boca Raton, Fla: 1994. p. 215. [Google Scholar]
- Estevez Y, Castillo D, Pisango MT, Arevalo J, Rojas R, Alban J, Deharo E, Bourdy G, Sauvain M. Evaluation of the leishmanicidal activity of plants used by Peruvian Chayahuita ethnic group. Journal of Ethnopharmacology. 2007;114:254–259. doi: 10.1016/j.jep.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Frasor J, Weaver AE, Pradhan M, Metha K. Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17β-estradiol and pro-inflammatory cytokines. Endocrinology. 2008;149:6272–6279. doi: 10.1210/en.2008-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U, Siems K, Solis PN, Gupta MP, Jenett-Siems K. New prenylated benzoic acid derivatives of Piper hispidum. Pharmazie. 2005;60:455–457. doi: 10.1002/chin.200541203. [DOI] [PubMed] [Google Scholar]
- Ganzera M, Khan IA. Analytical techniques for the determination of lactones in Piper methysticum forst. Chromatographia. 1999;50:649–653. [Google Scholar]
- Hänsel R, Pelter A. Cinnamylidenbutenolide aus Piper sanctum. Phytochemistry. 1971;10:1627–1634. [Google Scholar]
- Huang Y, Nikolic D, Pendland SL, Doyle BJ, Locklear TD, Mahady GB. Fukinolic acid derivatives and triterpenes from black cohosh extracts inhibit CYP 450 isozymes but are not cytotoxic to HepG2 cells in vitro. Current Drug Safety. 2010;4:XX. doi: 10.2174/157488610790936150. accepted, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett-Siems K, Mockenhaupt FP, Bienzle U, Gupta MP, Eich E. In vitro antiplasmodial activity of Central American medicinal plants. Tropical Medicine & International Health. 1999;4:611–615. doi: 10.1046/j.1365-3156.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- Lago JHG, Tanizaki TM, Young MCM, Guimaraes EF, Kato MJ. Antifungal piperolides from Piper malacophyllum (Prels) C. DC. Journal of the Brazilian Chemical Society. 2005;16:153–156. [Google Scholar]
- Machado SMF, Militao JSLT, Facundo VA, Ribeiro A, Morais SM, Machado MIL. Leaf oils of two Brazilian Piper species: Piper arboreum Aublet var. latifolium (C.DC) Yuncker and Piper hispidum Sw. Journal of Essential Oil Research. 1994;6:643–644. [Google Scholar]
- Mahady GB. Black cohosh: A review of the clinical data supporting safety and efficacy. Treatments in Endocrinology. 2005;4:117–123. doi: 10.2165/00024677-200504030-00006. [DOI] [PubMed] [Google Scholar]
- Medina RA, Sallander J, Benhamu B, Porras E, Campillo M, Pardo L, Lopez-Rodriguez ML. Synthesis of new serotonin 5-HT7 receptors ligand. Determinants of 5-HT7/5-HT1A receptor selectivity. Journal of Medicinal Chemistry. 2007;2:2384–2392. doi: 10.1021/jm8014553. [DOI] [PubMed] [Google Scholar]
- Michel J, Duarte RE, Bolton JL, Huang Y, Caceres A, Veliz M, Soejarto DD, Mahady GB. Medical potential of plants used by the Q’eqchi Maya of Livingston, Guatemala for the treatment of women’s health complaints. Journal of Ethnopharmacology. 2007;114:92–101. doi: 10.1016/j.jep.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney HA, Field C, Yanes CV, Chu C. Environmental controls on stomatal conductance in a shrub of the humid tropics. Proceedings of the National Academy of Sciences of the United States. 1983;80:1295–1297. doi: 10.1073/pnas.80.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MG, Burke BA. Antimicrobial Piper metabolite and related compounds. Journal of Agricultural and Food Chemistry. 1990;38:1093–1096. [Google Scholar]
- Nair MG, Mansingh AP, Burke BA. Insecticidal properties of some metabolites of Jamaican Piper spp., and the amides synthesized from 5,6-Z and E-butenolides of Piper fadyenii. Agricultural and Biological Chemistry. 1986;50:3053–3058. [Google Scholar]
- Navickiene HM, Alecio AC, Kato MJ, Bolzani VD, Young MC, Cavalheiro AJ, Furlan M. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry. 2000;55:621–626. doi: 10.1016/s0031-9422(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Pelter A, Al-Bayati R, Hansel R, Dinter H, Burke B. The structure and synthesis of fadyenolide, a new butenolide from Piper fadyenii. Tetrahedron Letters. 1981;22:1545–1548. [Google Scholar]
- Quijano-Abril MA, Callejas-Posada R, Miranda-Esquivel DR. Areas of endemism and distribution patterns for Neotropical Piper species (Piperaceae) Journal of Biogeography. 2006;33:1266–1278. [Google Scholar]
- Sengupta S, Ray AB. The chemistry of Piper species: a review. Fitoterapia. 1987;58:147–166. [Google Scholar]
- Vieira PC, De A, Marden A, Gottlieb OR, Gottlieb HE. 4-Hexadecenylphenol and flavonoids from Piper hispidum. Planta Medica. 1980;39:153–156. [Google Scholar]
- Wadt LHO, Ehringhaus C, Kageyama PY. Genetic diversity of “Pimenta longa” genotypes (Piper spp., Piperaceae) of the Embrapa Acre germplasm collection. Genetics and Molecular Biology. 2004;27:74–82. [Google Scholar]