Abstract
Infections with a range of common community viruses remain a major cause of mortality and morbidity after allogeneic hematopoietic stem cell transplantation. T cells specific for cytomegalovirus (CMV), Epstein-Barr virus (EBV) and adenoviruses can safely prevent and infections with these three most common culprits, but the manufacture of individual T cell lines for each virus would be prohibitive in terms of time and cost. We have demonstrated that T cells specific for all three viruses can be manufactured in a single culture using monocytes and EBV-transformed B lymphoblastoid cell lines (LCLs), both transduced with an adenovirus vector expressing pp65 of CMV, as antigen-presenting cells. Trivirus-specific T cell lines produced from healthy stem cell donors could prevent and treat infections with all three viruses, not only in the designated recipient, but in unrelated, partially-HLA-matched third party recipients. We now provide the details and logistics of T cell manufacture.
Keywords: Cytotoxic T cells (CTLs), viral infections, Immunotherapy, CMV, EBV, adenovirus, HSCT
Introduction
Opportunistic infections are frequent in allogeneic hematopoietic stem cell transplant (HSCT) recipients and are associated with significant morbidity and high mortality rates. Pharmacologic agents are standard therapy for some infections, but most have substantial toxicities, generate resistant variants and are not effective against all viruses. As the use of antivirals does not improve virus-specific immunity, infections frequently recur after termination of treatment. In contrast, reconstitution of HSCT recipients with antigen-specific T cells can offer an effective nontoxic strategy for providing both immediate and long-term protection. Here we outline the approach used at Baylor College of Medicine (BCM, Houston, TX, USA) to activate and expand antigen-specific T cells that simultaneously target three frequently detected viruses, the endogenous herpes viruses Epstein–-Barr virus (EBV) and cytomegalovirus (CMV), and adenovirus (Adv), which is increasingly detected post-transplant (1–4). Since 2004 these cytotoxic T lymphocytes (CTL) have been administered prophylactically to 26 patients at risk of developing CMV, EBV or Adv infections in a phase I/II clinical trial. Fourteen patients were treated during the dose-escalation phase (1 × 107/m2–1 × 108/m2). Then, to obtain further information regarding the immunologic effects after CTL, particularly in patients with low levels of viral re-activation at the time of CTL infusion, additional patients were treated with a fixed dose of 1 × 107/m2, chosen because there was no correlation between clinical response and higher cell doses and because of evidence of clinical efficacy seen at this dose in the dose-escalation phase. The clinical outcome of 11 patients has been described elsewhere (5); briefly, the infused cells expanded in vivo, correlating with a subsequent reduction in raised levels of all three viruses and with resolution of virus-associated signs and symptoms (5). Below we outline the CTL manufacturing process. We have made the detailed standard operating procedures (SOP) required available with appropriate URL links throughout the manuscript (see the supplementary material). These should facilitate the creation of protocols suitable for regulatory approval and provide the basis for good manufacturing practice (GMP) processing of trivirus-specific T cells.
Application
Increasing numbers of viral pathogens have been implicated in infectious complications after HSCT, largely because of the extension of this procedure to higher risk patients who require more intensive and prolonged post-transplant immunosuppression and often receive more extensively manipulated products. Several groups have produced and infused antigen-specific T cells, most commonly targeting either CMV or EBV (6–9). The goal of our study was to broaden the spectrum of viruses targeted in a single CTL product to include the three most common viral pathogens of stem cell recipients, namely EBV, CMV and Adv.
Patient eligibility
Trivirus-specific CTL are generated from CMV-seropositive HSCT donors. For the generation of CTL lines, blood is procured at the earliest appropriate time, usually prior to stem cell collection in order to allow sufficient time for the generation of an EBV-transformed lymphoblastoid cell line (EBV-LCL) that is an essential antigen-presenting cell (APC), and subsequently for trivirus-specific CTL production and cryopreservation. The protocol allows multiple samples to be drawn, so that sufficient T cells can be obtained for the generation of EBV-LCL and CTL, which may be generated from fresh or frozen aliquots. However, in general a single blood draw of 60 mL is sufficient for manufacturing purposes because the CTL are produced from healthy donors. The protocol is discussed with eligible stem cell donors and patients, and the informed consent required for participation in the study is obtained from both parties. The protocol is approved by the Recombinant DNA Advisory Committee (RAC), the Food and Drug Administration (FDA) and BCM ’ s Institutional Review Board (IRB). The National Marrow Donor Program (NMDP) has an IRB protocol and consent form that are used when obtaining blood for cell line preparation from an unrelated donor.
Manufacturing antigen-specific cytotoxic T cells using good manufacturing practices (GMP)
All cell culture and gene transfer manipulations are carried out in the Center for Cell and Gene Therapy GMP facility (BCM, The Methodist Hospital and Texas Children’s Hospital) using current SOPs (available online; see the supplementary material).
Blood procurement for CTL and APC generation
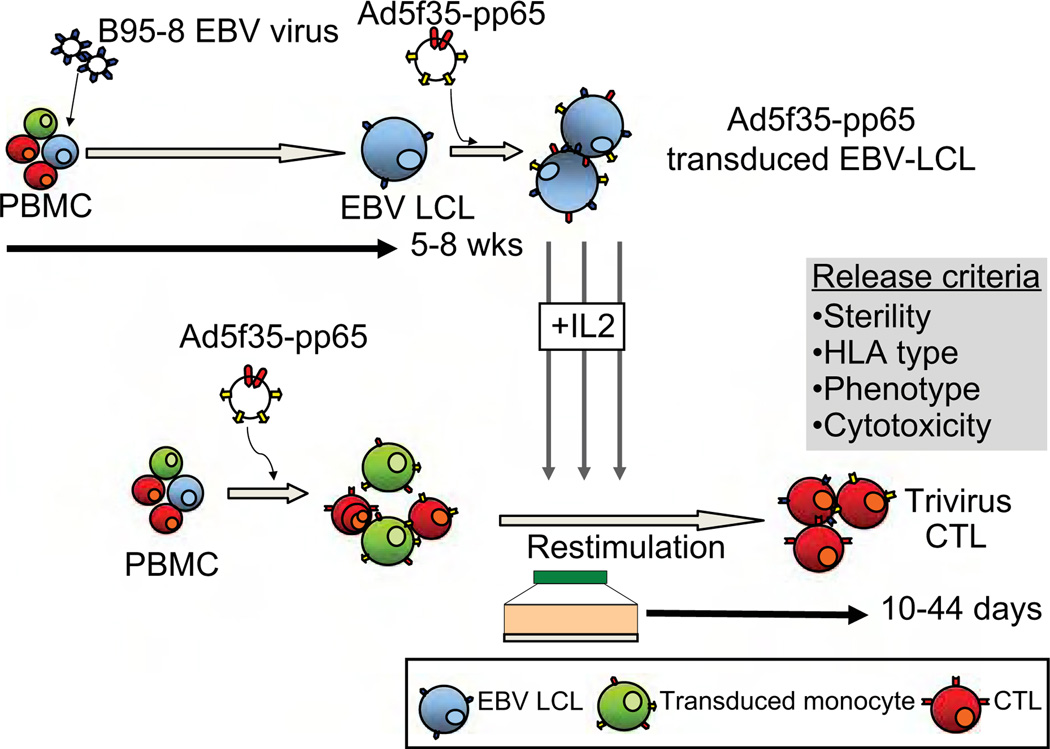
The generation of trivirus-specific CTL lines requires the production of several different components from peripheral blood mononuclear cells (PBMCs). The CTL line is initiated from the stem cell donor by transduction of adherent PBMC with an Adv vector expressing CMV-pp65. Within PBMCs, monocytes, which have been activated following overnight adherence, are preferentially transduced monocytes stimulate the T-cells component with both CMV-pp65 and Adv virion proteins. For the second and subsequent stimulations, autologous EBV-LCLs transduced with the same Ad5f35-pp65 vector are used to expand the trivirus-specific T cells (Figure 1).
Figure 1.
Generation of trivirus-specific CTL for clinical applications.
A maximum of 60 mL peripheral blood × 2 for a total maximum amount of blood of 120 mL is collected from the stem cell donor, who must be at least 12 kg (24 pounds) in weight. For donors < 18 years, a maximum of 3 mL/kg blood is taken in an 8-week period. PBMC are isolated on Ficoll (Lymphoprep, Cosmo Bio USA, Carlsbad, CA, USA) gradients. Each component, T cells, EBV-LCL and monocytes, can be prepared from fresh or cryopreserved PBMC.
CTL initiation
CTL specific for CMV-pp65, Adv and EBV are prepared according to GMP SOP D03.31 (see the supplementary material).
For donor-derived trivirus-specific CTL, donor PBMC are plated overnight in 24-well plates in X-Vivo 15 media (Bio Whittaker; Walkersville, MD, USA) at a concentration of 2 × 106 cells/well. This overnight adherence results in the transient up-regulation of the co-stimulatory molecules CD80 and CD83 on monocytes, which is crucial for optimal T-cell activation (10). The following day, the PBMC are harvested and activated monocytes are scraped from the wells. The bulk population is subsequently counted, pelleted and incubated with the Adv vector (Ad5f35-pp65), which preferentially transduces the activated monocyte fraction (10), at a viral particle (v.p.) to cell ratio of 10:1 for 2 h. After this time the cells are resuspended at a concentration of 1 × 106/mL in CTL media containing 45% Advanced RPMI (Invitrogen, Carlsbad, CA, USA), 45% Clicks Earle’s Ham’s amino acids (EHAA) (Irvine Scientific, Santa Ana, CA, USA), 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA) and 2 mm l-glutamine (Invitrogen). With this strategy, the transduced activated monocyte fraction of PBMC will express, process and present CMV-pp65 and Adv virion proteins to the CMV- and Adv-specific T cells contained within PBMC. Ideally, this step uses 20–40 × 106 PBMC from about 40 mL blood, but lower cell numbers have been effective.
Expansion of trivirus-specific CTL
For the second and subsequent stimulations, autologous EBV-LCL transduced with Ad5f35-pp65 are used as APC. In this setting, the EBV-LCL is a source of EBV antigenic stimulation for EBV-specific T cells that remain in the culture on day 9 after stimulation, and also processes and presents Adv virion proteins and CMV-pp65 to activated T cells. This stimulation can be performed either in 24-well plates or in a G-Rex gas permeable cell expansion device (11,12), according to SOP D03.32 (see the supplementary material).
Manufacture of APC
LCL are manufactured according to SOP D03.01 (see the supplementary material). EBV-LCL are derived from PBMC by infection with a clinical-grade laboratory strain of EBV (B95-8). About 5 × 106 PBMC, or 5–10 mL blood, are required to generate the EBV-LCL, and in the current study required a median of 6 weeks for establishment (range 5–8 weeks). The LCL are cultured in acyclovir for at least 2 weeks prior to use as APC to inhibit the release of infectious EBV. To expand the trivirus-specific T cells, EBV-LCL are transduced with the Ad5f35-pp65 vector at a v.p.:LCL ratio of 100:1, according to SOP D03.14 (see the supplementary material), although the multiplicity of infection is established for every new lot of vector. This transduction allows the EBV-LCL to present simultaneously EBV latent and early lytic cycle antigens, CMV (pp65) and Adv (virion) peptides to the T cells. The second CTL stimulation using transduced EBV-LCL is performed approximately 9–10 days after the first stimulation, with activated monocytes. Transduced LCL are harvested 48 h after transduction and used fresh, and can be cryo-preserved for future stimulations. Transduced LCL are irradiated (40 Gy; 80 Gy if used in G-Rex), washed and then co-cultured with the transduced monocyte-activated T cells at a responder to stimulator ratio of 4:1, if culturing cells in 24-well plates, or of 1:5, if culturing CTL in a G-Rex (SOP D03.32; see the supplementary material). The first interleukin (IL)-2 feed is performed 3–4 days after the first LCL stimulation (50–100 U/mL) and continued twice weekly thereafter. Earlier addition of IL-2 may result in the expansion of T cells specific for irrelevant antigens. Additional weekly LCL stimulations are usually required to expand CTL to the numbers required for clinical use. CTL are cryopreserved when sufficient cells for the dose level and quality control (QC) have been produced. For the 26 lines infused in this clinical study, clinical cryopreservation was performed following a median of three stimulations (range 2–6).
Quality assurance/QC and release criteria
Cryopreservation of CTL is performed according to SOP D03.05 (see the supplementary material) using a Cryomed (Thermo Fisher Scientific Inc., Waltham, MA, USA) and includes four washes to remove fetal bovine serum and phenol red. At the time of freezing, aliquots of the CTL, culture cells, cell culture supernatant, final wash and final cell product, are collected for sterility testing. Aliquots of cells are HLA typed for identity and phenotyped to assess T-cell subsets and confirm the absence of genetically modified EBV-LCL and monocytes. The CTL line is also tested for cytotoxic specificity. Lack of killing of patient-derived lymphoblasts (PHA blasts or OKT3 blasts) or fibroblasts is a release criterion to exclude alloreactivity. Samples are archived for immunologic characterization and for possible additional testing. The frequency of T cells directed against each of the viral components is determined by interferon (IFN)-γ ELIspot analysis using EBV-LCL as a stimulus as well as pepmixes (peptide libraries of 15 mers overlapping by 11 amino acids) spanning Adv hexon and penton (immunodominant T-cell targets if Adv) (13,14), CMV-pp65, EBV latent and lytic antigens LMP1, LMP2, EBNA1, EBNA3a, 3b, 3c and BZLF1, and multimer reagents, if available. Release criteria for administering the CTL to patients include viability > 70%, negative culture for bacteria and fungi after 7 days, endotoxin testing ≤ 5 Enzyme unit (EU)/mL, negative results for mycoplasma, < 10% killing of patient phytohemagglutinin (PHA) blasts or skin fibroblasts at a 20:1 ratio, < 2% CD19+ B cells, < 2% CD14+ monocytes and HLA identity. After quality assurance (QA) testing is complete, a certificate of analysis is issued.
Expected results
We expect no production failures because the system is robust and consistently generates CTL lines with trivirus specificity from seropositive individuals. Before the CTL line is released for infusion, all testing must have produced appropriate results or the line will be ineligible. Patient-derived lymphoblasts or fibroblasts should be killed only if expressing target viral antigens. CMV-pp65 is an immunodominant antigen and is overexpressed in the APC; therefore it is expected that the majority of T cells in the CTL line will be directed against pp65, but all lines should also contain reactivity against both Adv and EBV. The immune response to Adv is detected mainly in the CD4+ T-cell component, while CMV reactivity is often detected primarily in the CD8+ T-cell component, while EBV specificity is detected in both components (15–17). Inhibition of cytotoxicity to specific targets by antibodies to HLA class I and/or class II molecules confirms the HLA-restricted nature of the killing, but this is not a release criterion because the lines sometimes contain a component of natural killer (NK)-like activity from CD56+ CD3+ T cells, whose activity may be enhanced in the presence of antibody.
Potential problems and troubleshooting
One issue with this protocol is that the lines are often dominated by CMV specificity, with only minor fractions of EBV and Adv specificity (5,18), and in 1 of the 26 clinical CTL products, which was stimulated four times in vitro, the infused line lacked evidence of Adv specificity. We hypothesized that this loss of Adv activity was because of competition from the strong immunodominant CMV and EBV antigens for presentation by HLA molecules in APC (18). Indeed, in a follow-up study, removal of the CMV component resulted in an increase in both the EBV- and Adv-specific T-cell components within our CTL lines (19). However, in vivo we have seen that even low frequencies of adoptively transferred virus-specific T cells can expand substantially and protect or treat infections associated with all three viruses. Additionally, we were unable to grow PHA blasts from an appreciable number of patients, necessitating the evaluation of killing third-party (e.g. a haplo-identical parent) PHA blasts by the donor-derived CTL on a cytotoxicity assay. Only CTL lines that demonstrated < 10% killing of third-party PHA blasts at a ratio of 20:1 in cytotoxicity assays were administered, and no increased graft-versus-host disease (GvHD) was observed (20). Two lines that did not meet these criteria (i.e. exhibited killing of recipient target cells > 10%) were not infused.
Supplementary Material
Acknowledgments
This work was supported by NIH grants CA-61384 (HEH and CMR) and clinical grade CTLs were produced by Production Assistance for Cellular Therapies (NHLBI-PACT) (N01-HB-37163) (CMR and APG). The pp65 vector was provided by a grant from the National Gene Vector Laboratories (NIH-NCRR U42 RR16578). The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: Disclaimer: The information in this article is intended for educational purposes only and should not be considered as a standard of care or recommended treatment for any particular patient.
Supplementary material available online
D03.01.23. Generation of EBV-transformed cell lines (lymphoblastoid cell lines - LCL) 05.12.10
D03.05.22. Characterization and freezing of cytotoxic T lymphocytes 06.09.10
D03.14.23. Adv transduction of B95-8 + LCL for CTL stimulation 07.05.11
D03.31.22. Initiation of EBV- or antigen-specific cytotoxic T lymphocytes (CTL) 07.05.11
D03.32.21. Expansion of EBV- or antigen-specific cytotoxic T lymphocytes (CTL) 02.08.11
References
- 1.Leen AM, Tripic T, Rooney CM. Challenges of T cell therapies for virus-associated diseases after hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2010;10:337–351. doi: 10.1517/14712590903456003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers GD, Bollard CM, Wu MF, et al. Reconstitution of adenovirus-specific cell-mediated immunity in pediatric patients after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:677–686. doi: 10.1038/sj.bmt.1705645. [DOI] [PubMed] [Google Scholar]
- 3.Leen AM, Bollard CM, Myers GD, Rooney CM. Adenoviral infections in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:243–251. doi: 10.1016/j.bbmt.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Leen AM, Heslop HE. Cytotoxic T lymphocytes as immune-therapy in haematological practice. Br J Haematol. 2008;143:169–179. doi: 10.1111/j.1365-2141.2008.07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 6.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 7.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 8.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 9.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 10.Leen A, Ratnayake M, Foster A, et al. Contact-activated monocytes: efficient antigen presenting cells for the stimulation of antigen-specific T cells. J Immunother. 2007;30:96–107. doi: 10.1097/01.cji.0000211325.30525.84. [DOI] [PubMed] [Google Scholar]
- 11.Vera JF, Brenner LJ, Gerdemann U, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion culture-ware (G-Rex) J Immunother. 2010;33:305–315. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapteva N, Vera JF. Optimization manufacture of virus- and tumor-specific T cells. Stem Cells Int. 2011;2011:434392. doi: 10.4061/2011/434392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leen AM, Sili U, Vanin EF, et al. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- 14.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to generate multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther. 2009;17:1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein–Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leen A, Meij P, Redchenko I, et al. Differential immunogenicity of Epstein–Barr virus latent-cycle proteins for human CD4(+) T-helper 1 responses. J Virol. 2001;75:8649–8659. doi: 10.1128/JVI.75.18.8649-8659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein–Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 18.Leen AM, Christin A, Khalil M, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein–Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melenhorst JJ, Leen AM, Bollard CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116:4700–4702. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.