As people live longer, the question arises of how malleable aging is and whether it can be slowed or postponed. The classic evolutionary theories of aging (1-4) provide the theoretical framework that has guided aging research for 60 years. Are the theories consistent with recent evidence?
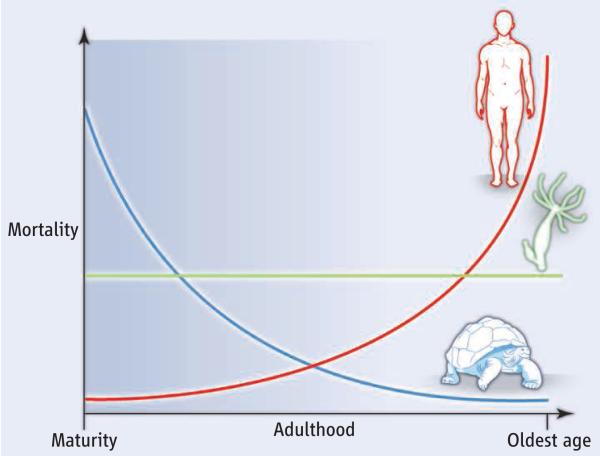
At the heart of the theories lies the observation that the old count less than the young: Unfavorable traits are weeded out by evolution more slowly at higher ages (2); traits that are beneficial early in life are selected for despite late life costs (3); and resources are used to enhance reproduction at younger ages instead of maintaining the body at ages that do not matter much for evolution (1). The decline in the force of selection with age is viewed as the fundamental cause of aging (4). It is why, starting at reproductive maturity, senescence—increases in susceptibility to death and decreases in fertility—should be inevitable in all multicellular species capable of repeated breeding (4). Yet, this is not the case. Increasing, constant, and decreasing mortality (and fertility) patterns (see the figure) are three generic variants that compose the rich diversity of life trajectories observed in nature. For vertebrates, reproductive trajectories are commonly hump-shaped, and death rates may start rising much later than reproductive maturity (5). Thus, a new view on the fundamental causes of aging is needed to explain the clash of theory and data.
Figure. Aging patterns.
The illustration is a schematic view of how species age in radically different ways. The life courses of humans and of many mammals and birds, at least at the oldest ages, are generally marked by rising mortality. For the tortoise Gopherus agassizii (17) and many other reptiles, amphibians, fish, and plants, mortality decreases throughout adult life. For the freshwater polyp Hydra vulgaris (18) and various other species across the tree of life, daily survival is more or less constant with age.
Allocation theory, which seeks to explain how resource limitations determine life-history patterns, provides a possible, promising perspective (6). Nature strikes compromises in allocating limited resources to growth versus maintenance versus reproduction versus escaping predators and pathogens. The scarcity of resources available for competing needs requires that an organism “makes difficult choices” at every moment of life. For instance, more energy dedicated to growth at one moment may reduce reproductive output but improve chances of survival to the next breeding opportunity, when conditions might be better and reproductive potential (because of growth) higher (6). Current theory of aging acknowledges the necessity of such compromises (7) but neglects their fundamental importance. Because the declining force of selection with age dominates evolutionary thinking about aging, classic theory focuses on life-history choices that specifically confer early-life advantages at the cost of late-life losses.
By widening horizons to consider not only early-versus late-life compromises but all the difficult choices an organism must make in allocating limited resources to competing needs over its life span, it is possible to gain insights into the diverse demographic patterns observed in nature (6, 8, 9). Even the effects of purely deleterious mutations that act only at older ages can be accounted for by appropriate allocation models (10). In such models, the force of selection declines with age, but though important, this decline is not decisive in molding fertility and mortality patterns.
What is decisive is the “option set” of a species, which can be summarized by the feasible combinations of survival and reproduction at all ages over the life span. Option sets differ widely: For some species, extra investment in repair and maintenance substantially reduces fertility; for other species there is little impact; for yet other species enhanced repair and maintenance decrease current but increase future fecundity. The details of such option sets shape age patterns of growth, fertility, and mortality (8, 11).
Little is known about what types of constraints favor a pattern of aging with increasing mortality and decreasing fertility (senescent) versus alternative patterns with constant or declining mortality and constant or increasing fertility (nonsenescent). Life-history models suggest that the marginal costs and benefits of energy allocation play a central role (8, 11). To test this and to explore other hypotheses, it would be informative to compare plants, for which growth and reproduction flexibly adapt to environmental conditions (12), to animals, for which growth and reproduction are more rigid and distinct (8). In contrast to vertebrates, plants capable of vegetative reproduction can create off-spring by splitting off body parts. Thereby an investment in growth effectively becomes an investment in reproduction. Species that are small but long-lived (such as hydra in the laboratory), that can reproduce either sexually or asexually (such as daphnia), or that face highly uncertain environments [such as desert plants (12)] may also be good candidates for studies of how allocation options shape patterns of aging.
Research on the evolution of aging should focus on unraveling those differences in species’ option sets that lead to senescent versus nonsenescent aging patterns. A major barrier in accomplishing this has been the lack of laboratory, zoo, and field evidence about age patterns of growth, maintenance, fertility, and mortality for species across the tree of life. New statistical methods and software now permit the extraction of mortality patterns from field data that are sporadic or are missing observations (13). Further development of life-history models hinges on more extensive and reliable data as well as on experiments to reveal how much allocation of additional resources to, say, faster growth or a more effective immune system affects lifetime fertility and survival. Fundamental understanding of why humans deteriorate so sharply (14) compared with other species, why human mortality has fallen so dramatically (15), and whether aging can be further delayed or even slowed (16) depends on knowledge of why some species senesce and others do not.
Acknowledgments
We thank L. Partridge, F. Colchero, D. Conde, D. Levitis, O. Jones, R. Salguero-Gomez, A. Scheuerlein, and the Evodemo group at the Max Planck Institute for Demographic Research. Supported by NIH grant AG-031719.
References and Notes
- 1.Kirkwood TBL. Nature. 1977;270:301. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 2.Medawar PB. Uniqueness of the Individual. Lewis; London: 1952. pp. 44–70. [Google Scholar]
- 3.Williams GC. Evolution. 1957;11:398. [Google Scholar]
- 4.Hamilton WD. J. Theor. Biol. 1966;12:12. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 5.Jones OR, et al. Ecol. Lett. 2008;11:664. doi: 10.1111/j.1461-0248.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 6.Stearns SC. The Evolution of Life Histories. Oxford Univ. Press; Oxford, New York: 1992. [Google Scholar]
- 7.Partridge L, Sibly R, Beverton RJH, Hill WG. Philos. Trans. Biol. Sci. 1991;332:3. [Google Scholar]
- 8.Baudisch A. Demographic Research Monographs. Springer; Berlin: 2008. Inevitable Senescence? Contributions to Evolutionary Demographic Theory. [Google Scholar]
- 9.Vaupel JW, et al. Theor. Popul. Biol. 2004;65:339. doi: 10.1016/j.tpb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Dańko MJ, et al. PLoS ONE. 2012;7:e34146. doi: 10.1371/journal.pone.0034146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baudisch A. Gerontology. 2012 doi: 10.1159/000341861. 10.1159/000341861. [DOI] [PubMed] [Google Scholar]
- 12.Salguero-Gómez R, Casper BC. J. Ecol. 2010;98:312. [Google Scholar]
- 13.Colchero F, et al. Methods Ecol. Evol. 2012;3:466. [Google Scholar]
- 14.Baudisch A. Methods Ecol. Evol. 2011;2:375. [Google Scholar]
- 15.Burger O, Baudisch A, Vaupel JW. Proc. Natl. Acad. Sci. U.S.A. 2012 doi: 10.1073/pnas.1215627109. 10.1073/pnas.1215627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaupel JW. Nature. 2010;464:536. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner FB, Berry KH, Randall DC, White GC. Report No. 87-RD-81”. Southern California Edison Company; 1987. “Population ecology of the desert tortoise at Goffs, California, 1983-1986. [Google Scholar]
- 18.Martínez DE. Exp. Gerontol. 1998;33:217. doi: 10.1016/s0531-5565(97)00113-7. [DOI] [PubMed] [Google Scholar]