Abstract
In vitro generation of hematopoietic stem cells (HSCs) from induced pluripotent stem cells (iPSCs) has the potential to provide novel therapeutic approaches for replacing bone marrow (BM) transplantation without rejection or graft versus host disease. Hitherto, however, it has proved difficult to generate truly functional HSCs transplantable to adult host mice. Here, we demonstrate a unique in vivo differentiation system yielding engraftable HSCs from mouse and human iPSCs in teratoma-bearing animals in combination with a maneuver to facilitate hematopoiesis. In mice, we found that iPSC-derived HSCs migrate from teratomas into the BM and their intravenous injection into irradiated recipients resulted in multilineage and long-term reconstitution of the hematolymphopoietic system in serial transfers. Using this in vivo generation system, we could demonstrate that X-linked severe combined immunodeficiency (X-SCID) mice can be treated by HSCs derived from gene-corrected clonal iPSCs. It should also be noted that neither leukemia nor tumors were observed in recipients after transplantation of iPSC-derived HSCs. Taken our findings together, our system presented in this report should provide a useful tool not only for the study of HSCs, but also for practical application of iPSCs in the treatment of hematologic and immunologic diseases.
Introduction
Direct reprogramming of somatic cells allows generation of patient-specific induced pluripotent stem cell (iPSC) lines similar to embryonic stem cells (ESCs).1,2,3 Such iPSCs are capable of self-renewal, large-scale expansion, and differentiation into all three germ layers, opening a way to cell therapy using a patient’s own cells.4,5,6 For instance, when iPSCs are propagated and gene-corrected in vitro, hematopoietic stem cells (HSCs) derived from them can be transplanted, as curative therapy, into patients with genetic hematologic disorders. Efficient differentiation of iPSCs into functional HSCs is the most important goal for future HSC-based cell or gene therapies. Although in vitro generation of HSCs from mouse ESCs through forced expression of HOXB4 has been reported, the resultant HSCs exhibited abnormal hematopoiesis.7,8 Furthermore, forced expression of HOXB4 is not helpful in generating human HSCs.9 Generation of fully functional HSCs from mouse or human iPSCs without any genetic modifications has rarely been accomplished.10,11 One of the reasons is that it is difficult to reproduce the microenvironment necessary for development of hematopoietic lineage cells during embryogenesis. In addition, recapitulation of an adult HSC niche in vitro has also been complicate by the fact that not all the cell types composing the HSC niche are known.
From these reasons, we considered use of teratomas as the differentiation site of hematopoietic lineage cells and engraftable HSCs from ESCs or iPSCs. Teratomas are benign tumors containing differentiated tissues of all three germ layers artificially formed by an injection of ESCs/iPSCs into immunodeficient mice. Erythrocytes, megakaryocytes, and blood vessels develop within teratomas formed in chickens or mice.12,13,14,15 We hypothesized that a microenvironment like that in developing embryos, that allows generation of hematopoietic lineage cells, might be formed in teratomas and that functional iPSC-derived HSCs, once generated, would eventually migrate to the bone marrow (BM) niche just as bona fide HSCs behave in vivo16,17 (Figure 1a).
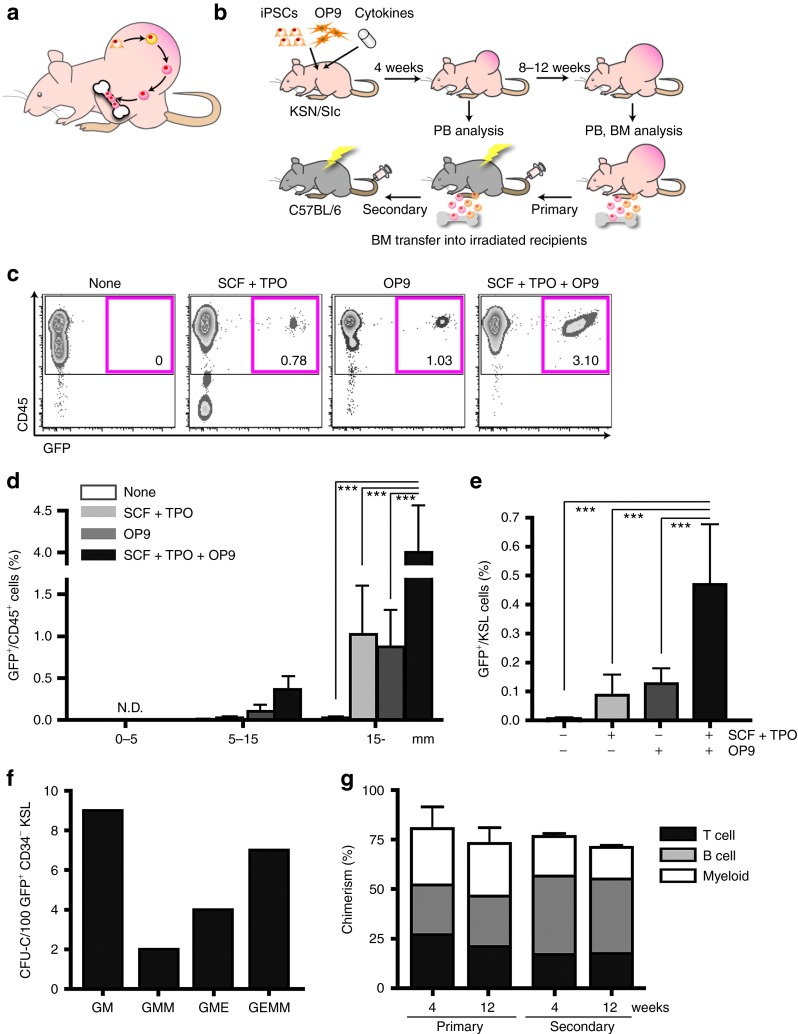
Figure 1.
Generation of transplantable hematopoietic stem cells (HSCs) from LG-iPSCs through teratoma formation. (a) The hypothesized model for the homing of iPSC-derived HSCs from teratoma into bone marrow (BM). (b) Strategy to induce HSCs from iPSCs through teratoma formation. With or without OP9 cells, iPSCs were subcutaneously injected into nude mice. Cytokines were administered for 2 weeks using a micro-osmotic pump. BM cells, among which iPSC-derived HSCs were detectable, were transplanted into irradiated mice. (c) Flow cytometric analysis of peripheral blood (PB) in teratoma-bearing nude mice 12 weeks after iPSC injection. Numbers represent percentages of GFP+/CD45+ cells. (d) Time course change of GFP+/CD45+ cells in PB dependent on teratoma size. The X-axis represents teratoma size (***P < 0.001). (e) Percentages of GFP+/KSL cells in BM of nude teratoma-bearing mice. Analysis conducted 12 weeks after iPSC injection (n = 5 per group). Error bars represent SEM (***P < 0.001). (f) Hundred GFP+ CD34− KSL cells in BM of teratoma-bearing mice were single-cell sorted and cultured for 10 days with cytokines for hematopoietic differentiation. (g) BM transplantation assay for chimerism of LG-iPSC–derived GFP+/CD45+ cells in PB of recipient mice. BM cells of teratoma-bearing mice were transplanted into irradiated mice. Secondary transplantation was also performed 12 weeks after primary transplantation. (Primary, n = 4; secondary, n = 10.) Error bars represent SEM. CFU-C, colony-forming unit in culture; GFP, green fluorescent protein; GM, granulocyte macrophage; GMM, granulocyte macrophage megakaryocyte; GME, granulocyte macrophage erythroid; GEMM, granulocyte erythroblast macrophage megakaryocyte multilineage; LG-iPSC, Lnk−/− GFP transgenic mice-induced pluripotent stem cell; N.D., not detectable; SCF, stem cell factor; TPO, thrombopoietin.
Here, we established an in vivo differentiation system to generate fully functional HSCs from iPSCs through teratoma formation. To monitor the system, we evaluated induction of HSCs with Lnk−/− iPSCs that have a high hematopoietic potential. We then successfully generated engraftable HSCs from mouse iPSCs without any genetic modifications. Moreover, we obtained a proof-of-concept for the next generation of gene therapy using iPSCs in an X-linked severe combined immunodeficiency (X-SCID) mouse model and also succeeded in inducing xenotransplantable HSCs from human iPSCs.
Results
Generation of iPSC-derived transplantable HSCs in Lnk−/− mice through teratoma formation with combined administration of hematopoietic cytokines and stromal cells
According to our speculation that HSC induction from iPSCs derived from wild-type mice might be a very rare event, we chose to use iPSCs derived from Lnk−/− GFP transgenic mice (LG-iPSCs). Lnk is an intracellular adaptor protein reported to be a negative regulator of HSC self-renewal.18 Lnk−/− mice overproduce HSCs19 due to their extreme self-renewing capability;20 thus once generated, we expected to be able to detect LG-iPSC–derived HSCs even when induction efficiency was very low. We first injected LG-iPSCs alone into KSN/Slc nude mice subcutaneously, but we were not able to detect donor-derived green fluorescent protein+ (GFP+) cells in the peripheral blood (PB) of teratoma-bearing mice. Co-culturing with OP9 stromal cells supplemented with stem cell factor (SCF) and thrombopoietin (TPO) has been used to induce hematopoietic progenitors from ESCs/iPSCs in vitro.7 In an attempt to increase the efficiency of HSC induction, we co-injected LG-iPSCs with OP9 cells and subcutaneously implanted a micro-osmotic pump to supply hematopoietic cytokines (Figure 1b). After 8–10 weeks, LG-iPSC–derived GFP+ CD45+ cells appeared in PB of mice treated with cytokines, OP9 cells, or a combination of both (Figure 1c). The frequency of GFP+ CD45+ cells in PB gradually increased with teratoma enlargement (Figure 1d and Supplementary Figure S1a). Combined administration of cytokines and OP9 cells was most efficient for the production of GFP+ CD45+ cells (average values 12 weeks after iPSC injection: none, 0.002 ± 0.01%; SCF + TPO, 1.02 ± 1.15%; OP9, 0.87 ± 0.79%; SCF + TPO + OP9, 4.26 ± 3.79%) (Figure 1d). In the BM of teratoma-bearing mice 12 weeks after iPSC injection, GFP+ cells could be detected among Lineage-negative (Lin−) cells as multipotent progenitors, Lin− c-Kit+ Sca-1+ (KSL) cells as hematopoietic stem progenitor cells, and CD34− KSL cells as long-term HSCs (Supplementary Figure S1b). The frequency of LG-iPSC–derived KSL cells was highest when both cytokines and OP9 cells were administered, correlating with the frequency of circulating iPSC-derived CD45+ cells in PB (Figure 1e). These data suggest that immunophenotypically defined HSCs, originating from LG-iPSCs and capable of homing to host BM through the blood circulation, were generated through teratoma formation and that the supplementation with hematopoietic cytokines and OP9 cells enhanced the process.
To test the function, GFP+ CD34− KSL cells in BM of the teratoma-bearing mouse were sorted as single cells and individual cells were cultured for 10 days with cytokines for colony formation. Among the seeded CD34− KSL cells, 22.9% formed large colonies exhibiting colony-forming unit (CFU)-granurocyte/erythroblast/macrophage/megakaryocyte (GEMM) differentiation (data not shown), with evidence of robust multipotency at the clonal level, along with other types of colonies with more restricted differentiation properties (Figure 1f). To determine whether LG-iPSC–derived HSCs had any marrow-repopulating ability, 1 × 107 BM cells of teratoma-bearing mice were transplanted into lethally irradiated C57BL/6 (B6) mice as primary recipients. Sequential analysis of PB cells of recipient mice detected LG-iPSC–derived hematopoietic cells at high frequencies (4 weeks, 77.7 ± 18.9%; 12 weeks, 73.0 ± 16.5% mean ± SEM, n = 4 each) with multilineage reconstitution (Figure 1g, primary; Supplementary Table S1). LG-iPSC–derived cells were also detected in spleen and BM of recipient mice, with the average percentage of GFP+ cells within CD34− KSL fractions as high as 45.0% (Supplementary Figure S1c,d and Supplementary Table S1). BM cells of these primary recipients (1 × 107 cells) were then serially transplanted into secondary recipients to test the self-renewal potential with LG-iPSC–derived HSCs. Multiple lineage reconstitution by LG-iPSC–derived cells in secondary recipient mice was observed for more than 12 weeks after transplantation (4 weeks, 76.6 ± 2.7%; 12 weeks, 60.6 ± 4.6% mean ± SEM, n = 10 each) (Figure 1g, secondary). These results demonstrated that HSCs with self-renewal and multilineage differentiation capabilities were generated from LG-iPSCs through teratoma formation.
Generation of functional HSCs from normal mouse iPSCs through teratoma formation
To determine whether functional HSCs can be induced from iPSCs without Lnk deficiency, we established iPSCs from B6 GFP transgenic mice (G-iPSCs) and assessed the generation of HSCs through teratoma formation. G-iPSC–derived hematopoietic cells, unlike LG-iPSCs, were rare but detectable in PB of teratoma-bearing mice, with the exception of those receiving G-iPSC alone (Figure 2a and Supplementary Figure S2a). The highest frequencies of GFP+ CD45+ cells were consistently observed in the presence of cytokines and OP9 cells (% GFP+/CD45+ cells: none, 0.003 ± 0.006%; SCF + TPO, 0.01 3 ± 0.01%; OP9, 0.025 ± 0.01%; SCF + TPO + OP9, 0.16 ± 0.09% mean ± SD, n = 4 each) (Figure 2a). GFP+ cells were also detected among Lin− cells, KSL cells, and CD34− KSL cells in BM of teratoma-bearing mice at 12 weeks after iPSC injection (Supplementary Figure S2b). Using G-iPSC–derived CD34− KSL cells from BM of teratoma-bearing mice, we evaluated early multilineage differentiation. In the presence of cytokines for hematopoietic differentiation, we observed single G-iPSC–derived CFU-GEMM mixed colonies (Figure 2b). Transplantation of BM cells obtained from teratoma-bearing mice (1 × 107 cells) led to engraftment of G-iPSC–derived blood cells in PB, spleen, and BM of primary recipient mice (Figure 2c, primary; Supplementary Figure S2c). Chimerism in this experiment was much lower than when LG-iPSC–derived HSCs were transplanted (Supplementary Table S1), presumably because wild-type mouse iPSC-derived HSCs have lower capability for self-renewal and for homing to the host BM niche. GFP+ cells were also detected in primitive hematopoietic fractions, including Lin− cells, KSL cells, and CD34− KSL cells within recipient BM (Supplementary Figure S2d). To examine the reconstitution activity of G-iPSC–derived HSCs, we transplanted 40 sorted GFP+ CD34− KSL cells from primary recipients along with 2 × 105 B6 BM cells into secondary recipient mice. This transplantation led to robust engraftment of GFP+ cells at multilineage levels, with chimerism maintained for between 4 and 12 weeks after transplantation (Figure 2c, secondary). These data suggest that our HSC induction system allows generation of engraftable HSCs even from iPSCs having no Lnk mutations. Leukemia and other abnormalities were not observed in any recipient mice in transplantation experiments (Supplementary Figure S2e), implying that induced HSCs have normal hematopoietic properties.
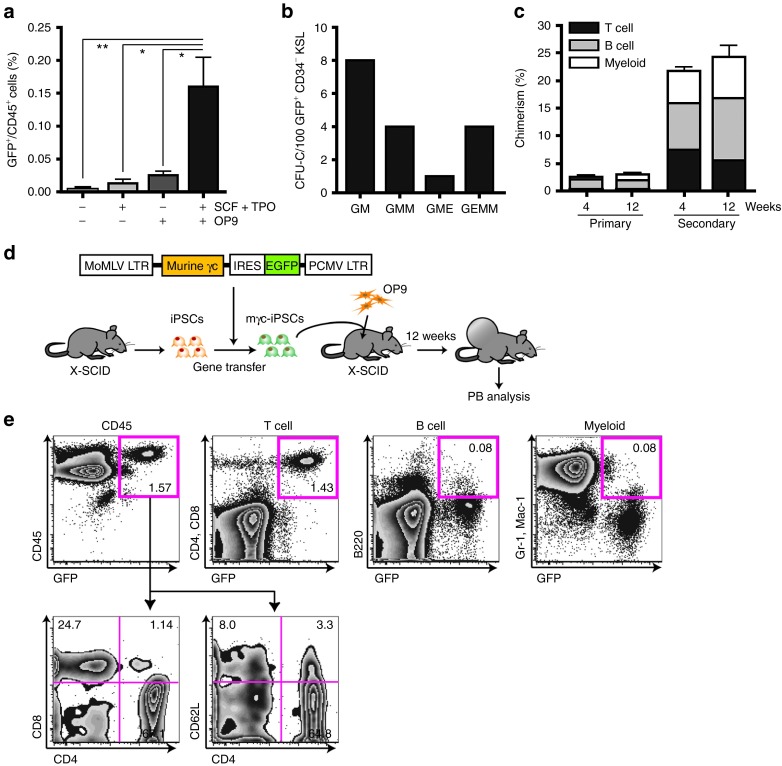
Figure 2.
Generation of transplantable hematopoietic stem cells (HSCs) from G-iPSCs through teratoma formation, with the gene therapy model of X-SCID. (a) Percentages of GFP+/CD45+ cells in peripheral blood (PB) of nude teratoma-bearing mice. Analysis was conducted 12 weeks after iPSC injection (n = 4 per group). Error bars represent SD (*P < 0.05, **P < 0.01). (b) Hundred GFP+ CD34− KSL cells in bone marrow (BM) of teratoma-bearing mice were single-cell sorted and cultured with cytokines for hematopoietic differentiation. Numbers and types of CFC-GEMM were evaluated on day 10. (c) BM transplantation assay for chimerism of G-iPSC–derived GFP+ hematopoietic cells/CD45+ cells in PB of recipient mice. Whole BM cells of teratoma-bearing mice were transplanted into primary recipient mice. Forty FACS-sorted GFP+ CD34− KSL cells in BM of primary recipient mouse were transplanted into secondary recipient mice. (Primary, n = 6; secondary, n = 5.). Error bars represent SD. (d) Flow chart explaining the generation of T cells from mγc-iPSCs in X-SCID mice. iPSCs were established from X-SCID mice and mγc was transduced, yielding mγc-iPSCs. mγc-iPSCs and OP9 cells were injected into X-SCID mice to generate teratomas. (e) Flow cytometric analysis of PB in teratoma-bearing mice 12 weeks after iPSC injection. Numbers represent percentages. CFC-GEMM, colony-forming cell-granulocyte erythroblast macrophage megakaryocyte multilineage; EGFP, enhanced green fluorescent protein; FACS, fluorescence-activated cell sorting; G-iPSC, GFP transgenic mice-induced pluripotent stem cell; IRES, internal ribosomal entry site; LTR, long terminal repeat; SCF, stem cell factor; TPO, thrombopoietin; X-SCID, X-linked severe combined immunodeficiency.
Reconstitution of lymphocytes in X-SCID mice by HSCs derived from gene-corrected clonal X-SCID-iPSCs
To validate the utility of our HSCs induction system in principle, we established a therapeutic model for X-SCID utilizing gene correction of disease-specific iPSCs. X-SCID causes severely impaired T and B cell immunity21 due to a mutation in the gene encoding the common gamma chain (γc), whose product is shared by receptors for multiple immunologically important cytokines. Stem cell gene therapy is effective in X-SCID, but its use has multiple risks.22,23 While gene therapy utilizing clonal disease-specific iPSCs screened for safety24 may be a solution, generation of genuine HSCs has been a major obstacle. We first generated iPSCs from X-SCID mice, and then established a clonal cell line expressing murine γc (mγc-iPSCs) by retroviral gene transfer (Figure 2d and Supplementary Figure S3). We then subcutaneously injected mγc-iPSCs into X-SCID mice with OP9 cells to generate teratomas. Twelve weeks after iPSC injection, mature T cells derived from functionally corrected mγc-iPSCs were clearly observed with normal distributions of CD4+ or CD8+ cells and evidence of naive T cell (CD4+ CD62L+) generation in PB of teratoma-bearing X-SCID mice (Figure 2e). Only trace levels of B- and myeloid-lineage cells expressing GFP appeared, likely due to competition with their residual counterparts in host BM. These results proved that, in principle, the immunodeficiency of X-SCID can be treated by HSCs generated from gene-corrected iPSC-derived teratomas.
Production of HSCs with BM reconstitution potential from human iPSCs
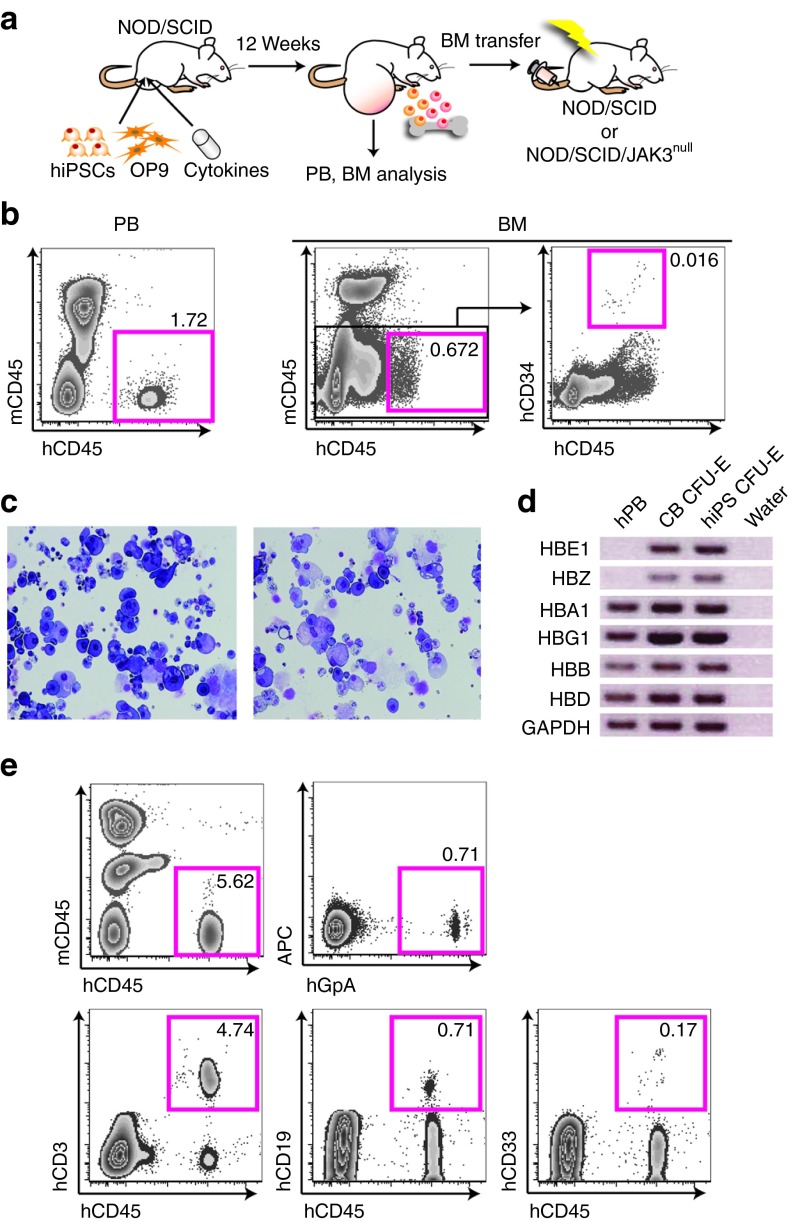
Then, to investigate whether functional HSCs could also be induced from human iPSCs, we injected human iPSCs into testes of NOD/SCID mice to generate teratomas and, with them, HSCs. The frequencies of iPSC-derived cells were then analyzed in PB and BM of teratoma-bearing mice after 12 weeks (Figure 3a). PB of two mice among 15 contained a detectable mCD45− hCD45+ cell population (Figure 3b, left panel and Supplementary Figure S4a). Furthermore, we could detect hCD45dull hCD34+ cells (a highly enriched human HSC population) and hCD45+ hCD34− cells (terminally differentiated blood cells) within a mCD45-negative population in BM of 11 of 15 teratoma-bearing mice (Figure 3b, right panel and Supplementary Figure S4a). These findings demonstrated that human iPSC-derived hematopoietic cells can be generated via teratoma formation, and that they circulate through xenogenic mouse PB and BM. Then, we evaluated the multilineage differentiation capacity of human iPSC-derived HSCs using colony assay and observed typical morphology of various subtypes including CFU-GEMM (Figure 3c). A previous study revealed that human ESC-derived erythroid-like cells coexpress high levels of embryonic and fetal globins, but no adult globins.25 We therefore examined expression of different globins in human iPSC-derived CFU-erythroid colonies. As a result, we could detect expression of all types in human iPSC-derived CFU-erythroid colonies as well as in cord blood-derived CFU-erythroid colonies, but no embryonic Hb Gower-1 (ζ2ε2) expression in human PB (Figure 3d). These data suggest that erythrocytes expressing adult type Hb-A (α2β2) were induced in iPSC-derived CFU-erythroid colonies, although cells expressing embryonic Hb Gower-1 and fetal Hb-F (α2γ2), and adult Hb-A co-existed in iPSC-derived colonies as well as cord blood-derived colonies due to in vitro differentiation. To confirm the repopulating ability of human iPSC-derived HSCs, we transplanted mCD45− hCD45+ hCD34+ BM cells (600 cells) obtained from teratoma-bearing mice into irradiated NOD/SCID or NOD/SCID/JAK3null mice (Figure 3a). This resulted in engraftment of human iPSC-derived blood cells with a multilineage repopulation including hGlycophorinA+ erythrocytes and hCD3+ T cells in PB of some recipient mice at 12 weeks after transplantation (Figure 3e and Supplementary Figure S4b–g). A higher proportion of engrafted mice was observed in NOD/SCID/JAK3null recipient mice (29.4%) than in NOD/SCID recipient mice (3.57%) (Supplementary Figure S4a). Our findings established that engraftable HSCs which could differentiate into all hematopoietic lineages had been induced from human iPSCs without any genetic modifications.
Figure 3.
Induction of engraftable hematopoietic stem cells (HSCs) from human iPSCs through teratoma formation. (a) Strategy to induce HSCs from human induced pluripotent stem cells (iPSCs) through teratoma formation. iPSCs were injected with OP9 cells into testes of NOD/SCID mice. Cytokines were administered for 2 weeks via a micro-osmotic pump. BM cells of teratoma-bearing mice were transplanted into sublethally (3 Gy) irradiated NOD/SCID or NOD/SCID/JAK3null mice. (b) Flow cytometric analysis of PB (left panel) and BM cells (right panels) in teratoma-bearing mice 12 weeks after human iPSC (hiPSC) injection. (c,d) Colony-forming assay with human iPSC-derived hCD45dull hCD34+ HSCs isolated from BM of teratoma-bearing mice. (c) Wright-Giemsa staining of cytospin preparations of CFU-GEMM mixed colonies. (d) Reverse transcription-PCR analysis for the expression of embryonic, fetal, and adult globins in cells taken from CFU-E colonies derived from human iPSCs. Human (h) PB cDNA was used as positive control. CB-derived CFU-E cDNA was used as control. Water is the negative control. HBE1: ε chain, HBZ: ζ chain, HBA1: α chain, HBG1: γ chain, HBB: β chain, and HBD: δ chain. (e) mCD45− hCD45+ hCD34+ 600 cells in BM of teratoma-bearing NOD/SCID mice were transplanted into irradiated NOD/SCID/JAK3null mice with 2 × 105 NOD/SCID/JAK3null BM cells. PB chimerism in recipient mice 12 weeks after BM transplantation. APC, allophycocyanin; BM, bone marrow; CB, cord blood; CFU-E, colony-forming unit-erythroid; PB, peripheral blood; SCID, severe combined immunodeficiency.
HSC niche-like cells exist in iPSC-derived teratomas
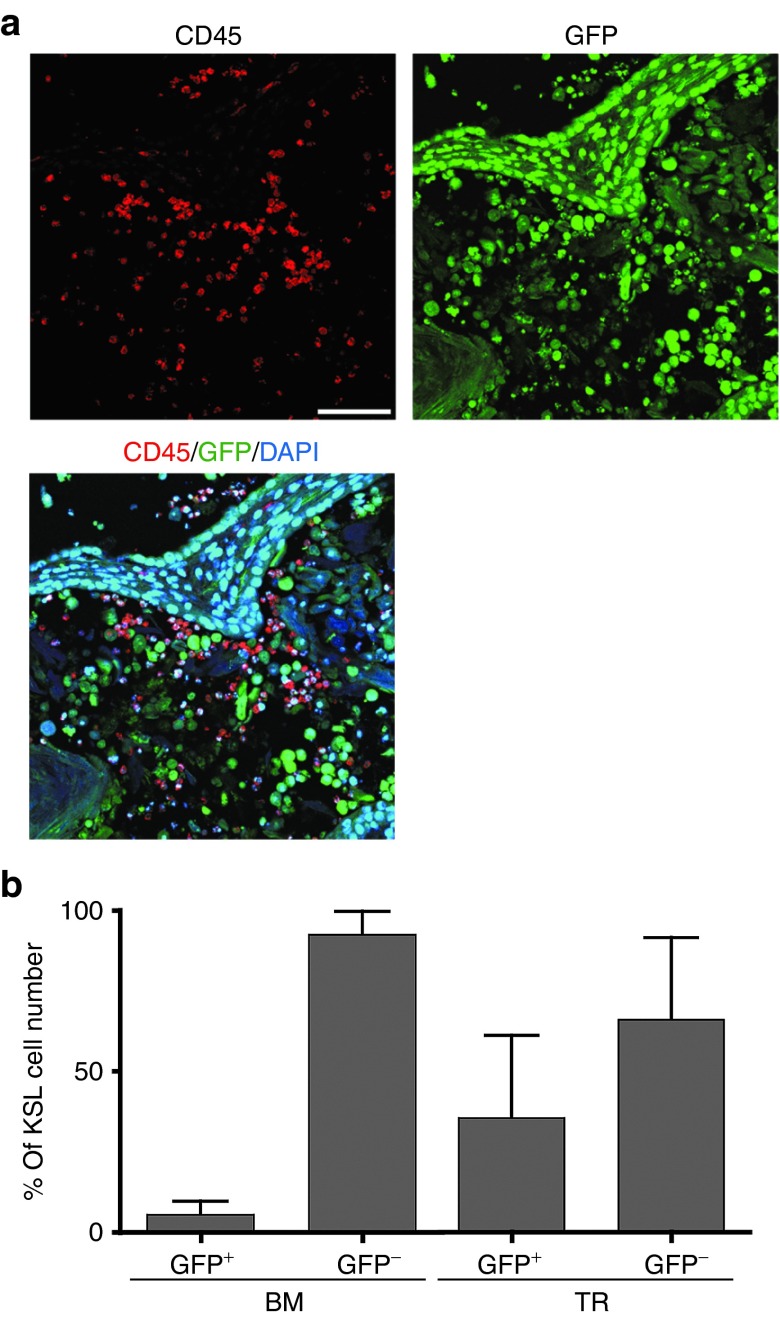
Finally, to study how iPSC-derived blood cells or HSCs are generated and ascertain whether they might exist in teratomas, we analyzed cells composing G-iPSC–derived teratomas formed in experimental mice. In addition to GFP+ CD45+ hematopoietic cells, GFP− CD45+ cells were detected by immunostaining at 12 weeks after iPSC injection (Figure 4a). These cells were thought to infiltrate within teratomas with the aid of host-derived myeloid cells. GFP+ KSL cells were also identifiable by fluorescence-activated cell sorting analysis, suggesting that HSCs were generated from iPSCs in teratomas (Figure 4b). Osteoblasts,26,27 endothelial cells,28,29 and nonmyelinating Schwann cells30 are believed to contribute to formation of the HSC niche.31 We immunohistochemically confirmed a large number of osteocalcin+ osteoblasts as well as VE-cadherin+ endothelial cells in G-iPSC–derived teratoma sections (Supplementary Figure S5c). Moreover, CD45+ c-Kit+ cells presumably containing HSCs were found localized near VE-cadherin+ cells and osteocalcin+ cells (Supplementary Figure S5d,e). In human iPSC-derived teratomas, CD45+ CD34+ HSCs were frequently observed near HSC niche-like cells (Supplementary Figure S5a,b). Collectively these data clearly indicate that HSCs and the previously reported niche components co-exist in teratomas.
Figure 4.
Hematopoietic stem cell (HSC) niche environment formation in GFP-iPSC–derived teratomas. (a) Immunostaining of CD45+ cells in G-iPSC–derived teratomas. Bar, 75 μm. (b) Flow cytometric analysis of iPSC-derived GFP+ and GFP− CD45+ cells and KSL cells in bone marrow (BM) and teratomas (TR). KSL cells in teratomas ranging from 93 to 620 cells. DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; G-iPSC, GFP transgenic mice-induced pluripotent stem cell.
Discussion
HSCs are the best-studied stem cells with a number of in vitro as well as in vivo assay systems. Yet, in vitro generation of HSCs from ESCs or iPSCs is difficult even with genetic modification. In this study, we succeeded in inducing HSCs by injecting iPSCs into immunodeficient mice and reproducing the HSC niche subcutaneously by way of teratoma formation. Although injection of iPSCs alone could not generate iPSC-derived HSCs, co-injection of iPSCs with OP9 stromal cells along with hematopoietic cytokines dramatically enhanced the induction efficiency. We confirmed that HSCs derived from both LG- and G-iPSCs could self-renew and reconstitute multiple lineages by transplanting BM cells of teratoma-bearing mice. Prospectively isolated LG- and G-iPSC–derived CD34− KSL cells could give rise to all blood lineages on secondary transfer. These findings strongly suggested that injected iPSCs partially differentiate into HSCs in teratomas and migrate into BM of teratoma-bearing mice, the iPSC-derived HSCs being indistinguishable from bona fide HSCs with regard to phenotype and biological characteristics. This is in contrast to the HSCs generated in vitro from PSCs, which lack homing capacity, thus requiring additional use of an intra-BM delivery method.32,33,34 Unlike in vitro culture systems, microenvironment present in a teratoma must have been very similar to that prevailing during normal embryonic development. During the course of the study, we performed transplantation of HSCs directly isolated from teratomas and found that these maintain a stable reconstitution ability. However, because of a significantly high level of lethality, we could not observe long-term repopulation. This is probably due to contamination of iPSC-like immature cells in HSCs fractions. These results suggest that only functional HSCs were selected in the process of BM homing. Similar findings were also reported by Amabile et al.,35 but here we carefully investigated the optimal conditions for in vivo induction of HSCs using Lnk−/− mice. Furthermore, we demonstrated, as proof-of-principle, that gene correction in X-SCID mice-derived iPSCs could overcome immunodeficiency by generation of T lymphocytes with HSCs through teratoma formation. Finally, human iPSCs also proved capable of generating functional HSCs in xenogenic mouse models, reconstituting myeloid and lymphoid cells in recipient mice with intravenous transplantation.
Gene therapy of HSCs is currently limited due to substantial risks of leukemogenesis caused by viral-mediated insertional mutagenesis.22,23 In contrast, clonal selection of gene-corrected iPSCs derived from patients with defined proviral insertion in the secure genome region may be more appropriate for therapeutic application because screened iPSCs can be expanded clonally ex vivo.24 In addition, leukemia and other hematopoietic abnormalities have not been observed in recipient mice, suggesting that these iPSC-derived HSCs generated in teratomas are functionally and developmentally normal. Compared with those developing in vitro, HSCs obtained from teratomas must have gone through the near-normal differentiation processes with appropriate epigenetic changes utilizing the microenvironment offered by a teratoma.
Since this system made it possible to generate engraftable HSCs from ESCs/iPSCs, it should provide a useful tool for the study of HSC biology. In addition, our differentiation system can expect to find application in elucidation of mechanisms and development of drugs for various hematopoietic diseases by analyzing iPSC-derived HSCs or terminally differentiated blood cells. There are a number of issues that remain to be clarified before this system could be used for generation of clinically usable human HSCs, but eventually we should be able to transplant a patient’s own gene-corrected iPSC-derived HSCs isolated from BM of teratoma-bearing immunodeficient animals.
We believe that our novel technique will be an exciting research tool having therapeutic potential which will open up a new era for both custom-tailored cell therapy and disease modeling using iPSCs.
Materials and Methods
Mice. C57BL/6 (B6) mice, KSN/Slc nude mice, and GFP transgenic mice were purchased from Japan SLC (Shizuoka, Japan). Lnk−/− GFP transgenic mice were bred and maintained in the Animal Research Facility of the Institute of Medical Science, University of Tokyo. Generation and characterization of X-SCID mice with B6 background were as described earlier. NOD/SCID mice were purchased from CLEA Japan (Tokyo, Japan) and NOD/SCID/JAK3null mice from Sankyo Labo Service (Tokyo, Japan). Animal care in our laboratory was in accordance with the guidelines of University of Tokyo for animal and recombinant DNA experiments.
Cell lines and culture conditions. Mouse iPSCs were obtained by reprogramming tail tip fibroblasts of Lnk−/− GFP transgenic B6 mice and GFP transgenic B6 mice with Oct3/4, Sox2, and Klf4, then maintained on mouse embryonic fibroblasts with iPSCs culture medium consisting of Dulbecco’s Modified Eagle Medium (GIBCO, Carlsbad, CA) supplemented with 15% Knockout Serum Replacement (GIBCO), 20 mmol/l HEPES buffer solution, 0.1 mmol/l minimum essential medium (MEM) non-essential amino acids solution, 0.1 mmol/l L-glutamine (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich, St Louis, MO), 0.1 mmol/l 2-mercaptoethanol, and 1,000 U/ml ESGRO (GIBCO).
Human iPSCs were obtained by reprogramming adult normal human epidermal keratinocytes (Lonza, Basel, Switzerland) with Oct3/4, Sox2, and Klf4. Established iPSCs were maintained on mouse embryonic fibroblasts with culture medium consisting of Dulbecco’s Modified Eagle Medium-F12 (Sigma-Aldrich) supplemented with 20% Knockout SR, 0.1 mmol/l MEM non-essential amino acids solution (Invitrogen), 0.2 mmol/l L-glutamine, 0.1 mmol/l 2-mercaptoethanol, and 5 ng/ml bFGF (PeproTech, Rocky Hill, NJ).
OP9 cells were maintained in growth medium consisting of MEM α-medium (α-MEM; Invitrogen), supplemented with 20% fetal bovine serum (HyClone, Logan, UT).
Histopathology and immunostaining. Histological findings were assessed by light microscopy of hematoxylin and eosin-stained sections of paraffin-embedded teratoma tissue.
Immunofluorescence staining of Nanog and SSEA-1 was performed using cryosections with an anti-mouse Nanog antibody (1:100; Cosmo Bio, Tokyo, Japan) and an anti-mouse SSEA-1 antibody (1:100; Abcam, Cambridge, MA), followed by incubation with anti-rabbit IgG coupled with Alexa Fluor 546 (1:300; Invitrogen) and with anti-mouse IgM-allophycocyanin (APC) (1:100; eBioscience, San Diego, CA). Nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) according to the manufacturer’s instructions. Sections were viewed and images were captured using an Olympus BX-51 fluorescence microscope and digital camera system DP-71 (OLYMPUS, Tokyo, Japan).
Teratoma tissues were embedded in Optimal Cutting Temperature (O.C.T.) compound (Sakura Finetek, Tokyo, Japan) and flash-frozen on dry ice. Then 7–8 μm sections produced using a CM3050 S cryostat (Leica Microsystems, Tokyo, Japan) were fixed with ethanol and immunostained. Each section was incubated with primary antibodies for 24 hours at 4 °C and with secondary antibodies for 30 minutes at room temperature. Primary antibodies, all anti-mouse, were directed against CD45 (1:50; BD Biosciences, San Jose, CA), osteocalcin (1:200; LifeSpan Biosciences, Seattle, WA), and VE-cadherin (1:200; Abcam). CD117 was stained with Alexa Fluor 488-conjugated anti-mouse antibody (1:10; BioLegend, San Diego, CA). Secondary antibodies were Alexa Fluor 546-conjugated goat anti-rat IgG and Alexa Fluor 647-conjugated goat anti-rabbit IgG (Invitrogen). After antibody treatment, sections were mounted employing a fluorescence-microscopy medium (Dako, Carpinteria, CA) containing DAPI (Sigma-Aldrich) for nuclear counterstaining. Sections were examined under a TCS SP2 AOBS confocal laser scanning microscope (Leica Microsystems).
Teratoma formation and differentiation of iPSCs to HSCs
Mouse iPSCs. 5 × 106 iPSCs were injected subcutaneously into KSN/Slc mice (4–5 weeks old). Differentiation of HSCs was induced under the following conditions: (i) only iPSCs were injected as a control; (ii) the hematopoietic cytokines SCF (200 ng) and TPO (200 ng) (PeproTech) were administered via a micro-osmotic pump (ALZET, Cupertino, CA) implanted subcutaneously for 2 weeks; (iii) 1 × 106 OP9 stromal cells were co-transplanted with iPSCs; and (iv) both cytokines and OP9 cells were administered.
Human iPSCs. 1 × 106 iPSCs and 5 × 105 OP9 stromal cells were co-injected into testes of NOD/SCID mice (5–7 weeks old). The hematopoietic cytokines human recombinant SCF (200 ng) and TPO (200 ng) (PeproTech) were administered via a micro-osmotic pump (ALZET) implanted subcutaneously for 2 weeks.
Flow cytometry analysis and sorting
Mouse iPSC-derived blood cells. Mouse PB and spleen cells were stained with anti-mouse antibodies, APC-conjugated anti-CD45 (BD Biosciences), APC-Cy7–conjugated anti-CD3e, Pacific Blue-conjugated anti-CD45R (eBioscience), phycoerythrin (PE)-Cy7–conjugated anti-Gr-1, and anti-Mac-1 (BioLegend). BM cells of teratoma-bearing mice were extracted by flushing out. They were stained with an antibody mixture consisting of anti-mouse biotinylated anti-Gr-1, anti-Mac-1, anti-CD45R, anti-CD4, anti-CD8, anti-IL-7R, and anti-TER119 antibodies (eBioscience) and Lin+ cells were then depleted using MACS anti-biotin microbeads and a LS-MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). The cells were further stained with anti-mouse Alexa Fluor 700-conjugated anti-CD34, Pacific Blue-conjugated anti-Sca-1, and APC-conjugated anti-CD117 antibodies, as well as with APC-Cy7–conjugated streptavidin antibody for biotinylated antibodies (eBioscience). Four color analysis and sorting were performed on a FACSAria (Becton Dickinson, Franklin Lakes, NJ).
hiPSC-derived blood cells. BM cells of teratoma-bearing mice were stained with APC-conjugated anti-mouse CD45, fluorescein isothiocyanate-conjugated anti-human CD34 (BD Biosciences), and Pacific Blue-conjugated anti-human CD45 antibodies (BioLegend). PB of recipient mice was stained with APC-conjugated anti-mouse CD45, PE-conjugated anti-human CD3, APC-H7–conjugated anti-human CD19, PE-Cy7–conjugated anti-human CD33 (BD Biosciences), and Pacific Blue-conjugated anti-human CD45 antibodies (BioLegend). Analysis and sorting were performed on a FACSAria (Becton Dickinson).
Teratoma-derived blood cells. Teratomas were minced into small pieces and digested in 0.2% collagenase (Wako Pure Chemical Industries, Osaka, Japan) for 30 minutes with shaking at 37 °C. The suspension was filtered with a cell strainer (Falcon 2350) and collected by centrifugation at 280g for 7 minutes at 4 °C. The pellet was immersed in 1 ml water (Sigma-Aldrich) for 5–10 seconds to burst the RBCs, after which 1 ml of 2× phosphate-buffered saline (dilution from Sigma-Aldrich) containing 4% fetal bovine serum was added, and the suspension was filtered through a cell strainer. Cell staining and analysis were same as BM cells.
Single-cell colony assay. Purified iPSC-derived CD34− KSL cells were clonally deposited into 96-well plates containing 200 μl of S-clone SF-O3 medium (Sanko Junyaku, Tokyo, Japan) supplemented with 1% bovine serum albumin and mouse SCF (50 ng/ml), TPO (50 ng/ml), IL-3 (10 ng/ml), and EPO (1 U/ml; all PeproTech). The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2. Ten days after incubation began the cells were cytospun onto slide glasses and stained using Hemacolor (Merck, Darmstadt, Germany).
BM transplantation assay
LG-iPSC–derived HSCs. 1 × 107 BM cells of teratoma-bearing mice were transplanted into lethally irradiated (9.5 Gy) wild-type B6 recipient mice. Four and 12 weeks thereafter, PB cells of recipient mice were analyzed by flow cytometry. 1 × 107 BM cells of primary recipient mice were secondarily transplanted 12 weeks after primary BM transplantation.
G-iPSC–derived HSCs. 40 GFP+ CD34− KSL cells of primary recipient mice were sorted and transplanted into secondary recipient mice with 2 × 105 B6 BM cells.
hiPSC-derived HSCs. 1 × 107 BM cells or 600 mCD45-depleted hCD45+ hCD34+ BM cells with 2 × 105 NOD/SCID BM cells of teratoma-bearing mice were transplanted into irradiated (2 Gy) NOD/SCID or NOD/SCID/JAK3null recipient mice. Eight weeks after transplantation, PB cells of recipient mice were analyzed by flow cytometry.
Statistical analysis. Mean values of two groups were compared by two-tailed unpaired t-testing. All statistical analyses were performed on Prism 4 software (Graphpad, San Diego, CA).
SUPPLEMENTARY MATERIAL Figure S1. Engraftable HSCs were induced from LG-iPSCs through teratoma formation. Figure S2. Engraftable HSCs were induced from G-iPSCs through teratoma formation. Figure S3. Establishment of gene-corrected mγc-iPSCs from X-SCID mice. Figure S4. Induction of engraftable HSCs from hiPSCs through teratoma formation. Figure S5. iPSC-derived hematopoietic cells including HSCs and HSC niche components were co-exist in teratomas. Table S1. Chimerism of iPS-derived hematopoietic cells in recipient mice 12 weeks after bone marrow transplantation.
Acknowledgments
We thank Kazuo Sugamura and Naoto Ishii for the X-linked severe combined immunodeficiency mice, Yumiko Ishii and Stephanie Napier for technical assistance, and Masataka Kasai, Hideo Ema, and A.S. Knisely for critical review of the manuscript. This work was supported in part by grants from the Ministry of Education, Culture, Sport, Science and Technology, Japan. The authors declared no conflict of interest.
Supplementary Material
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takayama N, Nishimura S, Nakamura S, Shimizu T, Ohnishi R, Endo H, et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Pilat S, Carotta S, Schiedlmeier B, Kamino K, Mairhofer A, Will E, et al. HOXB4 enforces equivalent fates of ES-cell-derived and adult hematopoietic cells. Proc Natl Acad Sci USA. 2005;102:12101–12106. doi: 10.1073/pnas.0505624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledran MH, Krassowska A, Armstrong L, Dimmick I, Renström J, Lang R, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Risueño RM, Sachlos E, Lee JH, Lee JB, Hong SH, Szabo E, et al. Inability of human induced pluripotent stem cell-hematopoietic derivatives to downregulate microRNAs in vivo reveals a block in xenograft hematopoietic regeneration. Stem Cells. 2012;30:131–139. doi: 10.1002/stem.1684. [DOI] [PubMed] [Google Scholar]
- Cudennec C, Nicolas JF. Blood formation in a clonal cell line of mouse teratocarcinoma. J Embryol Exp Morphol. 1977;38:203–210. [PubMed] [Google Scholar]
- Cudennec CA, Salaün J. Definitive red blood cell differentiation in a clonal line of mouse teratocarcinoma cultured in vivo in the chick embryo. Cell Differ. 1979;8:75–82. doi: 10.1016/0045-6039(79)90027-7. [DOI] [PubMed] [Google Scholar]
- Cudennec CA, Johnson GR. Presence of multipotential hemopoietic cells in teratocarcinoma cultures. J Embryol Exp Morphol. 1981;61:51–59. [PubMed] [Google Scholar]
- Li Z, Huang H, Boland P, Dominguez MG, Burfeind P, Lai KM, et al. Embryonic stem cell tumor model reveals role of vascular endothelial receptor tyrosine phosphatase in regulating Tie2 pathway in tumor angiogenesis. Proc Natl Acad Sci USA. 2009;106:22399–22404. doi: 10.1073/pnas.0911189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL. Niche recycling through division-independent egress of hematopoietic stem cells. J Exp Med. 2009;206:2837–2850. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Seita J, Ema H, Ooehara J, Yamazaki S, Tadokoro Y, Yamasaki A, et al. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc Natl Acad Sci USA. 2007;104:2349–2354. doi: 10.1073/pnas.0606238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki S, Morita H, Tezuka Y, Takatsu K. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. J Exp Med. 2002;195:151–160. doi: 10.1084/jem.20011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, et al. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell. 2005;8:907–914. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Chang KH, Nelson AM, Cao H, Wang L, Nakamoto B, Ware CB, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol. 2004;32:1226–1237. doi: 10.1016/j.exphem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Tian X, Woll PS, Morris JK, Linehan JL, Kaufman DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24:1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- Burt RK, Verda L, Kim DA, Oyama Y, Luo K, Link C. Embryonic stem cells as an alternate marrow donor source: engraftment without graft-versus-host disease. J Exp Med. 2004;199:895–904. doi: 10.1084/jem.20031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Nagao Y, Kitano Y, Hasegawa H, Shibata H, Takatoku M, et al. Hematopoietic microchimerism in sheep after in utero transplantation of cultured cynomolgus embryonic stem cells. Transplantation. 2005;79:32–37. doi: 10.1097/01.tp.0000144058.87131.c5. [DOI] [PubMed] [Google Scholar]
- Amabile G, Welner RS, Nombela-Arrieta C, D’Alise AM, Di Ruscio A, Ebralidze AK, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121:1255–1264. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.