Summary
Several solute carriers and ATP-binding cassette transporters have been implicated in the influx or efflux of platinum-based chemotherapeutic agents such as cisplatin, carboplatin, and oxaliplatin. Given that many of these proteins are highly polymorphic, the genetic status of these proteins could be an important contributor to the extensive interindividual pharmacokinetic variability associated with the clinical use of these agents. In this review article, we provide an updated overview of the various transporters that have shown promise in animal models or patient populations in facilitating the movement of platinum-based agents across cell membranes, and how their function is associated with drug disposition or pharmacodynamic effects.
Keywords: Solute carriers, Cisplatin, Carboplatin, Oxaliplatin, Drug disposition, SNPs, Pharmacodynamics
Introduction
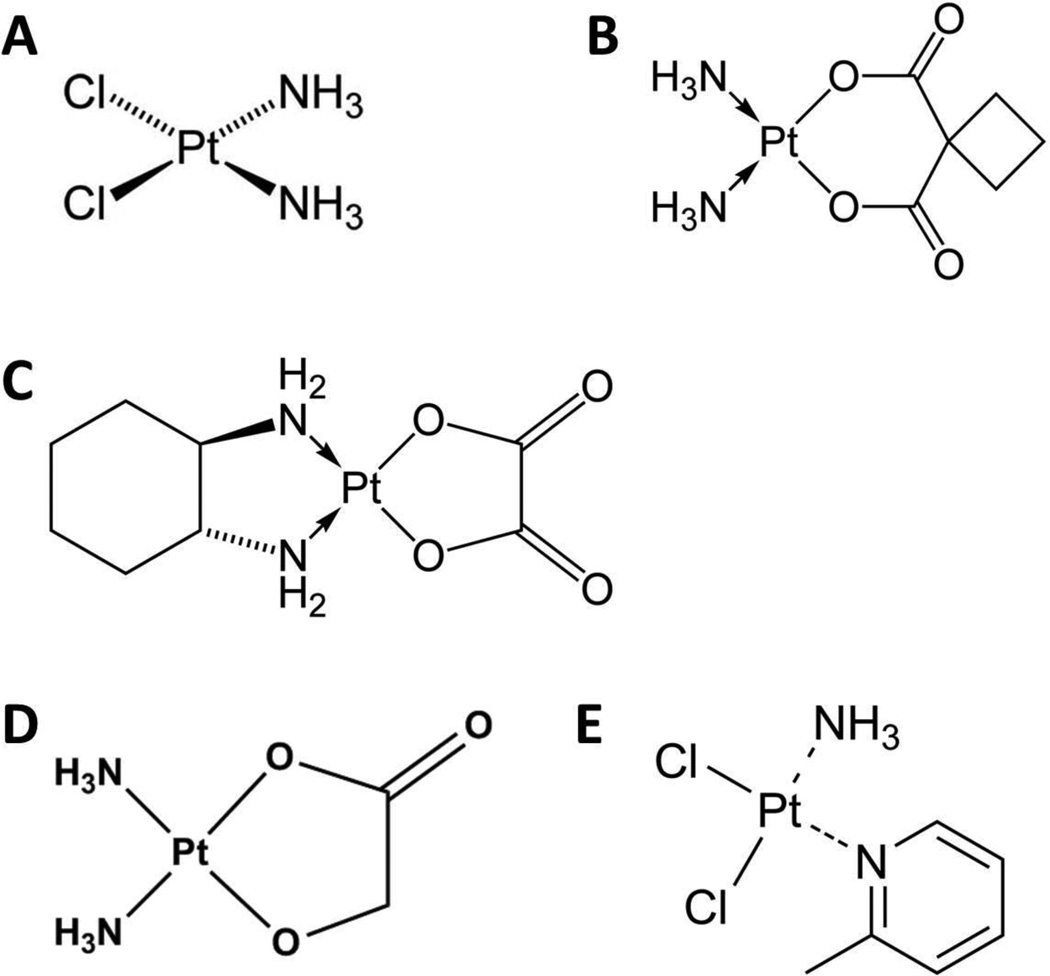
Platinum-based chemotherapeutic agents are among the most widely used compounds for treatment of human malignancies.1–6) It is believed that the dominant antineoplastic effects of this class of drugs are the result of their ability to form interstrand or intrastrand cross-linking with nuclear DNA which ultimately promotes DNA damage and initiates cell cycle arrest or apoptosis.7,8) Cisplatin [cis-diamminedichloroplatinum (II)] was the first platinum drug approved for clinical use (Fig. 1A) and remains one of the most widely used anti-cancer agents in the United States.9,10) The introduction of cisplatin was a significant contribution to improving treatment outcome for various diseases, most notably in patients with testicular cancer who now benefit from a 90–95% survival rate.11) Unfortunately, its use is limited by the onset of a series of debilitating adverse effects that include nephrotoxicity, neurotoxicity, gastrointestinal toxicity and ototoxicity.10) Due to the severity of these toxicities additional platinum-based compounds were developed. These include carboplatin (Fig. 1B), which was approved by the FDA in 1989 and has lower incidences of neurotoxicity, nausea, hearing loss and renal toxicity compared to cisplatin, although patients receiving carboplatin are at risk of developing severe thrombocytopenia and anemia.10,12,13) Carboplatin has a spectrum of antitumor activity that is partially overlapping with cisplatin and thus would be a candidate to replace cisplatin. However, for testicular and head–neck cancer, there is evidence for the inferiority of carboplatin compared with cisplatin. Furthermore, meta-analyses in non-small cell lung cancer suggest that cisplatin-based chemotherapy is superior to carboplatin-based chemotherapy in terms of response rate and, in certain subgroups, in prolonging survival without being associated with increased severe toxic effects. As a result, cisplatin is increasingly replacing carboplatin as the preferred platinum drug in routine polychemotherapy protocols for multiple indications despite the risk for the development of nephrotoxicity.12,14)
Fig. 1.
Chemical structures of cisplatin (A), carboplatin (B), oxaliplatin (C), nedaplatin (D) and picoplatin (E).
The most recently FDA approved platinum-based agent, oxaliplatin (Fig. 1C), has demonstrated efficacy in patients diagnosed with metastatic colorectal cancer, a disease that is characteristically unresponsive to other platinum chemotherapeutics, and retains its antineoplastic properties in tumors that have developed resistance to cisplatin and carboplatin.15–18) Although oxaliplatin is not associated with nephrotoxicity or ototoxicity, and is less myelosuppresive than carboplatin, its use is limited by the development of both acute and chronic sensory and motor neuropathies.15)
The antitumor efficacy of platinum-based chemotherapeutics has been directly linked with accumulation into target cells, and data acquired throughout the past decade has indicated that uptake of these agents across the cellular lipid bilayer membrane is at least partially mediated by specific carrier proteins.19–21) Additionally, proteins involved in the cellular efflux of platinum species are of great importance in regulating intracellular accumulation and cellular sensitivity. This manuscript provides an overview of recent studies that have enhanced our understanding of polymorphic influx and efflux transporters as regulators of the pharmacokinetic and pharmacodynamic properties of platinum drugs.
The Organic Cation Transporters
Organic Cation Transporter 1
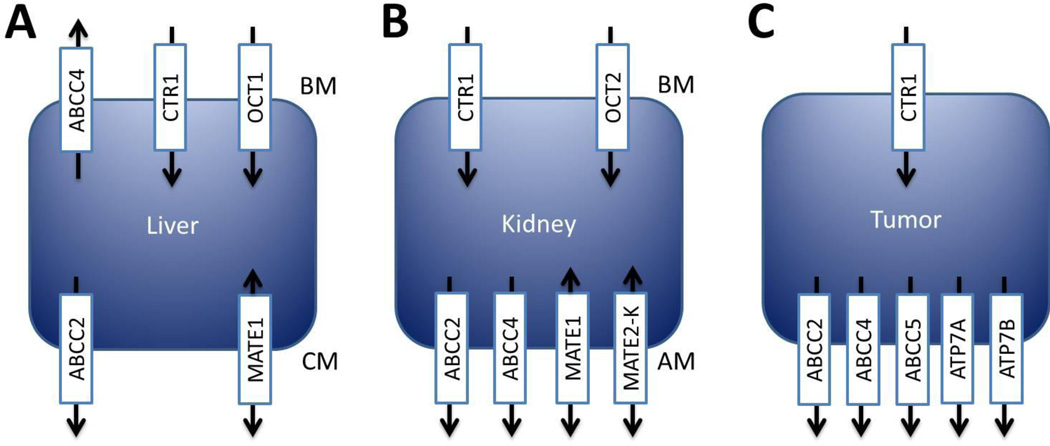
The organic cation transporter 1 (OCT1), encoded by the gene SLC22A1, mediates the intracellular uptake of a variety of structurally diverse organic cations. The discovery that organic cation transporters were highly expressed in tissues that were susceptible to platinum-based toxicities and that administration of cisplatin could inhibit uptake of tetraethyl ammonium (TEA) in rat kidneys, highlighted the possible clinical importance of these transporters and lead to the cloning of the murine homologue Oct1.22,23) Murine Oct1 is highly expressed in the basolateral membrane of renal proximal tubular cells of the kidney, in hepatocytes of the liver, as well as in the intestine,23) while the human homologue is predominantly localized to the basolateral membrane of hepatocytes and acts as the primary step to hepatic excretion of endogenous cations or xenobiotics (Fig. 2A).24) Interestingly, unlike in mice, immunohistochemical staining of human kidneys demonstrated OCT1 expression on the apical side of proximal and distal tubules.25)
Fig 2.
Transporters involved in the uptake or elimination of platinum-based chemotherapeutic agents in human liver (A), kidney (B), or tumor (C). Arrows depict the direction in which transport occurs at the basolateral (BM), canalicular (CM) or apical membrane (AM).
The role of OCT1 in mediating transport of platinum agents is largely conflicted. Multiple in vitro studies have concluded that both carboplatin and cisplatin are either weak substrates of human OCT1 or not transported by OCT1 at all.26,27) However, although human OCT1 does not appear to have an important role alone in the pharmacokinetics of cisplatin in vivo, loss of Oct1 function coupled with the loss of the organic cation transporter 2 (see below) seems to be necessary for protection of cisplatin-mediated nephrotoxicity or altered urinary elimination in mice (see below). Nonetheless, one recent study reported that common loss-of-function variants in the OCT1 gene were not associated with drug-induced nephrotoxicity in 79 patients who had received cisplatin.28)
Some studies have reported that carboplatin, oxaliplatin and nedaplatin (Fig. 1D) are not substrates of OCT1.27,29) In contrast, another study revealed that oxaliplatin, as well as picoplatin (Fig. 1E), may in fact be substrates of OCT1.30,31) In light of this latter finding, it was proposed that OCT1 present in colorectal tumors or cell lines could regulate efficacy of oxaliplatin. A follow-up study by Li et al indicated that Oct1 deficiency decreased uptake of oxaliplatin into primary mouse hepatocytes, although the administration of oxaliplatin directly in Oct1-deficient mice did not significantly alter accumulation in plasma, liver, kidney, intestine, heart, lung, spleen and muscles compared to wildtype controls, suggesting that OCT1 alone may not be sufficient in regulating platinum elimination via hepatocytes.32)
Organic Cation Transporter 2
The solute carrier known as the organic cation transporter 2 (OCT2) (gene symbol: SLC22A2) is strongly expressed and localized in the basolateral membrane of proximal tubule epithelial cells (Fig. 2B), as well as the inner ear of mice.33–35) Studies have been in general agreement that OCT2 greatly facilitates the movement of cisplatin and oxaliplatin, but not carboplatin, across the lipid bilayer.29,30,36,37) Interestingly, there is currently no existing clinical data to suggest that OCT2 expression or function is a critical determinant of cisplatin or oxaliplatin uptake in tumors, although an investigation of the NCI60 panel of cell lines has revealed that OCT2 was most highly expressed in ovarian cancer cell lines, suggesting that the efficacy of platinum-based therapy in this disease could be associated with tumoral expression of OCT2.37) However, tumor samples obtained from patients diagnosed with ovarian cancer were reported to have either low or absent expression of OCT2, and no correlation could be made between expression and treatment outcome. Meanwhile, oxaliplatin has been continuously shown as the superior substrate of OCT2 compared to cisplatin, and due to this efficiency some have hypothesized that the efficacy of oxaliplatin may be regulated by expression of OCT2 in colon cancer specimens.30,37) Unfortunately, although OCT2 expression was detectable in a subset of colon cancer tissue samples and is apparently not expressed in normal colon,30) no correlation between OCT2 expression and oxaliplatin efficacy in cell lines or patients has been reported. In contrast, a previous study reported that expression of OCT2 in various tumor types, including colorectal samples, was limited strictly to tumors of kidney origin, which suggests that OCT2 may not regulate platinum uptake into the majority of tumors.38) In agreement with the notion that OCT2 may not regulate tumoral uptake of platinum drugs, the OCT2 inhibitor cimetidine was shown to be ineffective in altering cisplatin accumulation within SK-OV-3 cells, a cell model with relatively high levels of OCT2 expression, and did not alter cisplatin efficacy or tumor uptake in rats bearing osteosarcoma SOSN2-xenografts.39,40)
Despite that its expression or function may not be involved in tumor uptake of platinum-agents, OCT2 does appear to play an important role in the elimination of cisplatin via the urine, as demonstrated in a murine model deficient of both the Oct1 and Oct2 transporters.38) These animals are also significantly protected from nephrotoxicity and show no changes in platinum plasma concentrations compared to wildtype mice. Likewise, BUN and serum creatinine levels, both markers of nephrotoxicity, have been reported to be lower in mice that received cisplatin in combination with cimetidine.40) Furthermore, OCT2 expression has also been detected in the inner ear of the mouse cochlea and loss of hearing associated with the use of cisplatin is ameliorated in mice deficient of Oct2 or in wildtype mice treated with cimetidine.35) Interestingly, the protective phenotype associated with platinum-induced nephrotoxicity in mice is dependent on the deficiency of not only Oct2 but Oct1 as well, which is expressed in the proximal tubules of mice unlike what is observed in humans who dominantly express OCT2 alone on the basolateral membrane. Nonetheless, OCT2 has demonstrated great importance for the accumulation of platinum drugs in humans and mouse models. For example, administration of cisplatin to Sprague-Dawley rats has been reported to reduce mRNA of Oct2 expression at 7 days suggesting that a defense response is initiated to reduce uptake and subsequent damage.41) It has also been observed that OCT2 expression is higher in male rats as opposed to females which may correlate with the fact that female patients have lower systemic clearance of cisplatin compared to male patients.36,42,43)
In agreement with observations made in the knockout mouse model, patients that possess an A270S OCT2 variant display protection from nephrotoxicity as measured by changes in serum creatinine while patients with the reference genotype do not.38) The A270S variant (rs316019) is caused by a G>T substitution at the 808 position of the SLC22A2 gene, which appears to occur at relatively high allelic frequency and has been associated with decreased transport of other OCT2 substrates.44) These findings were recently confirmed in a separate patient cohort.45) Interestingly, the A270S OCT2 variant has been reported to not have altered recognition of cisplatin, considering that no change in accumulation was reported in overexpressing HEK293 cells compared to cells overexpressing the wildtype protein.39) Despite this, another study that observed the A270S variant in a Chinese population who had received platinum therapy recently provided evidence supporting a decreased function role by revealing that patients with the A270S variant were protected from nephrotoxicity as measured by cystatin C levels. Moreover, combining cimetidine with cisplatin-based therapy also provided protection from nephrotoxicity.46) It is important to note however, that this study measured cystatin c only at a fixed time interval while it would have been more beneficial to assess whether this correlation holds true throughout treatment and over multiple time points. In contrast to the above studies, Tzvetkov et al were unable to significantly associate patients with the A270S OCT2 variant and protection from nephrotoxicity as measured by increased cystatin C or serum creatinine levels, although it was observed that patients who had received potential OCT2 inhibitors in combination with cisplatin showed a trend towards decreased estimate glomerular filtration rates and a lower increase of cystatin C levels than patients who were not exposed to these compounds.28) Likewise, associations between the A270S variant and altered systemic or renal clearance could not be made in another study, although no patients that were homozygous for the variant were included within the studied population.36) Taken together, OCT2 appears to play an important role in platinum-induced toxicities and functional variants, such as the A270S variant, may aid in identifying patients who are at reduced risk of nephrotoxicity.
Organic Cation Transporter 3
The human organic cation transporter 3 (OCT3) is encoded by the gene SLC22A3 and its transcript has been detected in the placenta, adrenal gland, liver, kidney, heart, lung, brain and intestine.47) Unlike the family members, OCT1 and OCT2, OCT3 has not been studied in great detail, although various studies have provided evidence that cisplatin and carboplatin are not substrates.27,29,30) Meanwhile, the same in vitro uptake studies that involved oxaliplatin have suggested that this compound may be a substrate for both the rat and human homologues of OCT3. Overexpression of OCT3 in HEK293 cells did not alter the cytotoxicity of oxaliplatin, suggesting that this transporter may not be clinically relevant in the pharmacokinetics of platinum drugs.30)
Organic Cation Transporters Novel 1 and 2
The two final SLC22 family members that have also been observed in association with the pharmacokinetics of platinum chemotherapeutics are the organic cation transporters novel 1 (OCTN1 encoded by the gene SLC22A4) and 2 (OCTN2 encoded by the gene SLC22A5). Both OCTN1 and OCTN2 are localized to the apical membrane of proximal tubules where OCTN2 prevents L-carnitine wasting using Na+ symport to recycle L-carnitine in proximal tubule cells.48) There has not been a great deal of studies performed in regards to platinum agents as substrates for these transporters, which is likely a result of initial observations suggesting that cisplatin may not be a substrate of OCTN1 or OCTN2.27,49) Likewise, oxaliplatin uptake has been shown to be unchanged in HEK293 cells overexpressing OCTN1 or OCTN2 and both carboplatin and oxaliplatin were unable to compete for carnitine uptake in OCTN2 overexpressing HEK293 cells.27,49) However, the possibility of these transporters in regulating platinum uptake was re-evaluated by a recent investigation in which oxaliplatin uptake and increased cytotoxicity was shown to be regulated by OCTN1 and OCTN2 expression and function.50) Despite these results, there is no current data to demonstrate that these transporters have clinical relevance in the uptake of platinum agents in humans.
Multidrug and Toxin Extrusion Transporters
Multidrug and Toxin Extrusion 1
The multidrug and toxin extrusion 1 (MATE1) transporter is encoded by the SLC47A1 gene and is expressed in the liver and the brush-border membrane of renal proximal tubules (Fig. 2A/B) as well as in skeletal muscle, adrenal gland and testis.51) This solute carrier is a major contributor to the efflux of a variety of cationic compounds and is driven by antiport of the proton gradient.52) Its role in regulating platinum agents remains uncertain as shown by the mixed reports involving cisplatin as a substrate of MATE1, although carboplatin is not considered a substrate.27,29) A recent study has demonstrated that Mate1 (−/−) mice who receive cisplatin, experience increased nephrotoxicity and increased renal accumulation compared to wildtype controls.53) To date various studies have identified numerous single nucleotide variants that can potentially alter the structure and function of MATE1, however only one study has attempted to associate MATE1 variants with cisplatin-induced toxicity. They revealed that the rs2289669 G>A MATE1 variant was not associated with adverse effects, despite being previously shown to result in decreased function.45) Unfortunately, this study did not take into account additional transporters such as MATE2-K (see below) that could possibly compensate for its loss of function.
While the role of MATE1 in the transport of cisplatin has been questioned, it has been typically unanimously shown that oxaliplatin is indeed a substrate of both murine Mate1, as well as the human homologue using over expressing cell lines.27,29) However, other than in vitro data, there is currently no data to indicate that MATE1 plays a role in oxaliplatin pharmacokinetics or adverse effects. Therefore, there is additional study required to elucidate the role of MATE1 in the regulation of platinum agents.
Multidrug and Toxin Extrusion 2
The multidrug and toxin extrusion 2 (MATE2), is transcribed by the gene transcript SLC47A2 that encodes for 602 amino acids that yields two additional isoforms known as MATE2-K and MATE2-B, which are made of 566 and 220 amino acids, respectively. Of these isoforms, only MATE2-K has been shown to have transport function and is almost exclusively expressed in the luminal membrane of renal proximal tubular epithelial cells in humans (Fig. 2B), while it is not expressed in mice.54) Although it does not appear to be a regulator of carboplatin transport and its ability to transport cisplatin is questionable, much like MATE1, oxaliplatin is considered to be an exceptional substrate of MATE2-K.27,29) Due to this high efficiency, it has been proposed that oxaliplatin is less nephrotoxic as a result of hastened efflux via MATE2-K as opposed to cisplatin which would be retained within the proximal tubules longer in comparison. Unfortunately, no clinical or animal data currently exists to verify this hypothesis. However, it is important to note that the affinity for the commonly used OCT2 inhibitor, cimetidine, has been reported to be superior to MATE1 and MATE2-K compared to OCT2 with IC50 values that are in the range that is similar to typical plasma concentrations.55–57) As a result, the use of cimetidine may in fact be potentially harmful when used with platinum agents if export of cellular platinum is inhibited.
Various non-synonymous single nucleotide polymorphisms have been identified for MATE2-K, some of which may alter protein function and have been identified in human heterozygous carriers.58,59) Unfortunately, non-synonymous homozygous MATE polymorphisms have not yet been discovered. While no clinical data is currently available that associates MATE2-K variants with platinum pharmacokinetics, some variants have been observed for their association with the pharmacokinetic analysis of metformin. These findings revealed that plasma concentrations and clearance did not differ between control patients and those heterozygous for these variants, indicating that MATE2-K may not regulate metformin pharmacokinetics or there are compensatory mechanisms.59) Nonetheless, future experiments are required to assess whether the same holds true for platinum-based chemotherapeutic agents.
The Copper Transporters
Copper Transporter 1
Copper transporter proteins (CTR) are members of the SLC31 family of solute carriers and play a major role in regulating cellular homeostasis of Cu+. CTR1, which is encoded by the gene SLC31A1, is made of three transmembrane domains that interact as a homotrimer to form a pore of nice helices through the lipid bilayer.60) The transport of substrates by CTR1 occurs independently of ATP, although its function is influenced by temperature, pH and the K+ gradient.61) CTR1 is ubiquitously expressed in all vertebrate tissues and its loss is embryonically lethal as demonstrated by the CTR1 knockout mouse (Fig. 2).62,63) The potential of CTRs to modulate platinum accumulation has undergone vigorous investigation since the discovery that deficiency of CTR1 in yeast reduced sensitivity and accumulation of cisplatin.64) Additional experiments have since provided evidence that CTR1 facilitates cellular import of not only cisplatin, but carboplatin and oxaliplatin as well.65–67)
Although, despite the compelling evidence that exists in vitro, evidence to show that CTR1 plays an important role in the pharmacokinetics of platinum drugs in vivo is lacking. CTR1 has been reported to be expressed on the basolateral side of proximal tubule cells and appears to modulate uptake of cisplatin in addition to OCT2.68) Furthermore, CTR1 has been shown to be expressed in a subset of neuronal cells in the dorsal root ganglia of rats that appear to be sensitive to the damage that occurs as a result of cisplatin and oxaliplatin exposure and contribute to neurotoxicity.69) Additionally, CTR1 expression has also been detected in outer hair cells, inner hair cells, stria vascularis, spiral ganglia and surrounding nerves in the mouse cochlea, suggesting a possible role of CTR1 in cisplatin-induced ototoxicity.70) Moreover, siRNA mediated knockdown of CTR1 in vitro significantly reduced cisplatin uptake, and intratympanic administration of copper sulfate before administration of cisplatin decreased the severity of hearing loss in mice. In regards to its role in tumor uptake, knockout of CTR1 has been shown to completely eliminate cisplatin tumor response using an in vivo murine model.65) However, a survey of tumors and cell lines with acquired resistance to cisplatin have revealed only few examples in which decreased CTR1 levels were associated with cisplatin resistance.71,72) Instead, recent findings suggest that this disconnect may be a consequence in the failure to glycosylate CTR1 at Thr27 which results in protealytic cleavage at the N-terminus and inactivates transport function.73) Therefore, it is possible that platinum resistant tumors may have decreased levels of glycosylation and while CTR1 expression is unchanged, the protein may in fact be non-functional. However, whether this possibility is of clinical relevance remains to be seen. Nonetheless, despite the conflicting data, one genetic variant of CTR1, rs10981694 A>C, has been associated with cisplatin induced severe toxicity in non-small cell lung cancer (NSCLC) patients in which patients carrying the C allele were more susceptible to ototoxicity.74) Interestingly, this variant was not reported to be associated with overall survival.
While CTR1 has undergone rigorous study, CTR2, a copper transporter that is capable of rescuing the loss of CTR1 has not been well studied. It is surprising, however, that deletion of CTR2 leads to increased accumulation of cisplatin and carboplatin.75) Although this is an interesting phenomenon, additional studies are required to elucidate its true role, if any, in platinum transport or accumulation.
ATP7A and ATP7B
While CTR1 regulates uptake of copper into the cell, its removal is mediated by two P-type ATPases known as ATP7A and ATP7B. These proteins, which share 55% homology, act as monomers and use ATP hydrolysis to drive copper against the concentration gradient. Despite their structural and functional similarities, ATP7A and ATP7B differ in their tissue localization as well as physiological function. ATP7A is expressed in the intestinal epithelium and other tissues with the exception of the liver while ATP7B is expressed in liver, kidney, and to a lesser extent the brain.76,77) Functional mutations of ATP7A, which typically plays a role in providing copper as a cofactor to various enzymes, results in Menkes disease, a disorder that manifests as numerous abnormalities which include neuronal degeneration, osteoporosis, and connective tissue defects.78) Likewise, mutations within the ATP7B gene can lead to the chronic copper toxicosis Wilson’s disease in the liver and central nervous system.
Much like CTR1, in addition to copper, both ATP7A and ATP7B have been shown to modulate accumulation of cisplatin, carboplatin, and oxaliplatin into cells.79,80) It is unknown whether these transporters contribute to platinum sensitivity by promoting efflux from the cell or by sequestering these agents into intracellular compartments, although, studies indicate that ATP7B is strongly correlated with resistance to platinum drugs by regulating efflux (Fig. 2C). Immunohistochemistry and mRNA measurements have shown that many tumor types with higher expression of ATP7B correlates with unfavorable response to platinum drugs.81,82) Increased expression of ATP7B has also been identified as a marker of cisplatin resistance in NSCLC xenograft models.83) Similarly, low mRNA expression of ATP7B has been associated with longer time to progression and more beneficial treatment response to oxaliplatin.84) While evidence for ATP7A is not as robust, immunohistochemical evidence also indicates that high expression of ATP7A is associated with poor treatment outcome in patients receiving platinum drugs when measured by clinical tumor response.85) Additionally, in a separate study, ATP7A expression was detected in 41.6% of non-small cell lung cancer patients which was not present in adjacent stroma or normal lung tissues and its expression was significantly associated with poor histological grade or poor response to platinum based chemotherapy.86) Moreover, ATP7A appears to be expressed in smaller neurons that show no neuronal damage induced by oxaliplatin and have no overlap of CTR1 expression.87) Surprisingly, studies involving analysis of the genetic variants of ATP7A and ATP7B in correlation with response or adverse effects are limited. However, an analysis of a Japanese population that identified 38 genetic variants of ATP7A as well as 61 genetic variants of ATP7B showed no association with treatment efficacy or toxicity.88)
ATP-Binding Cassette Proteins
ATP-Binding Cassette Protein Family C2
The ATP-Binding cassette family (ABC) member known as ABCC2 (or multidrug resistance protein 2; MRP2) is expressed at the apical membrane of renal proximal tubules (Fig. 2B) and the canalicular membrane of hepatocytes (Fig. 2A) where it is thought to regulate secretion of organic anions.89,90) Its role in platinum resistance has received a great deal of attention since the discovery that numerous cisplatin resistant cell lines and tumors had increased expression.91,92) Platinum agents are targets of glutathione conjugation which lead to the formation of inactive products and based on the physiological localization of ABCC2 and its ability to transport glutathione-conjugated compounds, this transporter has been continuously thought of as a regulator of cisplatin resistance or elimination.93) Various common single nucleotide polymorphisms have been identified in association with altered protein function, such as the −24C>T and 3972C>T variants. In fact, there are select patient based studies that have been performed where these alterations have shown associations with differential response or severity of toxicities. For example, the −24C>T variant, which has been linked with decreased ABCC2 transcript levels, was associated with higher response rates after irinotecan or platinum-based therapy.94,95) However, other studies have shown opposing results, indicating that the − 24C>T variant was instead correlated with decreased response to platinum-based therapy in a Japanese population with NSCLC.96) Additionally, the latter group also identified that the 3927C>T variant, despite being a silent mutation, was associated with increased overall toxicity in females and thrombocytopenia in both males and females. In light of the above conflicts, a more rigorous study was recently performed which demonstrated that loss of Abcc2 function alone in knockout mouse models or patients with function altering genetic polymorphisms does not regulate cisplatin-induced survival, response, or toxicity, suggesting that if ABCC2 does contribute to disposition, other factors exist that can compensate for its loss.97) In agreement with this study, others have also shown that ABCC2 variants were not linked with the onset or severity of oxaliplatin-induced neurotoxicity in patients with metastatic colorectal cancer, or progression-free and overall-survival in patients with ovarian cancer who received platinum-based chemotherapy.98,99)
ATP-Binding Cassette Protein Family C4
In addition to ABCC2, the ATP-binding cassette protein family member 4 (ABCC4; or MRP4) is also located on the brush border membrane of renal proximal tubular cells and its overexpression has been linked with increased resistance to cisplatin.100,101) In agreement with these observations, silencing of this transporter in cancer cells has been shown to reverse resistance associated with cisplatin.102) Likewise, overexpression of ABCC4 was correlated to oxaliplatin-resistance in an IGROV-1 cell line and silencing by siRNA increased sensitivity and accumulation.103) Meanwhile, overexpression of ABCC4 does not appear to alter sensitivity to carboplatin, although the reason as to why this may be is unclear.104) Due to its localization in the proximal tubular cells it has been suggested that ABCC4 could play a redundant role in the elimination of glutathione-conjugated platinum agents. ABCC4 expression has been reported to be increased following cisplatin treatment in kidney which may suggest that this protein may regulate and act to ameliorate platinum-induced stress.105) However, it appears that loss of ABCC4 alone does not alter cisplatin pharmacokinetics, at least in murine models.97) Although, despite its possible redundant role in platinum pharmacokinetics, genetic variants of ABCC4 found in 973 lung cancer patients were associated with changes in survival, indicating that altered expression or function can be important for platinum efficacy.106)
ATP-Binding Cassette Protein Family C5
The ATP-binding cassette protein family member 5 (ABCC5; or MRP5) is a membrane efflux protein that was originally isolated in 1997 through a human expressed sequence tag screening and is found in nearly every tissue of the body.92) The mRNA transcript of ABCC5 has been shown to increase after platinum drug treatment in both mice and humans.105,107) Unfortunately, there is mixed evidence as to its role in platinum drug resistance as demonstrated by a study that indicated that ABCC5 mRNA levels prior to chemotherapy was not predictive of platinum sensitivity in cancer cell lines or neuroblastoma patients.92,108,109) In contrast, increased transcript of ABCC5 in vitro correlated with inferior response to a combinational treatment of oxaliplatin and celecoxib, suggesting that ABCC5 does play a role in platinum efficacy.110) Furthermore, inhibition of the ABCC5/GS-X pump in both a hepatocellular carcinoma cell line and in a mouse model, has been shown to increase cisplatin anticancer efficacy.101,111) Together these studies indicate that, although baseline ABCC5 transcript levels do not have a predictive role in response to platinum chemotherapy, chronic rise in ABCC5 levels promotes increased efflux of platinum compounds and decreases efficacy. Despite the promise of the above studies in associating ABCC5 levels with platinum-mediated treatment response, to date no studies have looked into genetic variations of ABCC5 and how these variants could contribute to efficacy of platinum chemotherapies. Therefore, future studies will be required to identify its role as a mediator of platinum accumulation.
Organic Anion Transporters
The first organic anion transporter (OAT1, gene code: SLC22A6) was identified in 1997 in both the rat and flounder112–114), followed by OAT2 (gene code: SLC22A7), which was originally isolated from the rat in 1998.115) OAT1 is localized to the basolateral membrane of renal proximal tubules,116) and OAT2 is specific to the sinusoidal membrane of hepatocytes117). Cisplatin treatment has been shown to decrease the mRNA levels in kidneys of the mouse homologues Oat1 and Oat2.105) The purpose for this phenomenon remains unknown. However in agreement, the OAT1 inhibitor, thymoquinone, has also been shown to decrease mRNA levels of the rat homologue of Oat1 in kidneys and protect against cisplatin induced nephrotoxicity, suggesting that OAT1 may play a role in platinum drug associated toxicity.118) Furthermore, multiple studies have demonstrated that organic anions such as probenecid can significantly decrease the renal clearance of cisplatin without affecting GFR in animals119) and cancer patients,120) as well as reduce nephrotoxicity121,122) which suggests that the platinum-sulfhydryl group complexes formed are taken up by the kidney cells through an organic anion transport mechanism that is probenecid-sensitive.123) Unfortunately, follow-up studies have not been performed to provide direct evidence in the contribution of these transporters in platinum uptake, and there are currently no studies that have investigated the effect of SNPs in these transporters.
Conclusions and Future Directions
As the field of pharmacology becomes more directed to personalized medicine and identifying factors that regulate interpatient variability, it is evident that a sound understanding in the role of protein transporters as regulators of platinum uptake and disposition is required. The expression and function of many of the transporters discussed above have been identified as polymorphic proteins and therefore, the functional or expression altering variants could potentially play a dominant role in altering the intensity of toxicity or efficacy associated with the use of platinum drugs. Taken together, this review demonstrates that the consequence of genetic variants in association with efficacy or toxicity is greatly unexplored, highlighting the need to accelerate the identification of all variants and to test associations in adequate patient samples. While many solute carriers and ATP-mediated transporters have already been identified as key regulators in platinum-induced toxicity and efficacy, in the coming years their specific roles and the consequence of genetic variants will likely become more evident and the role of additional transporters will likely be identified which will be of great importance in determining or mediating effectiveness and adverse effects of platinum agents.
Acknowledgment
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC), United States Public Health Service (USPHS) Cancer Center Support Grant 3P30CA021765, and the National Institutes of Health (grant 5R01CA151633-03).
References
- 1.Horn L, Castellanos EL, Johnson DH. Update on new drugs in small cell lung cancer. Expert. Opin. Investig. Drugs. 2011;20:441–445. doi: 10.1517/13543784.2011.553185. [DOI] [PubMed] [Google Scholar]
- 2.Shelley MD, Cleves A, Wilt TJ, Mason MD. Gemcitabine chemotherapy for the treatment of metastatic bladder carcinoma. BJU Int. 2011;108:168–179. doi: 10.1111/j.1464-410X.2011.10341.x. [DOI] [PubMed] [Google Scholar]
- 3.Collins IM, Roberts-Thomson R, Faulkner D, Rischin D, Friedlander M, Mileshkin L. Carboplatin Dosing in Ovarian Cancer: Problems and Pitfalls. Int. J. Gynecol. Cancer. 2011 doi: 10.1097/IGC.0b013e31822127ad. [DOI] [PubMed] [Google Scholar]
- 4.Nichols C, Kollmannsberger C. First-line chemotherapy of disseminated germ cell tumors. Hematol. Oncol. Clin. North Am. 2011;25:543–556. doi: 10.1016/j.hoc.2011.03.011. viii. [DOI] [PubMed] [Google Scholar]
- 5.Chitapanarux I, Tharavichitkul E, Lorvidhaya V, Sittitrai P, Pattarasakulchai T. Induction chemotherapy with paclitaxel, ifosfamide, and cisplatin followed by concurrent chemoradiotherapy for unresectable locally advanced head and neck cancer. Biomed. Imaging Interv. J. 2010;6:e23. doi: 10.2349/biij.6.3.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J. Clin. Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Zwelling LA, Kohn KW. Mechanism of action of cis-dichlorodiammineplatinum(II) Cancer Treat. Rep. 1979;63:1439–1444. [PubMed] [Google Scholar]
- 8.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 9.Higby DJ, Wallace HJ, Jr, Albert DJ, Holland JF. Diaminodichloroplatinum: a phase I study showing responses in testicular and other tumors. Cancer. 1974;33:1219–1215. doi: 10.1002/1097-0142(197405)33:5<1219::aid-cncr2820330505>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol. Cancer. Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavan D. Testicular cancer: maintaining the high cure rate. Oncology (Williston Park) 2003;17:218–228. discussion 228–219, 234–215, passim. [PubMed] [Google Scholar]
- 12.Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, Radosavljevic D, Paccagnella A, Zatloukal P, Mazzanti P, Bisset D, Rosell R. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J. Natl. Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 13.Rajeswaran A, Trojan A, Burnand B, Giannelli M. Efficacy and side effects of cisplatin-and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer. 2008;59:1–11. doi: 10.1016/j.lungcan.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Schuler PJ, Trellakis S, Greve J, Bas M, Bergmann C, Bolke E, Lehnerdt G, Mattheis S, Albers AE, Brandau S, Lang S, Whiteside TL, Bier H, Hoffmann TK. In vitro chemosensitivity of head and neck cancer cell lines. Eur. J. Med. Res. 2010;15:337–344. doi: 10.1186/2047-783X-15-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim A, Hirschfeld S, Cohen MH, Griebel DJ, Williams GA, Pazdur R. FDA drug approval summaries: oxaliplatin. Oncologist. 2004;9:8–12. doi: 10.1634/theoncologist.9-1-8. [DOI] [PubMed] [Google Scholar]
- 16.Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, Fojo T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem. Pharmacol. 1996;52:1855–1865. doi: 10.1016/s0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- 17.Raymond E, Faivre S, Chaney S, Woynarowski J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol. Cancer Ther. 2002;1:227–235. [PubMed] [Google Scholar]
- 18.Mishima M, Samimi G, Kondo A, Lin X, Howell SB. The cellular pharmacology of oxaliplatin resistance. Eur. J. Cancer. 2002;38:1405–1412. doi: 10.1016/s0959-8049(02)00096-5. [DOI] [PubMed] [Google Scholar]
- 19.Gale GR, Morris CR, Atkins LM, Smith AB. Binding of an antitumor platinum compound to cells as influenced by physical factors and pharmacologically active agents. Cancer Res. 1973;33:813–818. [PubMed] [Google Scholar]
- 20.Binks SP, Dobrota M. Kinetics and mechanism of uptake of platinum-based pharmaceuticals by the rat small intestine. Biochem. Pharmacol. 1990;40:1329–1336. doi: 10.1016/0006-2952(90)90400-f. [DOI] [PubMed] [Google Scholar]
- 21.Dobson PD, Lanthaler K, Oliver SG, Kell DB. Implications of the dominant role of transporters in drug uptake by cells. Curr. Top. Med. Chem. 2009;9:163–181. doi: 10.2174/156802609787521616. [DOI] [PubMed] [Google Scholar]
- 22.Nelson JA, Santos G, Herbert BH. Mechanisms for the renal secretion of cisplatin. Cancer Treat. Rep. 1984;68:849–853. [PubMed] [Google Scholar]
- 23.Grundemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372:549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- 24.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 25.Tzvetkov MV, Vormfelde SV, Balen D, Meineke I, Schmidt T, Sehrt D, Sabolic I, Koepsell H, Brockmoller J. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin. Pharmacol. Ther. 2009;86:299–306. doi: 10.1038/clpt.2009.92. [DOI] [PubMed] [Google Scholar]
- 26.Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem. Pharmacol. 2005;70:1823–1831. doi: 10.1016/j.bcp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J. Pharmacol. Exp. Ther. 2006;319:879–886. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 28.Tzvetkov MV, Behrens G, O’Brien VP, Hohloch K, Brockmoller J, Benohr P. Pharmacogenetic analyses of cisplatin-induced nephrotoxicity indicate a renoprotective effect of ERCC1 polymorphisms. Pharmacogenomics. 2011;12:1417–1427. doi: 10.2217/pgs.11.93. [DOI] [PubMed] [Google Scholar]
- 29.Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem. Pharmacol. 2007;74:477–487. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.More SS, Li S, Yee SW, Chen L, Xu Z, Jablons DM, Giacomini KM. Organic cation transporters modulate the uptake and cytotoxicity of picoplatin, a third-generation platinum analogue. Mol. Cancer Ther. 2010;9:1058–1069. doi: 10.1158/1535-7163.MCT-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Chen Y, Zhang S, More SS, Huang X, Giacomini KM. Role of organic cation transporter 1, OCT1 in the pharmacokinetics and toxicity of cis-diammine(pyridine)chloroplatinum(II) and oxaliplatin in mice. Pharm. Res. 2011;28:610–625. doi: 10.1007/s11095-010-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, Inui K. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 2002;13:866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- 34.Nies AT, Herrmann E, Brom M, Keppler D. Vectorial transport of the plant alkaloid berberine by double-transfected cells expressing the human organic cation transporter 1 (OCT1, SLC22A1) and the efflux pump MDR1 P-glycoprotein (ABCB1) Naunyn Schmiedebergs Arch. Pharmacol. 2008;376:449–461. doi: 10.1007/s00210-007-0219-x. [DOI] [PubMed] [Google Scholar]
- 35.Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jurgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filipski KK, Loos WJ, Verweij J, Sparreboom A. Interaction of Cisplatin with the human organic cation transporter 2. Clin. Cancer Res. 2008;14:3875–3880. doi: 10.1158/1078-0432.CCR-07-4793. [DOI] [PubMed] [Google Scholar]
- 37.Burger H, Zoumaro-Djayoon A, Boersma AW, Helleman J, Berns EM, Mathijssen RH, Loos WJ, Wiemer EA. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) Br. J. Pharmacol. 2010;159:898–908. doi: 10.1111/j.1476-5381.2009.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franke RM, Kosloske AM, Lancaster CS, Filipski KK, Hu C, Zolk O, Mathijssen RH, Sparreboom A. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin. Cancer Res. 2010;16:4198–4206. doi: 10.1158/1078-0432.CCR-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsuda H, Yamashita M, Katsura H, Yu J, Waki Y, Nagata N, Sai Y, Miyamoto K. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol. Pharm. Bull. 2010;33:1867–1871. doi: 10.1248/bpb.33.1867. [DOI] [PubMed] [Google Scholar]
- 41.Huang Q, Dunn RT, 2nd, Jayadev S, DiSorbo O, Pack FD, Farr SB, Stoll RE, Blanchard KT. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol. Sci. 2001;63:196–207. doi: 10.1093/toxsci/63.2.196. [DOI] [PubMed] [Google Scholar]
- 42.Slitt AL, Cherrington NJ, Hartley DP, Leazer TM, Klaassen CD. Tissue distribution and renal developmental changes in rat organic cation transporter mRNA levels. Drug Metab. Dispos. 2002;30:212–219. doi: 10.1124/dmd.30.2.212. [DOI] [PubMed] [Google Scholar]
- 43.Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab. Dispos. 2006;34:477–482. doi: 10.1124/dmd.105.006932. [DOI] [PubMed] [Google Scholar]
- 44.Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, Shin JG. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin. Pharmacol. Ther. 2008;84:559–562. doi: 10.1038/clpt.2008.61. [DOI] [PubMed] [Google Scholar]
- 45.Iwata K, Aizawa K, Kamitsu S, Jingami S, Fukunaga E, Yoshida M, Yoshimura M, Hamada A, Saito H. Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin. Exp. Nephrol. 2012 doi: 10.1007/s10157-012-0638-y. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Zhou W. Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem. Toxicol. 2012;50:2289–2293. doi: 10.1016/j.fct.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 47.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 48.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 49.Lancaster CS, Hu C, Franke RM, Filipski KK, Orwick SJ, Chen Z, Zuo Z, Loos WJ, Sparreboom A. Cisplatin-induced downregulation of OCTN2 affects carnitine wasting. Clin. Cancer Res. 2010;16:4789–4799. doi: 10.1158/1078-0432.CCR-10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jong NN, Nakanishi T, Liu JJ, Tamai I, McKeage MJ. Oxaliplatin Transport Mediated by Organic Cation/Carnitine Transporters OCTN1 and OCTN2 in Overexpressing Human Embryonic Kidney 293 Cells and Rat Dorsal Root Ganglion Neurons. J. Pharmacol. Exp. Ther. 2011;338:537–547. doi: 10.1124/jpet.111.181297. [DOI] [PubMed] [Google Scholar]
- 51.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17923–17928. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuda M, Terada T, Asaka J, Ueba M, Katsura T, Inui K. Oppositely directed H+ gradient functions as a driving force of rat H+/organic cation antiporter MATE1. Am. J. Physiol. Renal Physiol. 2007;292:F593–598. doi: 10.1152/ajprenal.00312.2006. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem. Pharmacol. 2010;80:1762–1767. doi: 10.1016/j.bcp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 54.Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, Ogawa O, Inui K. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 2006;17:2127–2135. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 55.Matsushima S, Maeda K, Inoue K, Ohta KY, Yuasa H, Kondo T, Nakayama H, Horita S, Kusuhara H, Sugiyama Y. The inhibition of human multidrug and toxin extrusion 1 is involved in the drug-drug interaction caused by cimetidine. Drug Metab. Dispos. 2009;37:555–559. doi: 10.1124/dmd.108.023911. [DOI] [PubMed] [Google Scholar]
- 56.Ohta KY, Inoue K, Yasujima T, Ishimaru M, Yuasa H. Functional characteristics of two human MATE transporters: kinetics of cimetidine transport and profiles of inhibition by various compounds. J. Pharm. Pharm. Sci. 2009;12:388–396. doi: 10.18433/j3r59x. [DOI] [PubMed] [Google Scholar]
- 57.Tsuda M, Terada T, Ueba M, Sato T, Masuda S, Katsura T, Inui K. Involvement of human multidrug and toxin extrusion 1 in the drug interaction between cimetidine and metformin in renal epithelial cells. J. Pharmacol. Exp. Ther. 2009;329:185–191. doi: 10.1124/jpet.108.147918. [DOI] [PubMed] [Google Scholar]
- 58.Kajiwara M, Terada T, Ogasawara K, Iwano J, Katsura T, Fukatsu A, Doi T, Inui K. Identification of multidrug and toxin extrusion (MATE1 and MATE2-K) variants with complete loss of transport activity. J. Hum. Genet. 2009;54:40–46. doi: 10.1038/jhg.2008.1. [DOI] [PubMed] [Google Scholar]
- 59.Toyama K, Yonezawa A, Tsuda M, Masuda S, Yano I, Terada T, Osawa R, Katsura T, Hosokawa M, Fujimoto S, Inagaki N, Inui K. Heterozygous variants of multidrug and toxin extrusions (MATE1 and MATE2-K) have little influence on the disposition of metformin in diabetic patients. Pharmacogenet. Genomics. 2010;20:135–138. doi: 10.1097/FPC.0b013e328335639f. [DOI] [PubMed] [Google Scholar]
- 60.Nose Y, Rees EM, Thiele DJ. Structure of the Ctr1 copper trans’PORE’ter reveals novel architecture. Trends Biochem. Sci. 2006;31:604–607. doi: 10.1016/j.tibs.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002;277:4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 62.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larson CA, Blair BG, Safaei R, Howell SB. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol. Pharmacol. 2009;75:324–330. doi: 10.1124/mol.108.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin X, Okuda T, Holzer A, Howell SB. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol. Pharmacol. 2002;62:1154–1159. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- 67.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 68.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Renal Physiol. 2009;296:F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu JJ, Jamieson SM, Subramaniam J, Ip V, Jong NN, Mercer JF, McKeage MJ. Neuronal expression of copper transporter 1 in rat dorsal root ganglia: association with platinum neurotoxicity. Cancer Chemother. Pharmacol. 2009;64:847–856. doi: 10.1007/s00280-009-1017-6. [DOI] [PubMed] [Google Scholar]
- 70.More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsumoto S, Tanaka T, Kurokawa H, Matsuno K, Hayashida Y, Takahashi T. Effect of copper and role of the copper transporters ATP7A and CTR1 in intracellular accumulation of cisplatin. Anticancer Res. 2007;27:2209–2216. [PubMed] [Google Scholar]
- 72.Zisowsky J, Koegel S, Leyers S, Devarakonda K, Kassack MU, Osmak M, Jaehde U. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem. Pharmacol. 2007;73:298–307. doi: 10.1016/j.bcp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Maryon EB, Zhang J, Jellison JW, Kaplan JH. Human copper transporter 1 lacking O-linked glycosylation is proteolytically cleaved in a Rab9-positive endosomal compartment. The J. Biol. Chem. 2009;284:28104–28114. doi: 10.1074/jbc.M109.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X, Ren H, Zhou B, Zhao Y, Yuan R, Ma R, Zhou H, Liu Z. Prediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012 doi: 10.1016/j.lungcan.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 75.Blair BG, Larson CA, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of Cisplatin and Carboplatin. Clin. Cancer Res. 2009;15:4312–4321. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murata Y, Kodama H, Abe T, Ishida N, Nishimura M, Levinson B, Gitschier J, Packman S. Mutation analysis and expression of the mottled gene in the macular mouse model of Menkes disease. Pediatr. Res. 1997;42:436–442. doi: 10.1203/00006450-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki M, Gitlin JD. Intracellular localization of the Menkes and Wilson's disease proteins and their role in intracellular copper transport. Pediatr. Int. 1999;41:436–442. doi: 10.1046/j.1442-200x.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- 79.Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T, Akiyama S. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60:1312–1316. [PubMed] [Google Scholar]
- 80.Samimi G, Katano K, Holzer AK, Safaei R, Howell SB. Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol. Pharmacol. 2004;66:25–32. doi: 10.1124/mol.66.1.25. [DOI] [PubMed] [Google Scholar]
- 81.Kanzaki A, Toi M, Neamati N, Miyashita H, Oubu M, Nakayama K, Bando H, Ogawa K, Mutoh M, Mori S, Terada K, Sugiyama T, Fukumoto M, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human breast carcinoma. Jpn. J. Cancer Res. 2002;93:70–77. doi: 10.1111/j.1349-7006.2002.tb01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakayama K, Kanzaki A, Ogawa K, Miyazaki K, Neamati N, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) as a cisplatin based chemoresistance marker in ovarian carcinoma: comparative analysis with expression of MDR1, MRP1, MRP2, LRP and BCRP. Int. J. Cancer. 2002;101:488–495. doi: 10.1002/ijc.10608. [DOI] [PubMed] [Google Scholar]
- 83.Nakagawa T, Inoue Y, Kodama H, Yamazaki H, Kawai K, Suemizu H, Masuda R, Iwazaki M, Yamada S, Ueyama Y, Inoue H, Nakamura M. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) correlates with cisplatin resistance in human non-small cell lung cancer xenografts. Oncol. Rep. 2008;20:265–270. [PubMed] [Google Scholar]
- 84.Martinez-Balibrea E, Martinez-Cardus A, Musulen E, Gines A, Manzano JL, Aranda E, Plasencia C, Neamati N, Abad A. Increased levels of copper efflux transporter ATP7B are associated with poor outcome in colorectal cancer patients receiving oxaliplatin-based chemotherapy. Int. J. Cancer. 2009;124:2905–2910. doi: 10.1002/ijc.24273. [DOI] [PubMed] [Google Scholar]
- 85.Samimi G, Varki NM, Wilczynski S, Safaei R, Alberts DS, Howell SB. Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin. Cancer Res. 2003;9:5853–5859. [PubMed] [Google Scholar]
- 86.Li ZH, Qiu MZ, Zeng ZL, Luo HY, Wu WJ, Wang F, Wang ZQ, Zhang DS, Li YH, Xu RH. Copper-transporting P-type adenosine triphosphatase (ATP7A) is associated with platinum-resistance in non-small cell lung cancer (NSCLC) J. Transl. Med. 2012;10:21. doi: 10.1186/1479-5876-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ip V, Liu JJ, Mercer JF, McKeage MJ. Differential expression of ATP7A, ATP7B and CTR1 in adult rat dorsal root ganglion tissue. Mol. Pain. 2010;6:53. doi: 10.1186/1744-8069-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukushima-Uesaka H, Saito Y, Maekawa K, Kurose K, Sugiyama E, Katori N, Kaniwa N, Hasegawa R, Hamaguchi T, Eguchi-Nakajima T, Kato K, Yamada Y, Shimada Y, Yoshida T, Yamamoto N, Nokihara H, Kunitoh H, Ohe Y, Tamura T, Ura T, Saito M, Muro K, Doi T, Fuse N, Yoshino T, Ohtsu A, Saijo N, Matsumura Y, Okuda H, Sawada J. Genetic polymorphisms of copper- and platinum drug-efflux transporters ATP7A and ATP7B in Japanese cancer patients. Drug Metab. Pharmacokinet. 2009;24:565–574. doi: 10.2133/dmpk.24.565. [DOI] [PubMed] [Google Scholar]
- 89.van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J. Am. Soc. Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 90.Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 91.Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- 92.Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- 93.Taniguchi K, Wada M, Kohno K, Nakamura T, Kawabe T, Kawakami M, Kagotani K, Okumura K, Akiyama S, Kuwano M. A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 1996;56:4124–4129. [PubMed] [Google Scholar]
- 94.Han JY, Lim HS, Yoo YK, Shin ES, Park YH, Lee SY, Lee JE, Lee DH, Kim HT, Lee JS. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110:138–147. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
- 95.Sun N, Sun X, Chen B, Cheng H, Feng J, Cheng L, Lu Z. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2010;65:437–446. doi: 10.1007/s00280-009-1046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han B, Gao G, Wu W, Gao Z, Zhao X, Li L, Qiao R, Chen H, Wei Q, Wu J, Lu D. Association of ABCC2 polymorphisms with platinum-based chemotherapy response and severe toxicity in non-small cell lung cancer patients. Lung Cancer. 2011;72:238–243. doi: 10.1016/j.lungcan.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 97.Sprowl JA, Gregorc V, Lazzari C, Mathijssen RH, Loos WJ, Sparreboom A. Associations Between ABCC2 Polymorphisms and Cisplatin Disposition and Efficacy. Clin. Pharmacol. Ther. 2012 doi: 10.1038/clpt.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gamelin L, Capitain O, Morel A, Dumont A, Traore S, Anne le B, Gilles S, Boisdron-Celle M, Gamelin E. Predictive factors of oxaliplatin neurotoxicity: the involvement of the oxalate outcome pathway. Clin. Cancer Res. 2007;13:6359–6368. doi: 10.1158/1078-0432.CCR-07-0660. [DOI] [PubMed] [Google Scholar]
- 99.Tian C, Ambrosone CB, Darcy KM, Krivak TC, Armstrong DK, Bookman MA, Davis W, Zhao H, Moysich K, Gallion H, DeLoia JA. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecol. Oncol. 2012;124:575–581. doi: 10.1016/j.ygyno.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, Tsuruo T, Inazawa J. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- 101.Wakamatsu T, Nakahashi Y, Hachimine D, Seki T, Okazaki K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 2007;31:1465–1472. [PubMed] [Google Scholar]
- 102.Zhang YH, Wu Q, Xiao XY, Li DW, Wang XP. Silencing MRP4 by small interfering RNA reverses acquired DDP resistance of gastric cancer cell. Cancer Lett. 2010;291:76–82. doi: 10.1016/j.canlet.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Beretta GL, Benedetti V, Cossa G, Assaraf YG, Bram E, Gatti L, Corna E, Carenini N, Colangelo D, Howell SB, Zunino F, Perego P. Increased levels and defective glycosylation of MRPs in ovarian carcinoma cells resistant to oxaliplatin. Biochem. Pharmacol. 2010;79:1108–1117. doi: 10.1016/j.bcp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Tian Q, Zhang J, Tan TM, Chan E, Duan W, Chan SY, Boelsterli UA, Ho PC, Yang H, Bian JS, Huang M, Zhu YZ, Xiong W, Li X, Zhou S. Human multidrug resistance associated protein 4 confers resistance to camptothecins. Pharm. Res. 2005;22:1837–1853. doi: 10.1007/s11095-005-7595-z. [DOI] [PubMed] [Google Scholar]
- 105.Aleksunes LM, Augustine LM, Scheffer GL, Cherrington NJ, Manautou JE. Renal xenobiotic transporters are differentially expressed in mice following cisplatin treatment. Toxicology. 2008;250:82–88. doi: 10.1016/j.tox.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moyer AM, Sun Z, Batzler AJ, Li L, Schaid DJ, Yang P, Weinshilboum RM. Glutathione pathway genetic polymorphisms and lung cancer survival after platinum-based chemotherapy. Cancer Epidemiol. Biomarkers Prev. 2010;19:811–821. doi: 10.1158/1055-9965.EPI-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oguri T, Isobe T, Suzuki T, Nishio K, Fujiwara Y, Katoh O, Yamakido M. Increased expression of the MRP5 gene is associated with exposure to platinum drugs in lung cancer. Int. J. Cancer. 2000;86:95–100. doi: 10.1002/(sici)1097-0215(20000401)86:1<95::aid-ijc15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 108.Boiarskikh UA KI, Evshin IS, Sharipov RN, Komel'kov AV, Musatkina EA, Chevkina EM, Sukoian MA, Kolpakov FA, Kashkin KN, Filipenko ML. Prediction of a non-small cell lung cancer sensitivity to cisplatin and paclitaxel based on the marker genes expression. Mol. Biol. (Mosk) 2011;45:652–661. [PubMed] [Google Scholar]
- 109.de Cremoux P, Jourdan-Da-Silva N, Couturier J, Tran-Perennou C, Schleiermacher G, Fehlbaum P, Doz F, Mosseri V, Delattre O, Klijanienko J, Vielh P, Michon J. Role of chemotherapy resistance genes in outcome of neuroblastoma. Pediatr. Blood Cancer. 2007;48:311–317. doi: 10.1002/pbc.20853. [DOI] [PubMed] [Google Scholar]
- 110.Gradilone A PF. Lotti LV, Trifirò E, Martino S, Gandini O, Gianni W, Frati L, Aglianò AM, Gazzaniga P. Celecoxib upregulates multidrug resistance proteins in colon cancer: lack of synergy with standard chemotherapy. Curr. Cancer Drug Targets. 2008;8:414–420. doi: 10.2174/156800908785133178. [DOI] [PubMed] [Google Scholar]
- 111.Sugiyama T, Sadzuka Y. Theanine and glutamate transporter inhibitors enhance the antitumor efficacy of chemotherapeutic agents. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2003;1653:47–59. doi: 10.1016/s0304-419x(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 112.Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression Cloning and Characterization of a Novel Multispecific Organic Anion Transporter. J. Biol. Chem. 1997;272:18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- 113.Sweet DH, Wolff NA, Pritchard JB. Expression Cloning and Characterization of ROAT1. J. Biol. Chem. 1997;272:30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- 114.Wolff NA, Werner A, Burkhardt S, Burckhardt G. Expression cloning and characterization of a renal organic anion transporter from winter flounder. FEBS Lett. 1997;417:287–291. doi: 10.1016/s0014-5793(97)01304-5. [DOI] [PubMed] [Google Scholar]
- 115.Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett. 1998;429:179–182. doi: 10.1016/s0014-5793(98)00585-7. [DOI] [PubMed] [Google Scholar]
- 116.Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am. J. Physiol. 1999;276:F122–F128. doi: 10.1152/ajprenal.1999.276.1.F122. [DOI] [PubMed] [Google Scholar]
- 117.Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. J. Cell Sci. 1994;107:1065–1072. doi: 10.1242/jcs.107.4.1065. [DOI] [PubMed] [Google Scholar]
- 118.Ulu R, Dogukan A, Tuzcu M, Gencoglu H, Ulas M, Ä°lhan N, Muqbil I, Mohammad RM, Kucuk O, Sahin K. Regulation of renal organic anion and cation transporters by thymoquinone in cisplatin induced kidney injury. Food Chem. Toxicol. 2012;50:1675–1679. doi: 10.1016/j.fct.2012.02.082. [DOI] [PubMed] [Google Scholar]
- 119.Klein J, Bentur Y, Cheung D, Moselhy G, Koren G. Renal handling of cisplatin: interactions with organic anions and cations in the dog. Clin. Invest. Med. 1991;14:388–394. [PubMed] [Google Scholar]
- 120.Jacobs C, Coleman CN, Rich L, Hirst K, Weiner MW. Inhibition of cis-diamminedichloroplatinum secretion by the human kidney with probenecid. Cancer Res. 1984;44:3632–3635. [PubMed] [Google Scholar]
- 121.Ross DA, Gale GR. Reduction of the renal toxicity of cis-dichlorodiammineplatinum(II) by probenecid. Cancer Treat. Rep. 1979;63:781–787. [PubMed] [Google Scholar]
- 122.Jacobs C, Kaubisch S, Halsey J, Lum BL, Gosland M, Coleman CN, Sikic BI. The use of probenecid as a chemoprotector against cisplatin nephrotoxicity. Cancer. 1991;67:1518–1524. doi: 10.1002/1097-0142(19910315)67:6<1518::aid-cncr2820670610>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 123.Ban M, Hettich D, Huguet N. Nephrotoxicity mechanism of cis-platinum (II) diamine dichloride in mice. Toxicol. Lett. 1994;71:161–168. doi: 10.1016/0378-4274(94)90176-7. [DOI] [PubMed] [Google Scholar]