Abstract
Background
Saracatinib (AZD0530) is an orally available Src kinase inhibitor. A phase II study was conducted to evaluate saracatinib in patients with recurrent or metastatic head and neck squamous cell cancer (HNSCC).
Patients and Methods
This was an open-label, single-arm, phase II study. Patients received 175 mg saracatinib daily either orally or by percutaneous gastrostomy tube. Radiologic imaging for response was planned at the end of each eight-week cycle.
Results
Nine patients were enrolled. All patients had received prior radiotherapy and six patients had received prior chemotherapy for recurrent or metastatic disease. The most common adverse event was fatigue. Eight patients had progression of disease by response evaluation criteria in solid tumors (RECIST) within the first eight-week cycle and one patient was removed from the study after 11 days due to clinical decline with stable disease according to the RECIST criteria. Median overall survival was six months. The study was closed early due to lack of efficacy according to the early stopping rule.
Conclusion
Single-agent saracatinib does not merit further study in recurrent or metastatic HNSCC.
Keywords: HNSCC, phase II, Src, AZD0530, saracatinib
In patients with recurrent or metastatic head and neck squamous cell cancer (HNSCC) that is not suitable for surgery or further radiation, modest objective response rates are achieved with conventional cytotoxic drugs such as platinum agents, taxanes and antifolates (1, 2). Cetuximab, a monoclonal antibody directed against the extracellular domain of the epidermal growth factor receptor (EGFR), also has modest activity against advanced HNSCC, providing proof-of-principle regarding the potential utility of molecularly targeted agents in the management of this disease (3). However, even with the addition of cetuximab to conventional platinum plus 5-fluorouracil doublet therapy, median survival was only 10.1 months in a phase III study for patients who had received no prior chemotherapy for recurrent or metastatic disease (4). There is a clear need for new treatment strategies in HNSCC given the modest tumor control achieved with standard therapies (2).
Src is a non-receptor tyrosine kinase which attaches to the cytoplasmic aspect of the plasma membrane. Src regulates a wide variety of cellular processes central to the malignant phenotype, including proliferation, survival, adhesion, motility and invasion (5, 6). Increased Src activity in cancer may result from interaction with transmembrane receptors such as EGFR, platelet-derived growth factor receptor and fibroblast growth factor receptor (6). Src activity can be modulated by several other potential mechanisms, including inactivation by Src tyrosine kinase (6). Increased Src activity has been detected in a variety of human solid tumors, including head and neck, colon, breast, ovarian, pancreatic, gastric, esophageal, hepatocellular, bladder and lung carcinomas (6, 7).
Activated Src mediates proliferation and invasion of HNSCC cell lines. Inhibition of Src activity in human HNSCC 1483 cells, performed with either specific Src inhibitors or with a dominant-negative mutant c-Src, reduced cell proliferation and invasion in comparison to control cells (8, 9). In studies with dasatinib, a small-molecule inhibitor of Src family kinases, HNSCC cell lines were markedly more sensitive to growth inhibition than were non-small cell lung cancer cell lines (10). In four out of eight HNSCC cell lines treated with dasatinib, the IC50 for cell proliferation was less than 100 nM. Cell migration and invasion were inhibited in all eight HNSCC cell lines, independently of any effects on cell proliferation and survival. Immuno-histochemical studies confirmed that dasatinib treatment reduced Src autophosphorylation in these cell lines (10). Saracatinib (AZD0530), an orally available small molecule of the anilinoquinazoline class, is a potent and selective inhibitor of Src-family tyrosine kinases (Src, Yes, Lck) and Bcr-Abl, with IC50 values in the low nanomolar range (11, 12). Saracatinib acts through ATP competitive and reversible inhibition of the target enzyme. Saracatinib lacks any significant activity against a large panel of other non Src-family kinases. In preclinical models, saracatinib has demonstrated potent effects on cell motility and invasion (13).
In a phase I study of saracatinib for patients with advanced solid malignancies, the recommended daily phase II dose was 175 mg (14). The median number of prior chemotherapy regimens was 4 (range, 0-18) among enrolled patients. A total of 30 patients were treated in the dose escalation portion of the study (Part A). Dose-limiting toxicities (DLT) occurred in three patients at the 250 mg dose level (leukopenia, grade 5 septic shock with renal failure and asthenia) and in two patients at the 200 mg dose level (febrile neutropenia and dyspnea). An expansion cohort (Part B, n=51 patients) confirmed the 50, 125 and 175 mg doses to be tolerable. Eleven patients remained on study for more than three months, but no objective radiographic responses were seen. Pharmacokinetic data demonstrated that 175 mg daily dosing achieved therapeutic plasma levels and the median half-life after a single dose was approximately 40 h. Analysis of pre-treatment and post-treatment tumor biopsies, using both immunohistochemistry and Luminex methodologies, demonstrated that saracatinib treatment was associated with decreased phosphorylation of the Src substrates paxillin and FAK, which regulate cellular invasion (14).
Because of preclinical observations that saracatinib inhibits cellular motility and invasion, it is proposed that this agent may have value as a disease-stabilization agent in patients with advanced head and neck cancer. This report presents the results of a phase II study of saracatinib in patients with recurrent or metastatic HNSCC (ClinicalTrials.gov identifier NCT00513435). The primary endpoint was to determine whether saracatinib extends progression-free surival (PFS) in this patient population.
Patients and Methods
Patient eligibility
This was an open-label, single-institution trial approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center (MSKCC). All patients provided written informed consent. The study was open to adult patients (≥18 years of age) with recurrent or metastatic HNSCC that was not curable by surgery or radiation therapy. Pathologic confirmation at MSKCC of the HNSCC was required.
Patients were required to not have had more than one prior cytotoxic chemotherapy regimen in the recurrent, persistent or metastatic setting. Molecularly targeted agents (e.g., cetuximab) were not counted as prior cytotoxic therapy. Karnofsky performance status (KPS) ≥60 was required. Required laboratory parameters were: white blood cells ≥3×103/μl, absolute neutrophil count ≥1.5×103/μl, hemoglobin >9 g/dl, platelet count ≥100×103/μl, serum bilirubin within normal limits, AST(SGOT)/ALT(SGPT) ≤2.5 times the upper limit of normal, serum creatinine within normal limits or creatinine clearance ≥60 ml/min/1.73 m3 for patients with creatinine levels above the upper limits of normal. Measurable disease, defined by response evaluation criteria in solid tumors (RECIST) was required.
Exclusion criteria included: chemotherapy or radiotherapy within four weeks; brain metastases; symptomatic congestive heart failure; unstable angina; cardiac arrhythmia; QTc ≥480 m; history of myocardial infarction within one year; pulmonary fibrosis, pleural effusion (non-malignant) and or pneumonitis ≥grade 2; urine protein:creatinine ratio ≥1.0. HIV-positive patients on combination antiretroviral therapy were excluded because of the potential for pharmacokinetic interactions with saracatinib. Pregnant or lactating patients were excluded. Men and women of reproductive potential were required to use a medically acceptable form of birth control while on study and for eight weeks after discontinuation of the study drug.
Treatment plan
Saracatinib was supplied by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI, Bethesda, MD, USA). Patients were instructed to take saracatinib 175 mg per os (or via percutaneous gastrostomy tube) daily. Cycle length was eight weeks. During the first cycle, physical examination and toxicity evaluation were performed weekly for five weeks and during week seven.
Pretreatment assessment of all patients included complete medical history and physical examination. Baseline laboratory studies within 14 days of treatment included complete blood count with white blood cell differential and platelet counts; comprehensive metabolic profile (including electrolytes, bicarbonate, blood urea nitrogen, creatinine, glucose, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, total protein, albumin and glucose); prothrombin time and activated partial thromboplastin time and calculation of urine protein:creatinine ratio. Radiologic imaging of sites of measurable disease (preferably, computerized tomography (CT) or magnetic resonance imaging scan) was obtained within four weeks prior to therapy. All patients were required to obtain a baseline chest CT (with or without contrast agent) within eight weeks of starting treatment to assess for radiographic evidence of baseline pulmonary disease (fibrosis, pneumonitis or pleural effusions).
Evaluation of response
Disease response was assessed approximately every six to eight weeks with cross-sectional imaging of target lesions for response assessment by RECIST. Patients with disease progression as defined by an increase more than 20% in the sum of the products of the diameters of the lesions or the appearance of any new lesions were removed from the study.
Toxicity
Toxicities were evaluated according to NCI Common Terminology Criteria for Adverse Events, version 3.0 (15). Saracatinib dose reduction was required for non-hematologic toxicity ≥grade 3 (with exceptions, e.g., grade 3 nausea or fatigue) or hematologic toxicity as specified in the protocol. Treatment was held until the toxicity resolved to ≤grade 1 and then resumed at the next lower dose level. Dose level minus 1 was saracatinib 125 mg/day and dose level minus 2 was 100 mg/day.
Statistical considerations
The primary objective was to evaluate the effect of saracatinib on the median PFS. The expected PFS for this study population with conventional single-agent therapy was estimated to be approximately 60 days, based on a review of the published literature (16-18). The study aimed to determine whether saracatinib treatment is associated with PFS of at least 120 days. Under the proportional hazards model, this translates to the hypothesis that 70.7% of patients will be progression-free at 60 days. Using a single-stage binomial design, 28 patients were required to demonstrate the increase in 60-day PFS rate from 50% to 70% with type I and type II error rates of 10% and 20%, respectively.
Objective response rate and overall survival were secondary endpoints. The Kaplan-Meier method was used to estimate overall survival.
The study contained an early stopping rule in the event of lack of efficacy. It was hypothesized that the median PFS would improve from 60 days to 120 days. As mentioned above, under the proportional hazards model, this translated to the hypothesis that 70.7% of patients would be progression-free at 60 days. Using 50% and 70% 60-day progression-free proportions as undesirable and desirable rates, the two-stage minimax rule with 10% and 20% type I and II error rates was to stop if at most 7/15 patients (first stage) were progression-free. Thus, the early stopping for inefficacy was given by stopping the trial if at most 7 of the first 15 patients were progression-free at 60 days.
Results
Patient characteristics
Nine patients were enrolled between August 2007 and March 2009. Patient characteristics are summarized in Table I. Seven of the patients were male and two were female, and they had a median KPS of 80%. The most common anatomic subsite was oral cavity (n=4). All but two patients had at least ten pack-years of cigarette exposure. The median number of prior regimens for recurrent and/or metastatic disease was 1 (range, 0-2 regimens). The summary of prior regimens included targeted agents (e.g., prior cetuximab for two patients, prior AMG706 for one patient on another clinical trial), which are not classified as a cytotoxic therapy, as per the eligibility criteria. No patient received more than one cytotoxic chemotherapy regimen for recurrent and/or metastatic disease.
Table I.
Baseline characteristics.
| Characteristic | Summary |
|---|---|
| Gender (male:female) | 7:2 |
| Age (years) | Median 54 (range, 22-67) |
| Karnofsky performance status | Median 80% (range, 80-100) |
| Tobacco consumption (pack years) | Median 20 (range, 0-100) |
| None | 2 patients |
| >0 to ≤ 10 | 1 patients |
| >10 | 6 patients |
| Prior chemotherapy for recurrent or metastatic disease (number of regimensa) | Median 1 (range, 0-2) |
| 0 | 3 patients |
| 1 | 4 patients |
| 2 | 2 patients |
| Prior radiotherapy for head and neck cancer | 9 patients |
| Prior surgery for head and neck cancer | 4 patients |
| Distribution of disease | |
| Both loco-regional and distant disease | 6 patients |
| Distant metastatic disease only | 2 patients |
| Loco-regional disease only | 1 patients |
| Subsite of primary tumor | |
| Oral cavity | 4 patients |
| Oropharynx | 1 patients |
| Hypopharynx | 2 patients |
| Larynx | 2 patients |
Includes targeted (non-cytotoxic) agents.
Table II provides a summary of all adverse events experienced by more than one patient, felt to be possibly or probably related to the study drug. Fatigue was the most frequently observed adverse event. The most common ≥grade 3 adverse event was lymphopenia, occurring in two patients, but this was not clinically significant. Dose reduction from 175 mg to 125 mg was required in one patient due to a low platelet count (92×103/μl).
Table II.
Adverse events.
| Toxicity | Any grade (%) | ≥Grade 3 (%) |
|---|---|---|
| Fatigue | 6 (67) | 1 (11) |
| Hemoglobin, low | 4 (44) | 0 |
| Diarrhea | 4 (44) | 0 |
| Nausea | 4 (44) | 0 |
| AST, high | 4 (44) | 0 |
| Alkaline phosphatase, high | 4 (44) | 0 |
| Constipation | 3 (33) | 0 |
| Vomiting | 3 (33) | 0 |
| Lymphopenia | 2 (22) | 2 (22) |
| Platelets, low | 2 (22) | 0 |
| Creatinine, high | 2 (22) | 0 |
| Proteinuria | 2 (22) | 0 |
| Neuropathy, sensory | 2 (22) | 0 |
| Epistaxis | 2(22) | 0 |
There was one sudden death, occurring in a patient who had been removed from study due to progression of disease. The patient was a 62-year-old man with oropharyngeal cancer that had metastasized to the lung. Seventeen days after being removed from study due to progression of disease, the patient developed severely worsening shortness of breath and died at home on that day. The death was likely due to progression of disease and/or a sudden catastrophic event such as pulmonary embolus, but autopsy was not obtained. Due to the fact that the patient had been removed from study due to disease progression 17 days prior to the grade 5 event, it is unlikely that the death was due to the study drug.
Efficacy
Eight patients experienced progression of disease within the first 60 days on the study. As such, it was impossible that more than 7 of the first 15 patients would be progression-free at 60 days. In accordance with the early-stopping rule, the study was closed for lack of efficacy when the eighth patient had progression of disease by RECIST.
One patient did not have documented disease progression by RECIST criteria, but was removed from study after only 11 days due to clinical decline. The patient was a 52-year-old woman with recurrent oral cavity cancer squamous cell carcinoma. She was admitted to hospital due to cellulitis and clinical decline after 11 days of saracatinib. CT scans demonstrated stable neck disease by RECIST, but the patient was removed from study and transferred to a hospice due to clinical decline. The decision to close the study according to the early stopping rule was based upon the other eight patients who developed clear-cut disease progression by RECIST.
Median overall survival, plotted in Figure 1, was six months (range, 27 days to 680+ days). At the time of data analysis for this manuscript (September 1, 2010), only one of the enrolled patients in this study remained alive.
Figure 1.
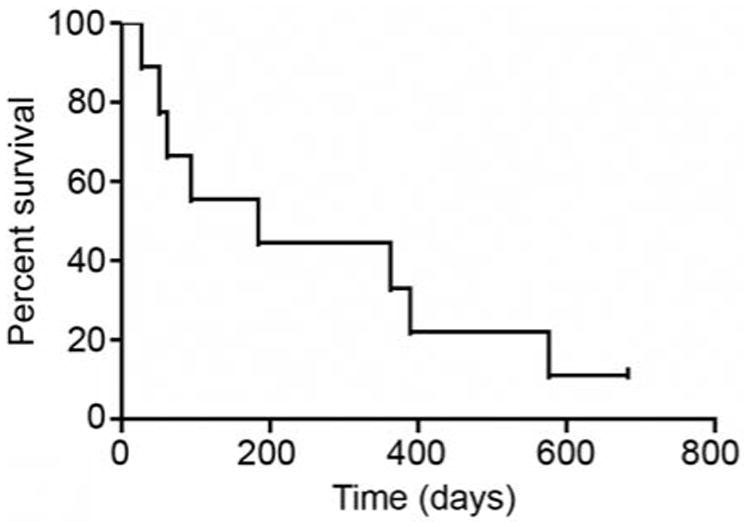
Kaplan-Meier plot for overall survival.
Discussion
This phase II trial described the clinical experience with oral saracatinib (175 mg per day) in patients with recurrent or metastatic HNSCC. The most common adverse event was fatigue. Treatment was generally well-tolerated, with only one patient requiring dose reduction due to thrombocytopenia. There was no evidence of clinical activity with saracatinib in this small study, which closed after accrual of nine patients due to lack of efficacy according to the early-stopping rule. The medial overall survival was six months.
There is no evidence that saracatinib altered the natural history of the disease among the patients enrolled in this study. Median survival of six months is not uncommon in studies that allow prior treatment for recurrent or metastatic HNSCC. Lack of efficacy of Src inhibition in advanced HNSCC was also observed in a phase II study of dasatinib, another inhibitor of Src family kinases (19). No responses were seen among 15 patients with advanced HNSCC who were treated with dasatinib monotherapy. The best response was stable disease at 12 weeks in one patient treated with dasatinib. The dasatinib study had a two-stage design and was closed for lack of efficacy after accrual of 15 participants.
It is increasingly appreciated that HNSCC is a heterogeneous disease in terms of both clinical and molecular characteristics (20, 21). For example, among oropharyngeal cancer patients, tobacco exposure is associated with resistance to treatment and poor prognosis, whereas infection with human papilloma virus-16 is associated with more favorable clinical outcomes. The patient population in the current study was not highly heterogeneous regarding risk factors; all but two patients had at least ten pack-years of tobacco use, and there was only one patient with oropharyngeal cancer. It is possible that saracatinib monotherapy may be active among HNSCC patients with clinical characteristics different from those of the patients enrolled in this study. However, taken together, the results of this study and the study of Brooks et al. (19) strongly suggest that Src inhibition has little or no efficacy as monotherapy among patients with advanced HNSCC.
The mechanism of resistance to saracatinib and dasatinib in HNSCC may be related to activation of compensatory feedback signaling loops in the setting of sustained Src inhibition. In studies of HNSCC cell lines in vitro, sustained inhibition of Src with siRNA resulted in paradoxical activation of signal transducer and activator of transcription 3 (STAT3), an important mediator of cell proliferation and tumor growth (22). Knock-down of STAT3 with siRNA enhanced the efficacy of dasatinib in these cell lines. HNSCC cells with depleted STAT3 were significantly more sensitive to dasatinib than control cells (22).
Src inhibition remains a research strategy to potentially reverse resistance to EGFR inhibitors in HNSCC. Preclinical data suggests that Src inhibition may enhance the efficacy of the EGFR tyrosine kinase inhibitor gefitinib as well as the anti-EGFR monoclonal antibody cetuximab. Treatment of HNSCC cell lines with saracatinib plus gefitinib was associated with greater reductions in cell proliferation than with either agent alone (23). Nuclear expression of EGFR has been proposed as a potential mechanism of resistance to cetuximab and dasatinib resulted in loss of nuclear localization of EGFR and enhanced the sensitivity of HNSCC cell lines to cetuximab (24). While combining Src inhibitors with EGFR inhibitors remains an interesting research topic, the apparent lack of activity of either saracatinib or dasatinib in advanced head and neck cancer provides an important note of caution regarding the development of Src kinase inhibitors in advanced HNSCC.
This phase II study demonstrated that saracatinib is generally well tolerated, but does not have evidence of clinical efficacy in an unselected population of patients with advanced HNSCC. Future clinical studies in HNSCC of saracatinib plus other drugs may be considered in the context of supporting preclinical data, but enthusiasm for this approach would be higher if saracatinib had significant single-agent activity. Further studies of saracatinib monotherapy in advanced HNSCC are not planned.
References
- 1.Fanucchi M, Khuri FR. Chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck. Semin Oncol. 2004;31:809–815. doi: 10.1053/j.seminoncol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644–2652. doi: 10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- 3.Cohen EEW. Role of epidermal growth factor receptor pathway-targeted therapy in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2659–2665. doi: 10.1200/JCO.2005.05.4577. [DOI] [PubMed] [Google Scholar]
- 4.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Racourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. New Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 5.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 6.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 7.van Oijen MG, Rijksen G, ten Broek FW, Slootweg PJ. Overexpression of c-Src in areas of hyperproliferation in head and neck cancer, premalignant lesions and benign mucosal disorders. J Oral Pathol Med. 1998;27:147–152. doi: 10.1111/j.1600-0714.1998.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Thomas SM, Xi S, Smithgall TE, Siegfried JM, Kamens J, Gooding W, Grandis JR. Src family kinases mediate epidermal growth factor receptor cleavage, proliferation, and invasion in head and neck cancer cells. Cancer Res. 2004;64:6166–6173. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 9.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, Kamens J, Grandis JR. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 10.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 11.Hennequin L, Allen J, Breed J, Curwen J, Fennell M, Green TP, Lambert-van der Brempt C, Morgentin R, Norman RA, Olivier A, Otterbein L, Ple PA, Warin N, Costello R. N-(5-chloro-1,3-benzodioxal-4-yl)-7-[2-(methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-src/abl kinase inhibitor. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- 12.Hennequin LF, Allen J, Costello GF, Fennell M, Green TP, Jacobs V, Morgentin R, Olivier A, Ple PA. The discovery of AZD0530: a novel, oral, highly selective and dual specific inhibitor of the Src and Abl family kinases. Proc Am Assoc Cancer Res. 2005;46:2537. [Google Scholar]
- 13.Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW, Elvin P, Boyer B, Carragher N, Ple PA, Bermingham A, Holdgate GA, Ward WH, Hennequin LF, Davies BR, Costello GF. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabernero J, Cervantes A, Hoekman K, Hurwitz HI, Jodrell DI, Hamberg P, Stuart M, Green TP, Iacono RB, Baselga J. Phase I study of AZD0530, an oral potent inhibitor of Src kinase: first demonstration of inhibition of Src activity in human cancers. J Clin Onco. 2007;25:18s. suppl; abstr 3520. [Google Scholar]
- 15.Ajani JA, Welch SR, Raber MN, Fields WS, Krakoff I. Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest. 1990;8:147–159. doi: 10.3109/07357909009017560. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, Goodnough LT, Mortimer JE, Einhorn LH, Schachter L, Cherng N, Dalton T, Burroughs J, Rozencweig M. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1992;10:257–263. doi: 10.1200/JCO.1992.10.2.257. [DOI] [PubMed] [Google Scholar]
- 17.Burtness B. The role of cetuximab in the treatment of squamous cell carcinoma of the head and neck. Expert Opin Biol Ther. 2005;5:1085–1093. doi: 10.1517/14712598.5.8.1085. [DOI] [PubMed] [Google Scholar]
- 18.Guardiola E, Peyrade F, Chaigneau L, Cupissol D, Tchiknavorian X, Bompas E, Madroszyk A, Ronchin P, Schneider M, Bleuze JP, Blay JY, Pivot X. Results of a randomized phase II study comparing docetaxel with methotrexate in pateints with recurrent head and neck cancer. Eur J Cancer. 2004;40:2071–2076. doi: 10.1016/j.ejca.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Brooks HD, Glisson B, Lu C, Sabichi F, Johnson F, Ginsberg L, Bekele B, Papadimitrakopoulou V. Phase II study of dasatinib in the treamtent of head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2009;27:15s. suppl; abstr 6022. [Google Scholar]
- 20.Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin N Am. 2008;22:1125–1142. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim HJ, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sen B, Saigal B, PArikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009;69:1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppikar P, Choi SH, Egloff AM, Cai Q, Suzuki S, Freilino M, Nozawa H, Thomas SM, Gooding WE, Siegfried JM, Grandis JR. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:4284–4291. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL. Nuclear EGFR contributes to resistance to cetuximab. Oncogene. 2009;28:3801–3813. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]


