Abstract
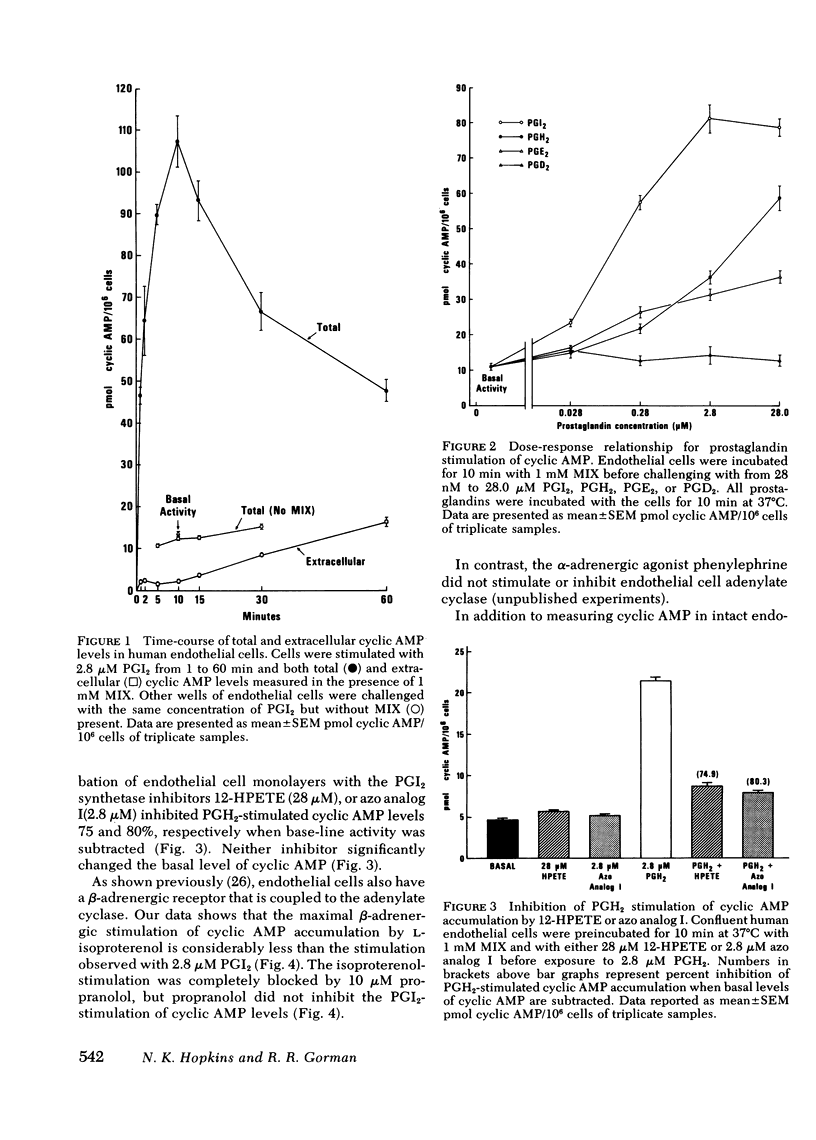
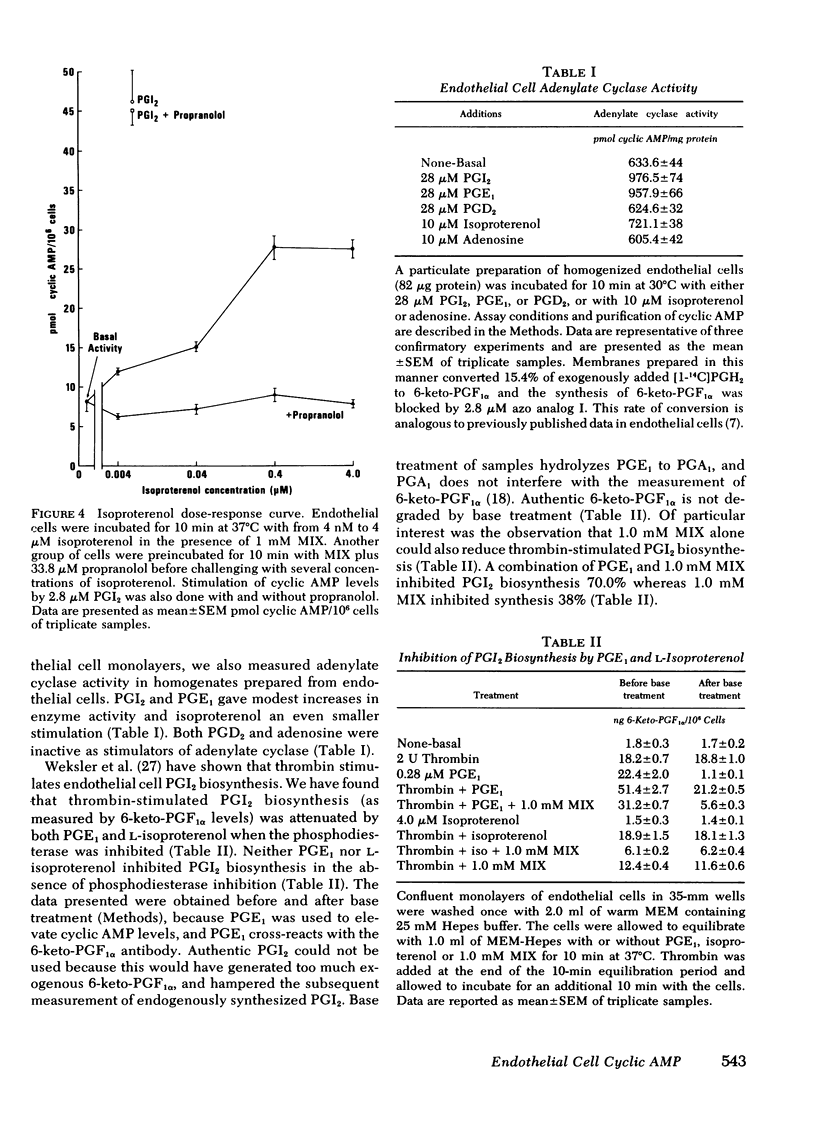
An analysis of prostaglandin-stimulated adenosine 3',5'-cyclic monophosphate (cyclic AMP) accumulation in cultured human umbilical vein endothelial cells showed prostacyclin (PGI2) to be the most potent agonist followed by prostaglandin (PG)H2, which was more potent than PGE2, while PGD2 was essentially inactive. The endothelial cells studied apparently have a high rate of cyclic AMP phosphodiesterase activity because significant PGI2-mediated increases in cyclic AMP could not be shown in the presence of the phosphodiesterase inhibitor isobutylmethylxanthine (MIX). Endoperoxide PGH2-stimulation of cyclic AMP accumulation was inhibited 75--80% by the prostacyclin synthetase inhibitors 12-hydroperoxyeicosatetraenoic acid or 9,11-azoprosta-5,13-dienoic acid. These data indicate that the PGH2-stimulation is due primarily to conversion to PGI2. The beta-adrenergic agonist L-isoproterenol stimulated cyclic AMP accumulation in the endothelial cells. This accumulation was completely blocked by propranolol. However, stimulation of cyclic AMP accumulation by the beta-adrenergic agent did not equal that induced by PGI2. Furthermore, the PGI2 response could not be blocked by propranolol. Thrombin-stimulated PGI2 biosynthesis was attenuated by PGE1 or isoproterenol in the presence of MIX. MIX alone was less effective than a combination of PGE1 or isoproterenol plus MIX. These data suggest two potential effects of PGI2 biosynthesis by endothelial cells: first, the PGI2 can elevate cyclic AMP in platelets, and second, endothelial cell cyclic AMP can be elevated as well, so that subsequent PGI2 synthesis will be attenuated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray F., Gerozissis K., Kouznetzova B., Mamas S., Pradelles P., Trugnan G. New approach to the RIA of prostaglandins and related compounds using iodinated tracers. Adv Prostaglandin Thromboxane Res. 1980;6:167–180. [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Hamilton R. D., Hopkins N. K. Stimulation of human foreskin fibroblast adenosine 3':5'-cyclic monophosphate levels by prostacyclin (prostaglandin I2). J Biol Chem. 1979 Mar 10;254(5):1671–1676. [PubMed] [Google Scholar]
- Gorman R. R., Sun F. F., Miller O. V., Johnson R. A. Prostaglandins H1 and H2. Convenient biochemical synthesis and isolation. Further biological and spectroscopic characterization. Prostaglandins. 1977 Jun;13(6):1043–1053. doi: 10.1016/0090-6980(77)90132-0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1906–1909. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H., Simon B. Effects of some naturally occurring prostaglandins of the D-, E-, and I-type and synthetic analogues on adenylate cyclase of human fat cell ghosts. Res Exp Med (Berl) 1979 Oct;176(1):25–29. doi: 10.1007/BF01852108. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A. Enzymatic conversion of prostaglandin endoperoxide H2 and arachidonic acid to prostacyclin by cultured human endothelial cells. J Biol Chem. 1978 Oct 25;253(20):7138–7141. [PubMed] [Google Scholar]
- Miller O. V., Aiken J. W., Hemker D. P., Shebuski R. J., Gorman R. R. Prostacyclin stimulation of dog arterial cyclic AMP levels. Prostaglandins. 1979 Dec;18(6):915–925. doi: 10.1016/0090-6980(79)90128-x. [DOI] [PubMed] [Google Scholar]
- Miller O. V., Aiken J. W., Shebuski R. J., Gorman R. R. 6-keto-prostaglandin E1 is not equipotent to prostacyclin (PGI2) as an antiaggregatory agent. Prostaglandins. 1980 Aug;20(2):391–400. doi: 10.1016/s0090-6980(80)80056-6. [DOI] [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A., Vane J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977 Jan 1;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Ortmann R. Effect of PGI2 and stable endoperoxide analogues on cyclic nucleotide levels in clonal cell lines of CNS origin. FEBS Lett. 1978 Jun 15;90(2):348–352. doi: 10.1016/0014-5793(78)80402-5. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker N., Bunting S., Salmon J., Moncada S., Vane J. R., Johnson R. A., Morton D. R., Kinner J. H., Gorman R. R., McGuire J. C. The chemical structure of prostaglandin X (prostacyclin). Prostaglandins. 1976 Dec;12(6):915–928. doi: 10.1016/0090-6980(76)90126-x. [DOI] [PubMed] [Google Scholar]


