Abstract
The influence of sex steroids on bone in both men and women has long been recognized. In men, however, the relative contribution of androgens versus estrogens in the regulation of bone metabolism remains uncertain. Animal studies demonstrate that both estradiol (E2), via activation of estrogen receptor-α, and testosterone (T), via activation of the androgen receptor, regulate bone mass in male rodents. The main focus of this review is to summarize and discuss recent findings from the osteoporotic fractures in men (MrOS) cohorts regarding the impact of serum sex steroids on bone health in elderly men. Collectively, these data demonstrate that serum E2 is directly associated with bone mineral density (BMD) and that low serum E2 associates with higher rates of bone loss and fracture. In addition, they substantiate the concept of a threshold E2 level that determines fracture risk in elderly men. We propose that the effect of E2 on fracture risk is at least partly mediated by its effect on BMD, whereas the more modest effect of T on fracture risk mainly is mediated by effects on muscle strength and risk of falls. Findings from the MrOS cohorts also demonstrate that racial and genetic variations in aromatase activity influence serum E2 levels in men. In conclusion, there is compelling evidence that not only androgens, but also estrogens, are important regulators of bone health in men. Consequently, E2 should not exclusively be regarded as the 'female hormone' but as a sex steroid that is necessary for maintenance of bone health in men.
Introduction
A role of estrogens for bone health in men was first clearly indicated by the important findings of Smith et al. in 1994,1 demonstrating that a man with an estrogen receptor (ER) mutation had reduced bone mineral density (BMD) and unfused growth plates. Similar results in men with aromatase deficiency have clearly demonstrated that ER activation is of importance for the male skeleton.1,2,3,4 Furthermore, although men have lower serum estradiol (E2) levels than premenopausal women, they have higher E2 levels than postmenopausal women.5,6 These findings suggested that E2 might be a hormone of importance for male bone health.
Animal studies
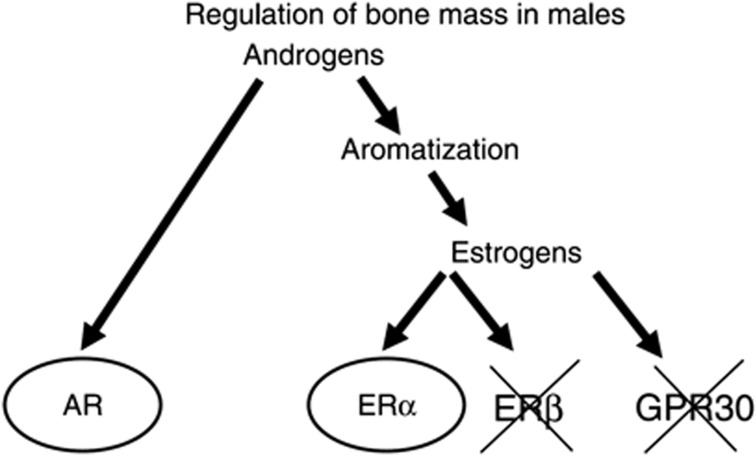
Testosterone (T) exerts its effect either directly through the androgen receptor (AR) or indirectly by aromatization to E2 and activation of ERα and/or β. All three of these sex-steroid receptors are expressed in bone.7,8,9,10 For more details regarding the downstream effects of sex steroids see Nilsson et al.,11 Riggs et al.7 and Khosla et al.12 Several experimental animal studies using mice with inactivated sex-steroid receptors demonstrated that activation of both the ERα and the AR results in a stimulatory effect on both the cortical and trabecular bone compartments in males.8,13,14,15,16,17 In contrast, ERβ is of no importance for the skeleton in male mice while it modulates the ERα action on bone in female mice.13,18,19,20 The relative physiological importance of ERα versus AR activation for bone mass acquisition in growing mice and for bone mass maintenance in adult mice is difficult to determine and depends on the animal model evaluated. In addition, it has been proposed that the membrane G protein-coupled receptor GPR30 (listed as GPER in the HUGO Database) is also a functional ER (Figure 1).21,22 Previous in vitro studies suggest that GPR30 also might be a functional ER. However, our in vivo analyses of GPR30-inactivated mice revealed no function of GPR30 for estrogen-mediated effects on bone mass but it is required for normal regulation of the growth plate and estrogen-mediated insulin secretion.23,24 Thus, several animal studies suggest that both E2, via activation of ERα but not ERβ or GPR30, and T, via activation of the AR, regulate bone mass in male rodents (Figure 1).
Figure 1.
Summary of possible pathways for androgens to regulate bone mass in males. Experimental animal studies using mouse models with inactivation of the androgen receptor (AR), estrogen receptor α (ERα), ERβ or the recently suggested ER GPR30 have demonstrated that the AR and ERα, but not ERβ or GPR30, are involved in the regulation of bone mass in males. (Reproduced from Ohlsson and Vandenput22 with permission from the European Society of Endocrinology.)
A major difference between the bone of men and women is bone size. The larger bone size in men is, at least partly, due to differences in sex-steroid exposure during sexual maturation.25 By stimulating periosteal bone formation, androgens are thought to increase bone size via AR activation as suggested by studies in rodents.26,27 In addition, we found that male ERα-inactivated mice displayed reduced cortical radial bone growth during sexual maturation, demonstrating that also ERα activation is required for radial bone expansion in males during growth.14
Given the fact that activation of ERα increases bone mass in male rodents, this receptor might be an interesting drug target for osteoporosis in men. Estrogen therapy in women is associated with side effects such as breast cancer and thromboembolism.28,29 Thus, it would be beneficial to develop a bone-specific estrogen treatment. To achieve this, it will be crucial to characterize the signaling pathways of estrogen in bone versus other tissues. ERα is the major ER for the bone-sparing effect of estrogens not only in males but also in females. ERα stimulates target gene transcription through two activation functions (AFs), AF-1 in the N-terminal and AF-2 in the ligand-binding domain. To evaluate the role of ERα AF-1 and ERα AF-2 for the effects of estrogen in bone and other estrogen-sensitive tissues in vivo, we recently analyzed mouse models lacking the entire ERα protein, ERα AF-1 or ERα AF-2. ERα AF-2 was required for the estrogenic effects on all parameters evaluated, whereas the role of ERα AF-1 was tissue-specific, with a crucial role in trabecular bone and reproductive tissues but not in cortical bone or for the vasculoprotective actions of E2.29,30 The mechanism behind the crucial role of ERα AF-1 in the trabecular but not the cortical bone is unknown. It might include different expression patterns of coregulators in trabecular versus cortical bone. In vitro studies have revealed that the steroid receptor coactivator (SRC)-1 is cooperatively recruited by AF-1 and AF-2 in ERα.31 Importantly, when ovariectomized SRC-1 knock-out mice were treated with E2, the normal estrogenic response was absent in trabecular bone, whereas it was normal in cortical bone.32 Thus, these estrogenic responses in the SRC-1 knock-out mice show a similar pattern as the results found for mice with a specific ERα AF-1 inactivation, suggesting that SRC-1 might be involved in the AF-1-dependent E2 effects in trabecular bone but not in the AF-1-independent E2 effects in cortical bone. The ERα AF-1-independent effect of E2 on cortical bone is of interest as Khosla et al.12 recently proposed that the main physiological target for estrogen in bone is cortical and not trabecular bone. This statement is based on a number of observations. First, detailed clinical investigations using computer tomography revealed that the trabecular bone loss begins in sex hormone-replete young adults of both sexes and might be the result of cell autonomous age-related factors as suggested by Manolagas.12,33 Second, the same studies, using computer tomography, demonstrated that the onset of cortical bone loss in humans is closely tied to estrogen deficiency.12 Thus, for cortical bone, which comprises more than 80% of the skeleton and is likely the major contributor to overall fracture risk, there are data supporting the view that estrogen deficiency is the major cause of bone loss.12 These findings suggest that selective ER modulators stimulating ERα with minimal activation of ERα AF-1 could retain clinical important beneficial actions in cortical bone and on vascular protection while minimizing the effects in reproductive organs.29,30
Clinical studies
Serum levels of free and bioavailable androgens decrease in aging men, and these changes coincide with an age-related increase in the level of sex hormone-binding globulin (SHBG).4,34 Levels of free and bioavailable estrogens decrease as well, whereas the decrease in total E2 levels in men is not well documented. In this section, the relative role of serum T and E2 for BMD, BMD loss and fracture risk in elderly men will be discussed. Most observational studies demonstrate that serum E2 and especially bioavailable E2 (bioE2) correlate better with BMD at various sites than serum T does in men.35,36,37,38,39,40,41,42,43,44 Moreover, prospective studies have shown that serum E2 is the best predictor of both the increase in bone mass in young men and the decrease of BMD in elderly men.5,22,45,46 In a key publication, Khosla et al.5 proposed that a threshold level for bioE2 of 40 pmol l−1 (11 pg ml−1) exists, below which the rate of bone loss at the radius and ulna is associated with levels of bioE2 in men. A similar threshold for E2 was reported in elderly men by Gennari et al.46 for bone loss at the femoral neck and lumbar spine.
The osteoporotic fractures in men (MrOS) cohorts in the US (n=5995), Sweden (n=3014) and Hong Kong (n≅2000) were initiated as large observational studies to identify determinants of fracture risk in older men. To investigate the relation between serum sex steroids and BMD, BMD loss and fracture risk in elderly men, these three large, well-characterized, prospective cohorts were recently evaluated.47,48,49,50 The protocols used for the MrOS cohorts are similar, but it should be noted that they are three separate cohorts and slightly different statistical models have been used in the resulting articles summarized in this section. Some early publications from MrOS US and MrOS Sweden presented associations between serum sex steroids analyzed by immunoassay-based techniques and BMD.43,51,52 However, later on it became apparent that especially for E2, there is a questionable specificity at lower concentrations when using immunoassay-based techniques. Therefore, golden standard mass spectrometry (MS)-based analyses of serum E2 and T were performed in all three MrOS cohorts. In the MrOS cohorts in Sweden and Hong Kong, MS analyses were performed in the laboratory of Professor Labrie in Quebec, Canada, whereas the serum samples of the MrOS US cohort were analyzed by MS at a commercial US-based company. To be able to compare the results between the different MrOS cohorts, thorough cross-calibrations between the two laboratories used were performed.53 In the following description and discussion of the results, we will focus on the results derived from the more reliable MS-based analyses of serum sex steroids.
Serum sex steroids and BMD/BMD loss in the MrOS cohorts.
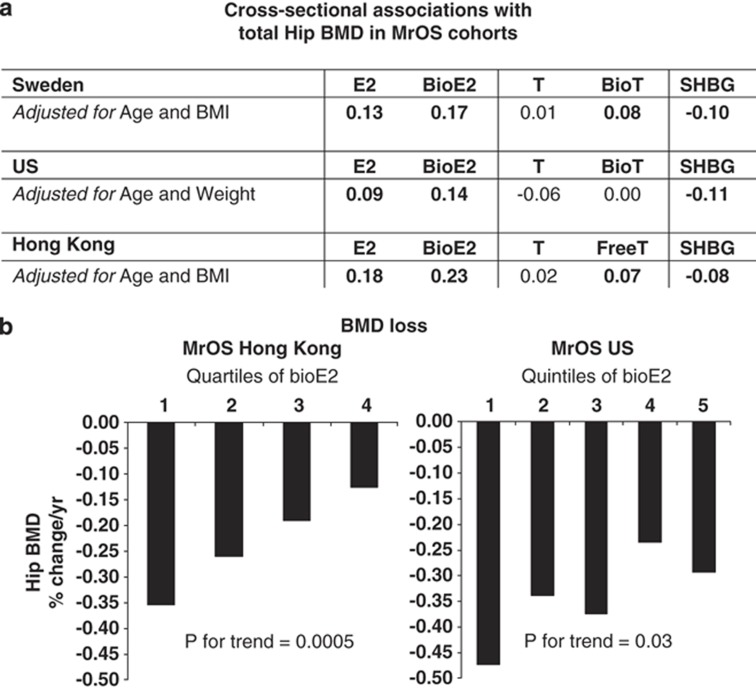
Cross-sectional studies demonstrated that E2 and bioE2 were directly associated with BMD in all three MrOS cohorts while no association or clearly weaker associations were seen for T and bioavailable T/free T (Figure 2a). The associations between serum sex steroids and BMD loss have thus far been evaluated in the MrOS Hong Kong and MrOS US cohorts. In the Hong Kong cohort, serum bioE2 was evaluated in quartiles and subjects in the lowest quartile had the highest hip BMD loss (Figure 2b).50 The average BMD loss in the lowest quartile was more than two times higher than that seen in the highest quartile. In the US cohort, serum bioE2 was evaluated in quintiles. The association seen was less pronounced but the lowest quintile had the highest hip BMD loss and the P for trend was significant (Figure 2b).49 In contrast, serum T was not independently associated with BMD loss in this population. These results from the MrOS cohorts support the notion that estrogen is crucial for the maintenance of BMD in elderly Caucasian and Asian men.
Figure 2.
Summary of the associations between serum sex steroids and total hip BMD in the three MrOS cohorts. (a) Cross-sectional association: partial correlation adjusted for the indicated covariates are given for the MrOS US and Sweden cohorts, whereas Spearman correlation coefficients are given for the MrOS Hong Kong cohort. Bold values indicate significant association. (b) BMD loss: serum bioavailable E2 was evaluated in quartiles in the MrOS Hong Kong cohort and in quintiles in the MrOS US cohort.49,50 (Figure 2b is adapted from Woo et al.50 with permission from the International Osteoporosis Foundation).
Serum sex steroids and fracture risk in the MrOS cohorts.
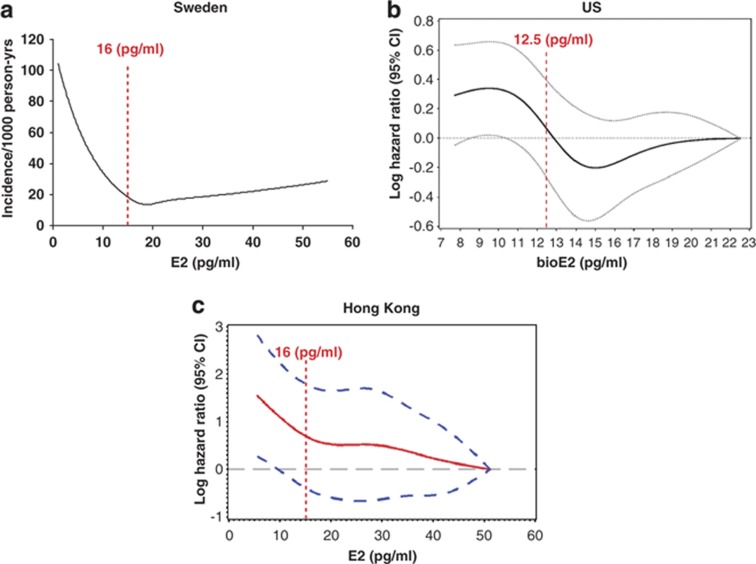
As fracture risk is not only dependent on BMD, it is important to determine the impact of serum sex steroids on risk of fractures. There are conflicting reports regarding the association between serum sex steroids and fracture risk as analyzed in prospective studies. Neither serum E2 nor serum T predicted risk of fractures in the Rotterdam study, which included 45 men with vertebral fractures,54 or in the Tromsö study, which included 105 men with non-vertebral fractures.55 An 18-year follow-up of the Framingham study, which included 39 men with hip fractures, showed that low E2, but not low T, was a predictor of fracture risk.56 By contrast, from the Dubbo Osteoporosis epidemiology study, which included 113 men with fractures, it was reported that serum levels of T, but not E2, predicted the risk of fractures.57 These contradictory findings might be explained by the fact that the above-mentioned prospective studies were underpowered and that these studies, except the Dubbo Osteoporosis study, measured participants' sex-steroid levels with immunoassay-based techniques. To investigate the relation between serum sex steroids and fracture risk in well-powered cohorts of elderly men with serum sex steroids analyzed by MS, we recently evaluated the three MrOS cohorts in independent analyses.47,48,50 The MrOS Sweden fracture study was population-based including 2639 men with an average age of 75 years. Subjects were followed in average for 3.3 years and 209 subjects with incident X-ray verified fractures were identified.47 The MrOS US fracture study was a case–cohort study including as many as 342 older men with non-vertebral fractures with an average follow-up of 4.7 years.48 The MrOS Hong Kong fracture study included 1489 older Chinese men who were followed in average for 4 years and included 118 men with non-vertebral fractures.50 The fracture data from the Swedish MrOS cohort was first reported in 2008, demonstrating that serum E2 as well as T (both total and free) were inversely associated with the risk of fracture when analyzed separately.47 However, low E2 but not low T was an independent predictor of fracture risk. The inverse relationship between serum E2 levels and fracture risk was nonlinear, supported by the fact that the association between E2 and yearly incidence of fractures was mainly seen for the 25% of the subjects with serum E2 lower than 16 pg ml−1 (59 pmol l−1, Figure 3a).
Figure 3.
Serum estradiol (E2) and fracture risk in the three MrOS cohorts. Red dotted lines indicate the threshold levels for the association between serum E2/bioE2 and fracture risk. (a) MrOS Sweden (adapted from Mellström et al.47 with permission from the American Society for Bone and Mineral Research). (b) MrOS US (adapted from LeBlanc et al.48 with permission from The Endocrine Society). (c) MrOS Hong Kong (adapted from Woo et al.50 with permission from the International Osteoporosis Foundation).
The main findings from the Swedish MrOS cohort were confirmed in the MrOS US fracture study, demonstrating that both low bioE2 and low bioT were associated with increased fracture risk.48 Analyses including both bioE2 and bioT suggested that low bioE2, but not low bioT, was an independent predictor of fracture. Spline models demonstrated that the relation between bioE2 and risk of fractures in the MrOS US cohort was nonlinear with an inverse association in the lower range (<12.5 pg ml−1) of bioE2 (Figure 3b). The MrOS Hong Kong fracture data was recently reported, essentially confirming the main findings from the MrOS Sweden and US cohorts.50 Spline models demonstrated that the fracture risk was clearly elevated below an E2 threshold of 16 pg ml−1 (Figure 3c), which is similar to the threshold seen in the Swedish MrOS cohort. For bioE2, this threshold was 12.5 pg ml−1, which is very similar to the MrOS US threshold.48,50 Thus, the findings from the three MrOS cohorts substantiate the important concept of a threshold E2 level that determines fracture risk in elderly men.
The effect of E2 on fracture risk was attenuated by adjustment for BMD in all three cohorts.47,48,50 However, in the MrOS Sweden and US cohorts, E2 remained significantly associated with fracture risk after BMD adjustment, suggesting that the effect of E2 on fracture risk is at least partly independent of areal BMD as analyzed by dual-energy X-ray absorptiometry. The fracture data from the three MrOS cohorts demonstrate that serum E2 is a risk marker for fractures not only in elderly Caucasian men but also in Asian men. Further studies should determine the clinical usefulness of adding serum E2/bioE2 analyses to established fracture risk markers and in detail characterize the E2 threshold for fracture risk in men. In addition, we propose that if serum E2 is going to be used in the clinical setting, standardized MS-based assays should be used.
Besides low serum E2/bioE2, high SHBG levels were consistently associated with an increased fracture risk in all three MrOS cohorts. In MrOS US, a significant interaction between SHBG and bioT resulted in that men with low bioT and high SHBG were at higher fracture risk. However, one should be cautious in the interpretation of this interaction as SHBG was actually used in the calculation of bioT and further studies are required to confirm and determine the impact of this possible interaction.48
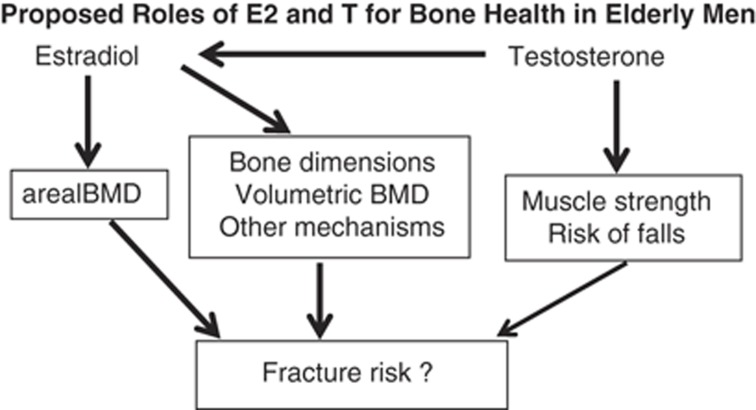
In Figure 4, we summarize our proposed roles of E2 and T for fracture risk in elderly men. E2 increases/preserves the areal BMD, which in turn reduces fracture risk. As the association between E2 and fracture risk partly remained after BMD adjustment, we believe that also other mechanisms are involved such as effects on bone dimensions and volumetric BMD, not revealed by dual-energy X-ray absorptiometry analyses. For T, there is no major direct influence on BMD. However, as T is the direct precursor of E2, T also has an effect on BMD indirectly via E2. In addition, there are convincing data demonstrating that T treatment increases muscle strength and in the MrOS US study, low serum T is associated with increased risk of falls; these non-bone parameters might contribute to the association with fracture risk described for low T in some studies (Figure 4).58
Figure 4.
The authors' proposed roles of estradiol (E2) and testosterone (T) for bone health in elderly men.
Regulation of Serum Sex Steroids in Men
As delineated above, it is clear that serum E2 is a major regulator of male bone health but little is known about the major determinants of serum E2 and/or its precursor T. Sex-steroid metabolism is complex and includes several enzymatic steps, which can be influenced both by genetic and environmental factors (Figure 5). Despite considerable racial and geographical differences in the incidence of diseases that may be associated with sex-steroid action in men, variations in sex-steroid levels among male populations are scarcely documented.59
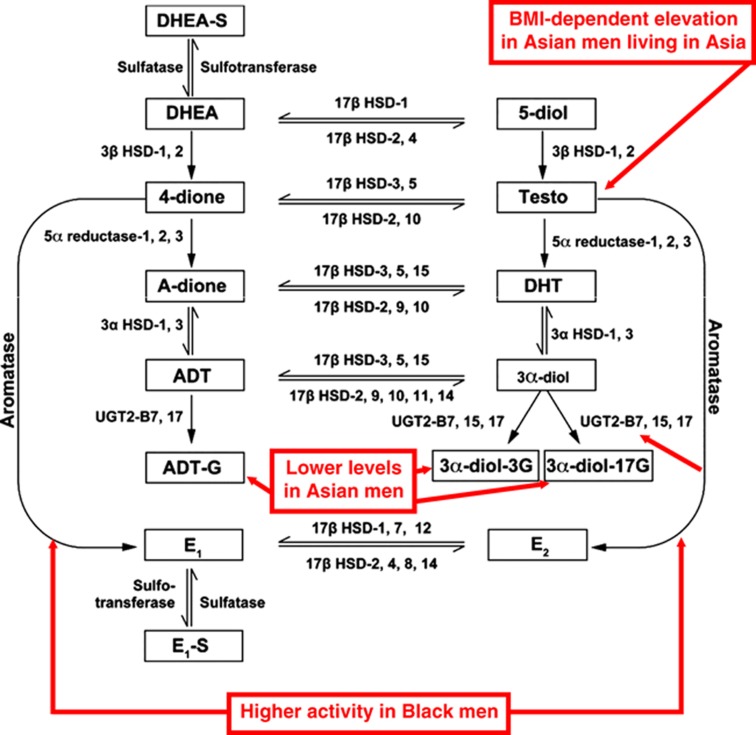
Figure 5.
Summary of sex-steroid metabolism in men. Geographical and racial differences in serum sex steroids in elderly men are indicated in red. 3α-DIOL-3G, androstane-3α,17β-diol-3glucuronide; 3α-DIOL-17G, androstane-3α,17β-diol-17glucuronide; 4-dione, androstenedione; 5-Diol, androst-5-ene-3β,17β-diol; A-dione, androstanedione; ADT, androsterone; ADT-G, ADT glucuronide; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; E2, estradiol; E1, estrone; E1-S, estrone sulfate; HSD, hydroxysteroid dehydrogenase; UGT, uridine glucuronosyl transferase. (Adapted from Orwoll et al.59 with permission from the Endocrine Society.)
To examine racial and geographic differences in sex hormone levels, we assembled a large international group of older men including, but not limited to, the three MrOS cohorts and measured the serum levels of estrogens and androgens as well as their precursors and metabolites (Figure 5).59 This design enabled us to, at least partly, separate racial factors from geographical/environmental factors. In total, 5003 older men were included with an average age of 74 years and morning samples were collected for serum sex steroid analyses. Thirteen different sex steroids, precursors and metabolites were analyzed by MS (Figure 5) and as described below we identified substantial geographical and racial variations in serum sex-steroid levels.
Geographical variations.
We found that there was geographical variation in the levels of serum T. Asian men in Hong Kong and Japan, but not in the United States, had levels of total T ∼20% higher than in other groups. However, this geographical variation in serum T disappeared after body mass index adjustment. Nevertheless, as a result of geographical heterogeneity, the proportion of men with T levels suggestive of low T (<230 ng dl−1)60 differed between populations (e.g., 5–6% in US and Swedish men; 3% in Hong Kong and Japanese men; Figure 5).
Racial variations.
In addition to the effects of geography, two consistent racial patterns were found. First, Black men had higher estrogen (E2 and estrone) levels than Caucasians or Asians. Moreover, in Blacks the ratios of total E2/total T and estrone/androstenedione (4-dione), suggestive of aromatase activity, were increased compared with other groups (Figure 5). Importantly, these differences in estrogen levels may have health implications, including effects on male bone metabolism. For instance, femoral cortical thickness and trabecular bone density are greater in Black men and they have lower hip fracture rates, findings that are consistent with higher estrogen levels.61,62,63 In our study, considerably fewer Black men had E2 levels below a threshold level reported to be associated with increased fracture risk.47,48,50 Second, Asian men had lower levels of glucuronidated androgen metabolites (Figure 5), suggesting that there are clear racial differences in androgen degradation. Of potential physiological importance, serum levels of glucuronidated androgen metabolites have been described to be more strongly related to bone parameters than serum T.64
Serum estrogens.
The majority of estrogens in elderly men do not originate from the testes but from the peripheral conversion of androgens, suggesting that genetic variations in peripheral aromatase activity might influence serum estrogen levels. This notion is supported by a report demonstrating that elderly men who have a high number of TTTA repeats in CYP19A1 (the aromatase gene) had higher E2 levels, lower rates of bone loss and fewer fractures than men with a low number of such repeats.65 In an extensive large-scale (n=5531 men) evaluation of 604 single-nucleotide polymorphisms in 50 sex-steroid-related candidate genes, we identified a single-nucleotide polymorphism in the I.4 promoter region of CYP19A1 that was strongly associated with serum E2 levels in men (P=10–14).53 Interestingly, men with the GG genotype of this CYP19A1 polymorphism not only had markedly elevated E2 levels, but also a higher lumbar spine BMD and fewer fractures than those with the GA or AA genotypes.4,53 These genetic findings in CYP19A1, together with the aforementioned higher estrogen levels and aromatase activity in Black men, demonstrate that genetic variations in aromatase are major determinants of serum E2 levels and suggest that these variations indirectly might influence male bone health via altered E2 activity.
Serum T.
Studies in male twins indicate that there is a strong heritability of serum T.66 However, the genetic determinants of serum T and the genetic risk factors for low concentrations are poorly understood. In an international consortium, we therefore recently performed a large-scale meta-analysis of genome-wide association studies to examine the effects of common genetic variants on serum T concentrations. By examining 14 429 men, we showed that genetic variants in the SHBG locus and on the X chromosome (near FAM9B) are associated with a substantial variation in serum T concentrations and increased risk of low T. Importantly, we identified the first known genetic variant that affects SHBG's affinity for binding T and the free T fraction and could therefore influence the calculation of free T. This finding suggests that individual-based SHBG-T affinity constants are required depending on the genotype of this single-nucleotide polymorphism.67
Summary
Both E2, via activation of ERα, and T, via activation of the AR, regulate bone mass in male rodents. The results from the MrOS cohorts demonstrate that E2 is directly associated with BMD and that low E2 is associated with higher rates of bone loss and an increased fracture risk in elderly men. In addition, they substantiate the concept of a threshold E2 level that determines fracture risk in elderly men. We propose that the effect of E2 on fracture risk is, at least partly, mediated via its effect on BMD, whereas the more modest effect of T on fracture risk mainly is mediated via effects on muscle strength and risk of falls. Findings from the MrOS cohorts demonstrate that racial and genetic variations in aromatase activity substantially influence serum E2 levels in men and there are indications that genetic differences in the CYP19A1 gene could influence male bone health. Thus, there is compelling evidence that not only androgens, but also estrogens, are important regulators of bone health in men. Consequently, E2 should not exclusively be regarded as the 'female hormone' but as a sex steroid that is also necessary for maintenance of bone health and perhaps other physiological functions in men.
Acknowledgments
This study was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, COMBINE, the ALF/LUA research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg's Foundation, the Novo Nordisk Foundation and the European Commission grant HEALTH-F2-2008-201865-GEFOS.
Footnotes
The authors declare no conflict of interest.
References
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994;331:1056–1061. [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 1995;80:3689–3698. [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 1997;337:91–95. [DOI] [PubMed] [Google Scholar]
- Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol 2009;5:437–443. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ III, Atkinson EJ, O′Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab 2001;86:3555–3561. [DOI] [PubMed] [Google Scholar]
- Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P et al. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J Steroid Biochem Mol Biol 2009;113:52–56. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ III. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002;23:279–302. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev 2004;25:389–425. [DOI] [PubMed] [Google Scholar]
- Nilsson LO, Boman A, Savendahl L, Grigelioniene G, Ohlsson C, Ritzen EM et al. Demonstration of estrogen receptor-beta immunoreactivity in human growth plate cartilage. J Clin Endocrinol Metab 1999;84:370–373. [DOI] [PubMed] [Google Scholar]
- Vidal O, Kindblom LG, Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res 1999;14:923–929. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G et al. Mechanisms of estrogen action. Physiol Rev 2001;81:1535–1565. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ III, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res 2011;26:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA, Clement-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 2003;111:1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB et al. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA 2000;97:5474–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J et al. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA 2003;100:13573–13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Alatalo S, Halleen J, Mohan S et al. Two different pathways for the maintenance of trabecular bone in adult male mice. J Bone Miner Res 2002;17:555–562. [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Weihua Z, Andersson N, Moverare S, Gao H, Vidal O et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 2002;174:167–178. [DOI] [PubMed] [Google Scholar]
- Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G. Female estrogen receptor beta-/- mice are partially protected against age-related trabecular bone loss. J Bone Miner Res 2001;16:1388–1398. [DOI] [PubMed] [Google Scholar]
- Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest 1999;104:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a 'ying yang' relationship between ERalpha and ERbeta in mice. Mol Endocrinol 2003;17:203–208. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005;307:1625–1630. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Vandenput L. The role of estrogens for male bone health. Eur J Endocrinol 2009;160:883–889. [DOI] [PubMed] [Google Scholar]
- Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B et al. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab 2009;296:E490–E496. [DOI] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009;150:687–698. [DOI] [PubMed] [Google Scholar]
- Seeman E. Clinical review 137: sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab 2001;86:4576–4584. [DOI] [PubMed] [Google Scholar]
- Turner RT, Wakley GK, Hannon KS. Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J Orthop Res 1990;8:612–617. [DOI] [PubMed] [Google Scholar]
- Venken K, De Gendt K, Boonen S, Ophoff J, Bouillon R, Swinnen JV et al. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res 2006;21:576–585. [DOI] [PubMed] [Google Scholar]
- Daly E, Vessey MP, Hawkins MM, Carson JL, Gough P, Marsh S. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348:977–980. [DOI] [PubMed] [Google Scholar]
- Borjesson AE, Windahl SH, Lagerquist MK, Engdahl C, Frenkel B, Moverare-Skrtic S et al. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci USA 2011;108:6288–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G et al. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci USA 2009;106:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metivier R, Penot G, Flouriot G, Pakdel F. Synergism between ERalpha transactivation function 1 (AF-1) and AF-2 mediated by steroid receptor coactivator protein-1: requirement for the AF-1 alpha-helical core and for a direct interaction between the N- and C-terminal domains. Mol Endocrinol 2001;15:1953–1970. [DOI] [PubMed] [Google Scholar]
- Modder UI, Sanyal A, Kearns AE, Sibonga JD, Nishihara E, Xu J et al. Effects of loss of steroid receptor coactivator-1 on the skeletal response to estrogen in mice. Endocrinology 2004;145:913–921. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 2010;31:266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005;26:833–876. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 1997;12:1833–1843. [DOI] [PubMed] [Google Scholar]
- Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston CC. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest 1997;100:1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Melton LJ III, Atkinson EJ, O′Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 1998;83:2266–2274. [DOI] [PubMed] [Google Scholar]
- Ongphiphadhanakul B, Rajatanavin R, Chanprasertyothin S, Piaseu N, Chailurkit L. Serum oestradiol and oestrogen-receptor gene polymorphism are associated with bone mineral density independently of serum testosterone in normal males. Clin Endocrinol (Oxf) 1998;49:803–809. [DOI] [PubMed] [Google Scholar]
- Center JR, Nguyen TV, Sambrook PN, Eisman JA. Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab 1999;84:3626–3635. [DOI] [PubMed] [Google Scholar]
- Amin S, Zhang Y, Sawin CT, Evans SR, Hannan MT, Kiel DP et al. Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Ann Intern Med 2000;133:951–963. [DOI] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 2000;85:3276–3282. [DOI] [PubMed] [Google Scholar]
- Szulc P, Munoz F, Claustrat B, Garnero P, Marchand F, Duboeuf F et al. Bioavailable estradiol may be an important determinant of osteoporosis in men: the MINOS study. J Clin Endocrinol Metab 2001;86:192–199. [DOI] [PubMed] [Google Scholar]
- Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 2006;21:529–535. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Travison TG, Leder BZ, McKinlay JB. Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. J Clin Endocrinol Metab 2008;93:2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pottelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. J Clin Endocrinol Metab 2003;88:3075–3081. [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, Martini G, Gonnelli S, Franci B, Campagna S et al. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab 2003;88:5327–5333. [DOI] [PubMed] [Google Scholar]
- Mellstrom D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Oden A et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 2008;23:1552–1560. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab 2009;94:3337–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Ewing SK, Taylor BC, Fink HA, Ensrud KE, Bauer DC et al. Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density--the osteoporotic fractures in men study. J Clin Endocrinol Metab 2010;95:4314–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwok T, Leung JC, Ohlsson C, Vandenput L, Leung PC. Sex steroids and bone health in older Chinese men. Osteoporos Int 2011. doi:10.1007/s00198-011-1552-y. [DOI] [PubMed] [Google Scholar]
- Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 2006;91:3908–3915. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Lewis CE, Lambert LC, Taylor BC, Fink HA, Barrett-Connor E et al. Endogenous sex steroids, weight change and rates of hip bone loss in older men: the MrOS study. Osteoporos Int 2006;17:1329–1336. [DOI] [PubMed] [Google Scholar]
- Eriksson AL, Lorentzon M, Vandenput L, Labrie F, Lindersson M, Syvanen AC et al. Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J Clin Endocrinol Metab 2009;94:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goderie-Plomp HW, van der Klift M, de Ronde W, Hofman A, de Jong FH, Pols HA. Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. J Clin Endocrinol Metab 2004;89:3261–3269. [DOI] [PubMed] [Google Scholar]
- Bjornerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fonnebo V, Jorgensen L et al. A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men: the Tromso Study. Eur J Endocrinol 2007;157:119–125. [DOI] [PubMed] [Google Scholar]
- Amin S, Zhang Y, Felson DT, Sawin CT, Hannan MT, Wilson PW et al. Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am J Med 2006;119:426–433. [DOI] [PubMed] [Google Scholar]
- Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med 2008;168:47–54. [DOI] [PubMed] [Google Scholar]
- Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett-Connor E, Cauley J et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med 2006;166:2124–2131. [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, Cummings SR et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab 2010;95:E151–E160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl 2009;32:1–10. [DOI] [PubMed] [Google Scholar]
- Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE et al. Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res 2008;23:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T et al. Racial differences in fracture risk. Epidemiology 1994;5:42–47. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health 1990;80:871–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenput L, Labrie F, Mellstrom D, Swanson C, Knutsson T, Peeker R et al. Serum levels of specific glucuronidated androgen metabolites predict BMD and prostate volume in elderly men. J Bone Miner Res 2007;22:220–227. [DOI] [PubMed] [Google Scholar]
- Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A et al. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab 2004;89:2803–2810. [DOI] [PubMed] [Google Scholar]
- Bogaert V, Taes Y, Konings P, Van Steen K, De Bacquer D, Goemaere S et al. Heritability of blood concentrations of sex-steroids in relation to body composition in young adult male siblings. Clin Endocrinol (Oxf) 2008;69:129–135. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JRB, Koster A et al. Genetic determinants of serum testosterone concentrations in men. PLoS Genet 2011;7:e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]