Abstract
The Notch signaling pathway regulates several distinct cellular programs that are indispensible for proper embryonic development and maintenance of adult tissue homeostasis. Among the various organs of the human body, the pathway has an important role in the bone microenvironment, managing cell-fate decisions in two bone-specific cells. Significantly, pathological activation of the Notch pathway in these cells by metastatic tumor cells promotes osteolytic colonization of the bone. Armed with this knowledge, disruption of the Notch pathway, and other bone microenvironment signaling components that facilitate Notch-mediated bone metastasis, may serve as a viable therapeutic intervention in this aggressive, incurable disease.
The notch signaling pathway
The evolutionarily conserved Notch pathway is traditionally known for its ability to facilitate short-range signaling between neighboring cells, coordinating spatial and temporal regulation of cell fates during embryonic development. As such it has been implicated in specifying the development of several different tissues and organisms.1 In a context-dependent manner, the pathway regulates a broad spectrum of fundamental processes and diverse cellular programs including proliferation, apoptosis, migration, growth and differentiation. Only recently has the pathway been appreciated for its role in the maintenance of adult tissues during postnatal life and aberrant activation in the pathogenesis of human diseases such as cancer.
The Notch pathway is activated when a signal-sending cell expressing a Notch membrane-bound ligand physically interacts with a signal-receiving cell expressing a Notch receptor.2 Upon ligand binding, the transmembrane Notch receptor is cleaved sequentially, first by an extracellular matrix metalloprotease3 and then by the transmembrane protease complex γ-secretase, releasing the Notch intracellular domain (NICD).4,5 After being liberated, NICD translocates to the nucleus where it interacts with the DNA-binding protein CSL (Rbp-Jκ in mice; CBF1 in humans), converting it from a transcriptional repressor to activator by recruiting cofactors such as Mastermind-like proteins.6,7 The most prominent targets of the Notch pathway include a set of basic helix-loop-helix factors of the hairy and enhancer of split (Hes) and Hes-related repressor protein (Hey) families.8,9 These transcription factors carry out Notch signaling functions, including maintenance of stem cells, specification of cell fate, differentiation, proliferation and apoptosis.10
Notch signaling in bone physiology
The Notch pathway regulates distinct cellular programs in individual cell types found in the bone microenvironment. For example, activation of the Notch pathway in murine stromal cells has been reported to promote osteoblast differentiation, either independently11 or in a bone morphogenetic protein-dependent manner.12 Of pathological importance, Notch signaling in prostate cancer cells was shown to confer osteogenic properties, a phenomenon known as osteomimicry, in an ERK-dependent mechanism.13 Conversely, loss- and gain-of-function experiments in transgenic mice demonstrated that Notch signaling directly inhibits osteoblast differentiation and indirectly influences osteoclast differentiation.14,15 These studies suggest a context-dependent role of Notch signaling in osteoblast function.16
The pathway is also an important determinant of osteoclast maturation and function in the physiological setting.16,17,18,19,20 Osteoclasts are bone-resorbing, multinucleated cells that differentiate from monocyte or macrophage lineage precursors. Research has shown that Notch signaling prevents the differentiation of osteoclast precursors into mature osteoclasts using primary bone marrow macrophages.18 Cell culture studies showed that Notch signaling desensitizes bone marrow macrophages to osteoclastogenic cytokines. Bone marrow macrophages were isolated from transgenic mice with functional loss of Notch1, Notch2 and Notch3 (individually or in combination) or control mice and cultured with increasing amounts of macrophage colony-stimulating factor and/or RANKL. At lower concentrations of macrophage colony-stimulating factor or RANKL, there were a significantly higher number of osteoclasts that developed from bone marrow macrophages isolated from Notch-deficient transgenic mice relative to controls. These earlier studies also found that osteoclast differentiation in Notch-deleted osteoclast precursor cells led to a functional increase in their capacity to breakdown bone in vitro and in vivo.18 In contrast, another group showed that Jagged1-mediated Notch2 signaling can promote RANKL-mediated osteoclast activation.19 These controversial results support, once again, a potential context-dependent role for the Notch pathway in osteoclastogenesis.
Another aspect of the bone microenvironment that is influenced by Notch signaling is the hematopoietic stem cell niche. Jagged1 expressed by osteoblasts was shown to regulate the expansion of hematopoietic stem cells in the bone microenvironment through Notch signaling.20 Interestingly, Jagged1 expression by osteoblasts has been shown to be regulated by parathyroid hormone signaling, a pathway that is also co-opted in pathological bone metastasis. Further research in the area of the Notch pathway and bone homeostasis is necessary to define additional molecular mediators and physiological contexts that implicate Notch signaling in the hematopoietic stem cell niche.
Pathological role for notch signaling in bone metastasis
Physiological activation of the Notch pathway is carried out with calculated accuracy and under precise regulation to prevent inappropriate signaling, the hallmark of pathological scenarios involving the pathway. The etiology of pathological Notch signaling can be underlying genomic mutations, as was revealed in T-cell acute lymphoblastic leukemia in which 50% of patients were shown to harbor activating mutations in Notch1.21,22 The mechanism of cancer development in these scenarios is through cell-autonomous pathway activity within the tumor cells. Beyond tumorigenesis, cell-autonomous Notch signaling has been implicated in cancer progression. In a prominent example, the pathway regulates the epithelial-to-mesenchymal transition, a highly conserved cellular program that confers mesenchymal features, such as migratory capacity, to epithelial cells during development and tumor cells during cancer invasion. Several factors have been shown to participate in Notch-mediated epithelial-to-mesenchymal transition, such as transforming growth factor beta (TGF-β) signaling,23,24 β-catenin activity,25 the transcription factor Slug26 and most recently hypoxia.27,28 Intriguingly, Notch pathway has also been known to be crucial for the regulation and maintenance of both normal stem cells and cancer stem cell.29,30,31 For example, in normal mammary gland, Notch1 activation directs the differentiation of the luminal cell lineage, whereas Notch4 activity is important to maintain stem and progenitor cells.32,33,34,35 In breast cancer, Notch 4 was shown to be hyperactive in breast cancer stem cells and inhibition of Notch 4 signaling significantly reduced tumor growth.31 On the basis of these findings, Notch pathway inhibitors are currently being tested in clinical trials based on their hypothesized role in maintaining CSCs.29 Despite these examples, we are yet to discover the mechanism of Notch signaling activation in solid tumors as, unlike acute T lymphoblastic leukemia, there is little evidence of underlying genomic mutations in patients.
Alternatively, the Notch pathway can be activated by aberrant environmental signaling cues. Broadly speaking, these conditions for Notch activation often depend on non-cell autonomous signaling through ligand-dependent mechanisms. In head and neck squamous carcinoma, activation of the mitogen-activating protein kinase pathway by various growth factors inappropriately induces Jagged1 levels, which in turn promotes Notch signaling in associated endothelial cells and ultimately results in tumor-driven angiogenesis.36 Furthermore, our group37 recently uncovered another mechanism by which aberrant signaling factors from the bone microenvironment can facilitate breast cancer metastasis to the bone through the actions of Notch signaling (Figure 1). TGF-β is sequestered in the bone matrix during bone formation, and often times released in response to bone degradation, a process that is largely at play during osteolytic bone metastasis.38 We found that bone-derived TGF-β aberrantly upregulates Jagged1 levels in breast cancer cells via the SMAD4 canonical signaling axis. Even more telling, enforced expression of Jagged1 in SMAD4-knockdown breast cancer cells, which are severely impaired in their ability to form productive osteolytic bone metastases due to defective TGF-β signaling,39,40 restored the bone metastasis-promoting faculties of these malignant cells, establishing Jagged1 as a critical mediator of TGF-β signaling in bone metastasis.
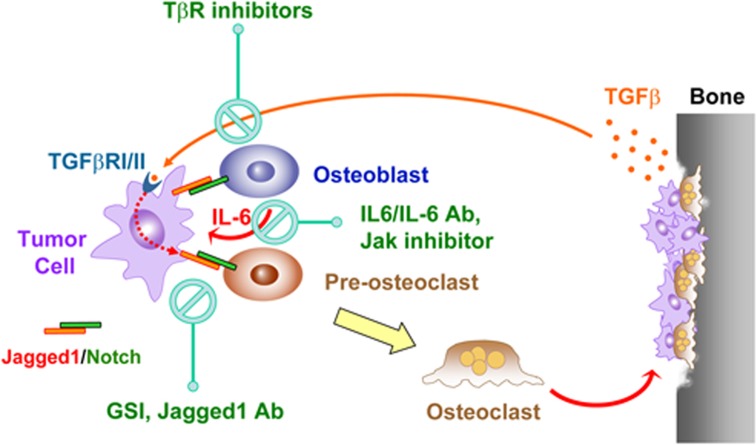
Figure 1.
A network of tumor–stromal interactions mediated by Notch, TGF-β and IL-6 signaling pathways in osteolytic bone metastasis. Jagged1 is overexpressed in breast tumor cells that are highly metastatic to bone and engages bone stromal cells by activating Notch signaling in these cells. Notch signaling promotes the expression and secretion of IL-6, which feeds back to tumor cells to stimulate growth and resistance to chemotherapy. Meanwhile, Notch signaling directly activates osteoclast maturation, thereby exacerbating osteolytic bone metastasis. The destruction of bone matrix releases abundant amount of TGF-β, which further activates the expression of Jagged1 in tumor cells through a Smad-dependent manner. The findings indicate the therapeutic potential of reducing osteolytic bone metastasis by targeting Notch, TGF-β and IL-6 through different classes of inhibitors. Figure is taken from Sethi et al.37
Of clinical importance, elevated expression of Jagged1 was associated with an increased incidence of breast cancer relapse and bone metastasis in two independent patient data sets. Functional studies in mice showed that Jagged1-expressing breast cancer cells promoted bone metastasis by activating the Notch signaling pathway in bone-specific cells. In particular, Jagged1-mediated activation of the Notch pathway in osteoblasts conferred a growth advantage to bone metastatic tumor cells. Further mechanistic investigation revealed that the proliferation-inducing mediator was interleukin (IL)-6, which was transcriptionally activated and subsequently secreted by osteoblasts in a Notch- and Hey1-dependent manner. Interestingly, activation of other developmental pathways in osteoblasts has also shown to promote bone metastasis. Recently, paracrine Sonic Hedgehog signaling by prostate cancer cells was shown to promote bone metastasis by inducing osteoblast differentiation through a Gli1-dependent mechanism.41
Jagged1-mediated bone metastasis also demonstrated a severe osteolytic phenotype in mice, suggesting a potential effect of tumor-derived Jagged1 on osteoclastogenesis. Coculture experiments between Jagged1-expressing tumor cells and primary bone marrow cells demonstrated a strong induction of osteoclastogenesis. Although one could have postulated a possible indirect mechanism throughosteoblast-dependent regulation of RANKL and osteoprotegerin signaling, the results showed a potent impact of Jagged1 on osteoclastogenesis by directly engaging pre-osteoclasts. More definitively, the direct application of recombinant Jagged1 protein to pre-osteoclasts demonstrated a strong upregulation of osteoclast maturation, which was unequivocally confirmed by transcriptional profiling of osteoclast differentiation markers. Integrating these findings, it appears that tumor-derived Jagged1 directly engages Notch pathway in pre-osteoclasts, promoting their differentiation into mature, multinucleated osteoclasts, a mechanism that can explain the severe osteolytic phenotype observed in Jagged1-mediated bone metastasis.
Outside the bone, a role for the Notch pathway in the tumor-associated stroma has also been shown to facilitate cancer progression. For example, activation of the Notch pathway in endothelial cells supports tumor neovascularization and angiogenesis in head and neck squamous cell carcinoma.36 Similarly, Delta-mediated Notch signaling was shown to facilitate tumor angiogenesis by maintaining the sprouting integrity of the developing tumor vasculature.42
Targeting the notch pathway and associated mediators in the treatment of bone metastasis
Ultimately, the molecular mechanisms underlying Notch signaling in bone metastasis inform new directions for therapy in patients with metastatic cancer involving the bone. The pro-metastatic functions of Notch signaling activation in the bone microenvironment begged the question of whether inhibition of the pathway in the stroma could reduce bone metastasis. γ-Secretase inhibitors potently disrupt the Notch pathway and are gaining popularity as they have shown anticancer potential,43 although its role in targeting the tumor-associated stroma has only recently been explored.37 Treatment by γ-secretase inhibitors reversed the bone metastasis-promoting functions of Jagged1 by disrupting the Notch pathway in bone stromal cells. Gene expression analysis of bone metastases demonstrated that γ-secretase inhibitor-treated mice displayed a downregulation of Notch target genes in the stromal but not in the tumor compartment. Overall, the results revealed a stromal-dependent mechanism for the Notch pathway in supporting tumor outgrowth in the bone and suggest that targeting the Notch pathway in the tumor-associated stroma may improve treatment for breast cancer bone metastasis.
Importantly, other mediators and regulators were shown to support Notch signaling in bone metastasis. First, Jagged1 was shown to be upregulated in bone metastatic breast cancer cells and is an ideal target for monoclonal antibody therapy as it is a cell-surface protein. Of significance, monoclonal antibodies targeting Delta-like 4, another Notch pathway ligand, were shown to disrupt tumor growth demonstrating the potential therapeutic benefit of targeting Notch signaling ligands.42 Second, TGF-β signaling has been targeted by therapeutic agents currently being tested in clinical trials39,46 and would be a candidate pathway to disrupt in combination with Notch signaling in the treatment of breast cancer bone metastasis. Third, the cytokine IL-6 is released by osteoblasts in response to Jagged1-mediated bone metastasis and a potential therapeutic target. Importantly, IL-6 is associated with a poor prognosis in breast cancer45,46 and is capable of supporting tumor growth in both autocrine- and stromal-dependent mechanisms.47,48,49 In neuroblastoma47 and multiple myeloma,50 stromal-derived IL-6 has been shown to be an important mediator between cancer cells and the bone microenvironment by supporting tumor survival and affecting osteoclast differentiation, respectively. Considering these collaborating findings, the pathological function of IL-6 should also be targeted in the treatment of bone metastatic disease. Tocilizumab is a humanized monoclonal antibody against IL-6 receptor, which is approved to be used as an immunosuppressive drug for the treatment of rheumatoid arthritis. Additional inhibitors of IL-6 and its downstream Jak-Stat pathways are also actively under clinical development. It is imperative to design appropriate clinical trials to test the therapeutic effect of these agents for the treatment of bone metastasis, either alone or in combination with other inhibitors targeting the cross-talk between tumor and bone stromal cells.
Footnotes
The authors declare no conflict of interest.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science (New York, NY) 1999;284:770–776. [DOI] [PubMed] [Google Scholar]
- Gray GE, Mann RS, Mitsiadis E, Henrique D, Carcangiu ML, Banks A et al. Human ligands of the Notch receptor. Am J Pathol 1999;154:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 2000;5:207–216. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998;393:382–386. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell 2000;5:197–206. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature 1995;377:355–358. [DOI] [PubMed] [Google Scholar]
- Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet 2000;26:484–489. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 2003;194:237–255. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009;137:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer 2003;3:756–767. [DOI] [PubMed] [Google Scholar]
- Tezuka K, Yasuda M, Watanabe N, Morimura N, Kuroda K, Miyatani S et al. Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res 2002;17:231–239. [DOI] [PubMed] [Google Scholar]
- Nobta M, Tsukazaki T, Shibata Y, Xin C, Moriishi T, Sakano S et al. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem 2005;280:15842–15848. [DOI] [PubMed] [Google Scholar]
- Zayzafoon M, Abdulkadir SA, McDonald JM. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem 2004;279:3662–3670. [DOI] [PubMed] [Google Scholar]
- Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 2008;14:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 2008;14:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F, Lee B. NOTCHing the bone: insights into multi-functionality. Bone 2010;46:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M et al. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood 2003;101:2227–2234. [DOI] [PubMed] [Google Scholar]
- Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 2008;283:6509–6518. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S et al. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol 2008;28:6402–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425:841–846. [DOI] [PubMed] [Google Scholar]
- Gridley T. Kick it up a Notch: NOTCH1 activation in T-ALL. Cancer Cell 2004;6:431–432. [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JP IV, Silverman LB, Sanchez-Irizarry C et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004;306:269–271. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 2004;23:1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 2004;18:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest 2005;115:3166–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med 2007;204:2935–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 2008;105:6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest 2008;118:3660–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 2011;8:97–106. [DOI] [PubMed] [Google Scholar]
- Harrison H, Farnie G, Brennan KR, Clarke RB. Breast cancer stem cells: something out of notching? Cancer Res 2010;70:8973–8976. [DOI] [PubMed] [Google Scholar]
- Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res 2010;70:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 2008;3:429–441. [DOI] [PubMed] [Google Scholar]
- Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K et al. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol 2006;293:565–580. [DOI] [PubMed] [Google Scholar]
- Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 2008;3:109–118. [DOI] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res 2004;6:R605–R615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell 2005;8:13–23. [DOI] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011;19:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med 2009;15:960–966. [DOI] [PubMed] [Google Scholar]
- Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 2005;102:13909–13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003;3:537–549. [DOI] [PubMed] [Google Scholar]
- Zunich SM, Douglas T, Valdovinos M, Chang T, Bushman W, Walterhouse D et al. Paracrine sonic hedgehog signalling by prostate cancer cells induces osteoblast differentiation. Mol Cancer 2009;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006;444:1032–1037. [DOI] [PubMed] [Google Scholar]
- Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res 2007;67:1879–1882. [DOI] [PubMed] [Google Scholar]
- Korpal M, Kang Y. Targeting the transforming growth factor-beta signalling pathway in metastatic cancer. Eur J Cancer 2010;46:1232–1240. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res 1999;19 (2B): 1427–1432. [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer 2003;103:642–646. [DOI] [PubMed] [Google Scholar]
- Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res 2009;69:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res 2008;68:9087–9095. [DOI] [PubMed] [Google Scholar]
- Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J 2007;21:3763–3770. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. Eur J Cancer 2006;42:1564–1573. [DOI] [PubMed] [Google Scholar]