Abstract
Increases in L-cell release of GLP-1 are proposed to serve as a negative feedback signal for postprandial changes in gastric emptying and/or motility. Previous ex vivo data suggests that direct electrical stimulation (E-stim) of ileal segments stimulates secretion of GLP-1. This suggests potential feed-forward increases in GLP-1 driven by intestinal neuronal and/or motor activity. To determine if E-stim could increase GLP-1 levels in an in vivo setting, we administered E-stim and nutrients to male Long-Evans rats (300–350g) under general anesthesia. Nutrient infusion into the duodenum or ileum significantly increased plasma GLP-1 levels, but E-stim applied to these locations did not (p<0.05). However, the combination of E-stim and nutrient infusion, in either the ileum or duodenum, significantly increased plasma GLP-1 when compared to nutrient infusion alone (p<0.05), and this effect was not blocked by either norepinephrine or atropine. To test the impact of intestinal motor activity, the effect of extra-luminal mechanical stimulation (M-stim) on GLP-1 levels was assessed. In the duodenum, but not the ileum, M-stim plus nutrient infusion significantly increased GLP-1 over nutrient infusion or M-stim alone (p<0.05). Thus, both E- and M-stim of the duodenum, but only E-stim of the ileum augmented nutrient-stimulated GLP-1 release. These data demonstrate that factors beyond enteral nutrients could contribute to the regulation of GLP-1 secretion.
Keywords: electrical stimulation, GLP-1
Introduction
Glucagon-like peptide-1 (GLP-1) is a neuropeptide that is secreted in a nutrient-dependent manner from the small intestine(1,2). GLP-1 is integral to regulation of glucose homeostasis primarily via stimulation of insulin secretion(3,4) but also via pancreatic-independent effects on glucose turnover (5–7). The importance of the GLP-1 system in regulating glucose homeostasis is illustrated by loss of function experiments (8) and by its therapeutic effectiveness for treatment of type 2 diabetes mellitus(9,10). For example, long-acting GLP-1 receptor agonists and small molecule enzyme blockers (dipetidyl peptidase-IV inhibitors) that increase the half-life of endogenously secreted GLP-1 have been introduced in the last decade and are useful for improving glucose homeostasis in diabetic patients.
Despite the clinical efficacy of increasing endogenous GLP-1 levels, there is only limited understanding of the control of GLP-1 release from the intestine. GLP-1 is secreted from intestinal enteroendocrine L-cells that are present throughout the intestine in increasing density from the proximal to distal gut. The higher density of L-cells in the lower intestine has led to the widespread belief that the proximal L-cells contribute less to post-prandial rises in GLP-1 than do more distal ones, and there is some evidence that this is the case (11). However, plasma and lymph (12) GLP-1 levels peak rapidly after a meal and likely before significant nutrients reach the distal gut (13). This mismatch between GLP-1 secretion and the timing of nutrients reaching the distal gut has led to hypotheses that GLP-1 secretion is stimulated via neural or paracrine mechanisms (14,15). Studies using ex vivo intestinal preparations have demonstrated that nutrient-induced GLP-1 secretion is potentiated by electrical stimulation (16), and 77 GI-tract electrical stimulation devices have been proposed to cause weight loss(17). This is consistent with a model of meal-induced GLP-1 secretion whereby nutrient sensing in the upper gut activates the more dense populations of L-cells in the distal intestine via enteric neural activity. The purpose of this study was to determine if GLP-1 secretion could be stimulated electrically in an in vivo setting.
Materials and Methods
Animal Preparation
Male (~300g) Long Evans rats were purchased from Harlan (Indianapolis, IN). Animals were singly-housed in the University of Cincinnati Laboratory Animals for Medical Science Facility at the Metabolic Diseases Institute under controlled conditions (12:12 light-dark cycle, 50–60% humidity, 25° C) with free access to standard rodent chow diet and water except where noted. All procedures for animal use were approved by The University of Cincinnati Institutional Animal Care and Use Committee and the principles of laboratory animal care as stated by the NIH were followed.
Electrical Stimulation Studies
Animals were placed on a liquid diet for 3d prior to study, were fasted overnight, and the morning of the study were placed under general anesthesia with isofluorane. Positive and negative electrodes (~1cm long) were implanted in a silicone cuff that wrapped around the outside of the intestine such that the electrodes were perpendicular to the long-axis of the ileum or duodenum. A catheter for infusion of nutrients was placed directly into the intestine just distal to the electrode. Electrodes and catheters were placed in all animals regardless of treatment group. Blood was sampled via an implanted portal vein catheter (all ileal infusion studies) or via the tail vein (all duodenal infusion studies). For the ileal studies two groups of animals were used (n=8/group): animals that had E-stim or nutrient infusion (3ml of ensure at 0.1ml/min× 30min) from 0–30 minutes followed by nutrient infusions from 30–60min. For the duodenal E-stim studies there were 4 groups of animals (n=6/group): 1) E-stim alone for 60 min; 2) nutrient infusion alone for 60 min (3ml of Ensure at 0.5ml/min× 6min at 0 and 30min); 3) E-stim from 0–30 minutes and a combination of E-stim plus nutrient infusions from 30–60min or 4) nutrient infusion from 0–30 minutes and a combination of E-stim plus nutrient infusions from 30–60min. The nutrient infusion rates were chosen because our preliminary data demonstrated that this rate was sufficient to increase GLP-1 levels when infused into the respective intestinal locations. Where indicated, the electrical stimulation was run as voltage regulated square pulses (5V, 5ms, 40Hz) that were generated using a function generator (model 33210A, Agilent Technologies, Santa Clara, CA). The electrical stimulation parameters were chosen based on ex vivo studies (16) and on our own preliminary data in an effort to maximize stimulation without tissue damage.
We also studied the role of the autonomic nervous system in mediating the effects of E-stim on GLP-1 levels within the ileum. To do this another group of animals were given an IV infusion of norepinephrine (2μg/min × 10min; Sigma-Aldrich, St. Louis, MO;) or saline and then were studied as above with nutrients (0.1μl/min Ensure) and with or without E-stim (n=6/group). In a second study, an identical protocol was followed except that an IP injection of atropine sulfate (1mg/kg; Sigma-Aldrich, St. Louis, MO) or saline (n=8/group) was administered before the E-stim/nutrients. This dose of atropine effectively reduces gastric emptying rate in rats (18).
Mechanical Stimulation
In our preliminary experiments, we found that excessive handling of the intestine during the experimental preparation lead to variability in GLP-1 levels. In order to determine if this could be replicated experimentally, we devised a cuff with an inflatable bladder that could be placed around the outside of the duodenum (n=10/group) or ileum (n=5/group), respectively and tested the hypothesis that mechanical manipulation of the gut would induce GLP-1 secretion. In anesthetized animals we placed the cuff around the outside of the intestine, and using a syringe pump, an air-filled syringe was used to repeatedly inflate and then deflate (~7X/min) the cuff bladder (+M-stim). Another group of rats had the cuff placed around the intestine but the air bladder was never inflated (M-stim controls). M-stim or control animals were then administered nutrients (as above for the respective intestinal region) or not for 30min.
To determine if M-stim influences nutrient transit time through the small intestine, toluidine blue dye, an indigestible indicator to monitor gut motility, was added to the duodenal nutrient infusion in animals with and without M-stim (n=5/group). After the experiment, the intestine was removed and the distance that the dye travelled down the intestinal tract was visually measured with a ruler and expressed relative to the total length of the small intestine (pylorus to cecum).
To determine if the internal mechanical manipulation of the duodenum could increase plasma GLP-1, a foley catheter was placed inside the intestinal lumen and an air-filled syringe attached to an infusion pump was used to repeatedly inflate and then deflate the balloon to visible external distention (~10X/min). In another group of animals, the foley catheter was inserted into the intestine but left in a deflated state. All animals were then either administered nutrients alone or in combination with mechanical manipulation for 30min (n=8/group).
Hormone Analysis
Total GLP-1 and GIP plasma levels were measured using an electrochemiluminescent detection by Meso Scale Discovery (Gaithersburg, MD).
Statistical Analysis
The data were analyzed using mixed-model ANOVAs with a Tukey’s post-hoc analysis where appropriate. Statistical significance was set at p < 0.05 for all analyses. Data are presented as mean ± SE.
Results
Duodenal E-stim
Nutrient, but not electrical stimulation, of the duodenum increased GLP-1 levels during the 1st experimental period (0–30 min) with no further increase during the 2nd experimental period (30–60 min; p<0.05 vs. baseline values; Figure 1a). However, the combination of nutrients + E-stim given during the 2nd experimental period caused a significant increase in GLP-1 compared to nutrients alone (p <0.05).
Figure 1. Response of GLP-1 and GIP to Duodenal E-stim.
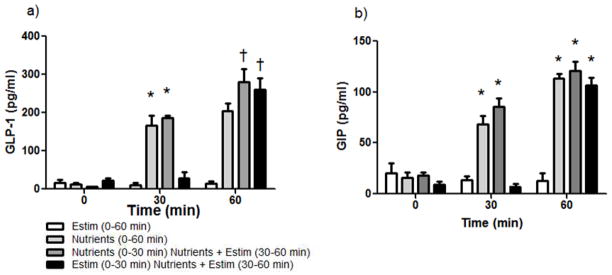
a) Nutrients, but not Estim of the duodenum increased GLP-1 levels at 30 min (light and dark grey bars vs. white and black bars; *p<0.05). At 60 min, the combination of nutrients + Estim given during the 2nd experimental period caused a significant increase in GLP-1 vs. when nutrients were given without E-stim (dark grey and black bars vs. light grey bar at 60 min and vs. both light and grey bars at 30 min; †p<0.05). b) Plasma GIP was significantly increased with nutrient infusions at 30 and 60 min (*p<0.05). Time×group interaction with Tukey post hoc.
GIP has been proposed to be an endocrine regulator of GLP-1 secretion (19). However, while nutrient infusion significantly increased GIP levels over baseline, there was no additional increase in GIP when nutrients and E-stim were administered together (Figure 1b).
Duodenal M-stim
Placing the cuff around the outside of the intestine with or without inflation of the air bladder had no independent effect on GLP-1 levels (Figure 2a). However, the addition of nutrient infusion to M-stim significantly increased GLP-1 levels over the increase seen with nutrient infusion alone (Figure 2a; p<0.05). Internal manipulation of the intestine by repeatedly inflating/deflating a foley catheter had no significant independent effect on GLP-1 levels but did significantly decrease nutrient-induced GLP-1 excursions (Figure 2b; p<0.05). Lastly, M-stim, but not E-stim, significantly increased intestinal transit of nutrients (Figure 3; p<0.05 for nutrients vs. M-stim+Nutrients).
Figure 2. Response of GLP-1 to M-stim.
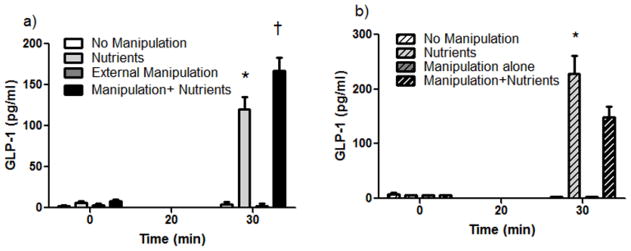
a) Nutrients significantly increased GLP-1 levels vs. M-stim alone (*p<0.05). M-stim plus nutrients significantly increased GLP-1 levels vs. all other groups (†p<0.05). Time×group interaction with Tukey post hoc. b) Nutrients alone significantly increased GLP-1 levels vs. all other groups. *p<0.05. Time×group interaction with Tukey post hoc.
Figure 3. The impact of E-stim and M-stim on intestinal transit of nutrients.
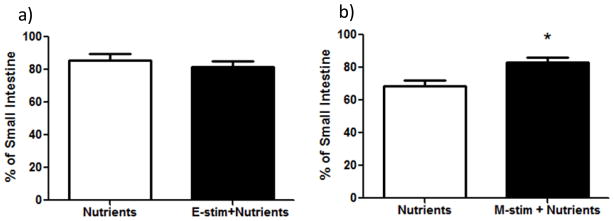
a) % of distance along the small intestine that nutrients travelled was not significantly different with and without E-stim. b) Nutrients travelled a greater % distance with M-stim vs. without M-stim (*p<0.05).
Ileal E-stim
Like the duodenum, E-stim of the ileum alone did not significantly affect GLP-1 levels from 0–30 min (Figure 4a). However, when nutrients and E-stim were combined during the 2nd experimental period (30–60 min), GLP-1 levels were significantly greater than when compared to when nutrients were given alone (Figure 4a). Norepinephrine given just prior to the nutrient infusion or prior to the combination of E-stim plus nutrient infusion had no effect on nutrient or nutrient plus E-stim-induced increases in GLP-1 (Figure 4b). We also tested whether atropine, a muscarinic cholinergic receptor antagonist, could block the combined effect of nutrient and E-stim to increase GLP-1 levels. There was a significant main effect of atropine to lower overall GLP-1 levels regardless of time and E-stim (p<0.05; Figure 5a), but when corrected for this baseline reduction, atropine did not specifically block the combined effect of E-stim plus nutrients on GLP-1 levels (Figure 5b).
Figure 4. The impact of E-stim in the ileum on GLP-1 levels.
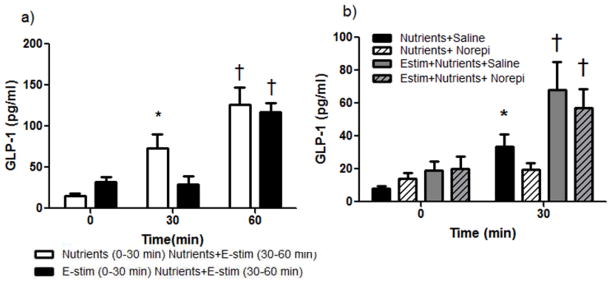
a) Nutrients, but not Estim of the ileum increased GLP-1 levels at 30 min (light vs. black bar at 30 min; *p<0.05). At 60 min, the combination of nutrients + E-stim given caused a significant increase in GLP-1 vs. when nutrients were given without E-stim at 30min (†p<0.05). b) Nutrients plus an IV injection of saline increased GLP-1 levels over baseline (*p<0.05). E-stim plus nutrients significantly increased GLP-1 levels over baseline and over nutrients alone whether given with an IV injection of saline or norepinephrine (†p<0.05).
Figure 5. The impact of atropine on E-stim-induced increases in GLP-1.
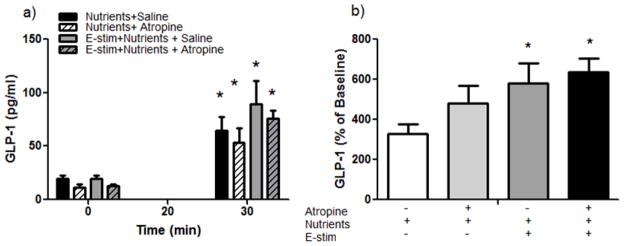
a) Nutrients and E-stim plus nutrients significantly increased GLP-1 levels over baseline (main effect of time; *p<0.05). There was also a significant main effect of drug with atropine lowering GLP-1 levels (p<0.05). b) When calculated as a percentage of baseline, E-stim plus nutrients significantly increased GLP-1 levels over nutrients alone (*E-stim plus nutrients with and without atropine vs. nutrients with saline; *p<0.05). Atropine had no effect on GLP-1 levels when expressed as a percentage of baseline.
Ileal M-stim
Mechanical stimulation had no significant impact on nutrient-induced GLP-1 levels (Figure 6).
Figure 6. Ileal M-stim and GLP-1 levels.
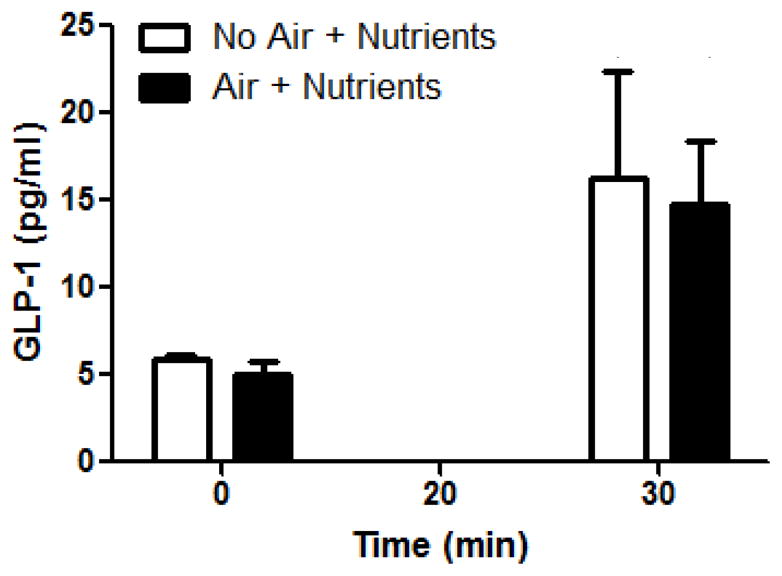
Nutrients, but not M-stim plus nutrients, significantly increased GLP-1 levels over baseline (main effect of time; *p<0.05).
Discussion
In the current study, we demonstrate that electrical stimulation of both the duodenum and ileum augmented nutrient-induced increases in plasma GLP-1. Our findings suggest that this process is nutrient-dependent since E-stim alone did not change plasma GLP-1. Interestingly, in the duodenum but not the ileum, external mechanical stimulation also augmented nutrient-induced increases in plasma GLP-1. Taken together with the increase in intestinal transit of nutrients following M-stim, these data suggest that mechanical manipulation of the duodenum increases motor activity of the intestine. These data support a model in which GLP-1 secretion is regulated by mechanisms in addition to direct stimulation by enteral nutrients.
A novel aspect of our study is the use of electrical and mechanical manipulation of the gut to stimulate GLP-1 release in intact animals. Because of the developmental nature of our E-stim and M-stim devices we used anesthetized rats to generate proof-of-concept results. The demonstration of E-stim/M-stim effects in this model raises the possibility that these responses would be greater without the neuromodulating actions of anesthesia. While we only measured GLP-1, there is good evidence that other preproglucagon peptides (oxyntomodulin, GLP-2) and peptide YY are co-secreted with GLP-1 (20,21). If this were true, the ability to stimulate the release of multiple L-cell peptides could have more wide-spread applications. Extending this work will require the development of stimulatory devices that can be placed in free-living animals for extended periods of time to evaluate the experimental therapeutic potential of devices that can stimulate endogenous GLP-1 secretion.
Our findings do not clearly define the precise molecular events that lead to an increase in nutrient-induced GLP-1 secretion from the intestine, but rather suggest broad mechanisms through which this could occur. The neural stimulation that we used likely caused depolarization of multiple membrane types including neuronal and endocrine and in the end resulted in a nutrient dependent increase in GLP-1 levels. Despite the incremental increase in preproglucagon expression from the proximal to distal gut, postprandial GLP-1 responses are rapid and happen over a time course that would preclude direct nutrient stimulation of cells in the distal intestine. For this reason, alternatives mechanisms linking passage of nutrients into the gut with L-cell secretion have been sought. Several groups have proposed that a neural connection best fits the meal-induced profile of GLP-1 secretion (14,15,22), and the findings presented here support that view.
It has been demonstrated previously that direct electrical stimulation of isolated segments of the distal ileum increased nutrient induced GLP-1 secretion (16). These authors showed that tetrodotoxin did not block the additive effect of electrical stimulation and nutrients to increase GLP-1 secretion from their intestinal preparation, suggesting that this effect was independent of sodium channel-mediated neural transmission. In another ex vivo study, electrical stimulation of mixed extrinsic nerves of isolated perfused porcine ileum inhibited basal secretion of GLP-1 (23), and this effect was inhibited by the α-adrenergic antagonist phentolamine and stimulated by norepinephrine. The authors of this study concluded that sympathetic nervous system activity contributed to the regulation of GLP-1 secretion. In the present study we were unable to detect effects of supraphysiologic doses of norepinephrine on basal (nutrient independent) GLP-1 release, or the combined stimulation of GLP-1 secretion by E-stim and nutrients. Thus our findings do not support a prominent role for regulation of basal or stimulated GLP-1 by the sympathetic nervous system.
Muscarinic receptors have been reported to mediate GLP-1 secretion (24). Acetylcholine serves as a neurotransmitter for both extrinsic vagal input to the intestine, and the enteric nervous system through muscarinic receptors. Consistent with this we observed that atropine blunted nutrient induced GLP-1 secretion, but it did not block the additive effect of E-stim and nutrients on GLP-1 secretion. Taken together, our data suggest that ileal E-stim-induced increases in GLP-1 is independent of the classical neurotransmitters of the autonomic nervous system. Rather, it seems more likely that the effects of E-stim are mediated by non-cholinergic signals in the intrinsic nervous system of the gut or direct effects on L-cells that are independent of neural innervation.
While external manipulation of the duodenum increased GLP-1 levels, internal M-stim blunted nutrient-induced increases in GLP-1 levels. The inhibitory effect of internal manipulation on GLP-1 levels may have been due to intestinal distention which elicits pain responses (25). In the duodenum, external M-stim also increased nutrient transit suggesting an increase in motor function that could move nutrients more rapidly towards distal L-cells. If so, this would suggest that the effects of M-stim overcame the inhibitory effect of GLP-1 on gastro-intestinal motility. It is also possible that the dye plus nutrient infusion technique we used to measure intestinal transit was not sensitive enough to detect the time-dependent dynamic changes in the regulatory loop between GLP-1 and intestinal nutrient transit. In contrast, to what was observed in the duodenum there was no effect of mechanical manipulation of the ileum. This would indicate that duodenal M-stim indirectly resulted in increased GLP-1, a mechanism that would not occur with ileal M-stim. Previous studies have also reported different effects of specific stimuli on GLP-1 release when applied in the upper vs. lower intestine (11,26,27). Thus, our results add to other evidence supporting distinct mechanisms of L-cell activation in different regions of the gut.
Several gastric E-stim devices to treat obesity and/or diabetes have been proposed to work via regulation of gastric emptying (28). However, duodenal E-stim has also been demonstrated to reduce food intake and body weight and again this was proposed to be due to a slowing of gastric emptying rate (29–31). However, the exact mechanisms for these effects remain unknown. Our data add the interesting possibility that intestinal E-stim leads to an increase in GLP-1 (and possibly other intestinal peptides) and this benefits overall energy homeostasis.
In summary, we show that the combination the electrical or mechanical stimulation of the duodenum, but only electrical stimulation of the ileum significantly augments nutrient-induced increases plasma GLP-1 levels. These results indicate that mechanisms beyond direct luminal effects of nutrients on enteroendocrine cells play a role in GLP-1 release and raise the possibility that these could be targets for therapeutic interventions. To this end it will be important to extend our present studies to determine the specific targets of E-stim and M-stim- neural, L-cell or both, and to understand the bases of these interventions in normal physiology or for therapeutic development.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK75365 to D.S.; DK54080, DK54890 and DK056863 to RJS; and DK 57900 to D.A.D.), and by research funding from Ethicon Endo-Surgery (to D.S., R.J.S., and D.A.D.).
Footnotes
Darleen Sandoval: study design, collected and analyzed data, prepared manuscript
Adam Dunki-Jacobs: study design, data collection, edited manuscript
Joyce Sorrell, study design, data collection, edited manuscript
Randy Seeley: study design, data analysis, edited manuscript
Dave D’Alessio: study design, data analysis, edited manuscript
References
- 1.D’Alessio DA, Thirlby R, Laschansky EC, Zebroski H, Ensinck JW. Response of GLP-1 to nutrients in humans. Digestion. 1993;54:377–9. [Google Scholar]
- 2.Dubé PE, Brubaker PL. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Hormone and Metabolic Research research. 2004;36(11–12):755–60. doi: 10.1055/s-2004-826159. [DOI] [PubMed] [Google Scholar]
- 3.Kreymann B, Ghatei MA, Williams G, Bloom SR. Glucagon-like peptide-1 7–36: A physiological incretin in man. Lancet. 1987;2:1300–3. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 4.Mojsov S, Weir GC, Habener J. Insulinotropin: Glucagon-like peptide 1 (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. Journal of Clinical Investigation. 1987;79:616–9. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57(8):2046–54. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115(12):3554–63. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmeister Ma, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Amer J Physiol Endo and Metab. 2012 Feb;302(3):E334–43. doi: 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- 8.Scrocchi LA, Hill ME, Saleh J, Perkins B, Drucker DJ. Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes. 2000;49(9):1552–60. doi: 10.2337/diabetes.49.9.1552. [DOI] [PubMed] [Google Scholar]
- 9.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, RItzel R, et al. Glucagon-like peptide-1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. American Physiological Society. 1997:E981–8. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006 Nov 11;368(9548):1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 11.Hira T, Mochida T, Miyashita K, Hara H. GLP-1 secretion is enhanced directly in the ileum but indirectly in the duodenum by a newly identified potent stimulator, zein hydrolysate, in rats. American Journal of Physiology3: Gastrointestinal and Liver Physiology. 2009 Oct;297(4):G663–71. doi: 10.1152/ajpgi.90635.2008. [DOI] [PubMed] [Google Scholar]
- 12.D’Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, et al. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. American Journal of Physiology: Regulatory, integrative and comparative physiology. 2007 Dec;293(6):R2163–9. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- 13.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. The American Journal of Physiology. 1990 Nov;259(5 Pt 1):G822–37. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- 14.Hansen L, Holst JJ. The effects of duodenal peptides on glucagon-like peptide-1 secretion from the ileum. A duodeno--ileal loop? Regulatory Peptides. 2002;110:39–45. doi: 10.1016/s0167-0115(02)00157-x. [DOI] [PubMed] [Google Scholar]
- 15.Brubaker PL, Anini Y. Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can J Physiol Pharmacol. 2003;81(11):1005–12. doi: 10.1139/y03-107. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz A, Ort T, Kajekar R, Wade PR, Hornby PJ. Electrical stimulation of the isolated rat intestine in the presence of nutrient stimulus enhances glucagon-like peptide-1 release. Physiol Meas. 2010;31:1147–59. doi: 10.1088/0967-3334/31/9/006. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Liu J, Chen JDZ. Neural mechanisms involved in the inhibition of intestinal motility induced by intestinal electrical stimulation in conscious dogs. Neurogastroenterology and Motility. 2006 Jan;18(1):62–8. doi: 10.1111/j.1365-2982.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Cao A, Zhou J, Hu Z, Wu D. Effect of jatrorrhizine on delayed gastrointestinal transit in rat postoperative ileus. Journal of Pharmacy and Pharmacology. 2012 Mar;64(3):413–9. doi: 10.1111/j.2042-7158.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 19.Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993;133(1):233–40. doi: 10.1210/endo.133.1.8319572. [DOI] [PubMed] [Google Scholar]
- 20.Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia. 2013 Mar 22; doi: 10.1007/s00125-013-2887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim B-J, Carlson OD, Jang H-J, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. The Journal of clinical endocrinology and metabolism. 2005 Dec;90(12):6665–71. doi: 10.1210/jc.2005-0409. [DOI] [PubMed] [Google Scholar]
- 22.Dirksen C, Hansen DL, Madsbad S, Hvolris LE, Naver LS, Holst JJ, et al. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33(2):375–7. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen L, Lampert S, Mineo H, Holst JJ. Neural regulation of glucagon-like peptide-1 secretion in pigs. American journal of physiology. Endocrinology and Metabolism. 2004 Nov;287(5):E939–47. doi: 10.1152/ajpendo.00197.2004. [DOI] [PubMed] [Google Scholar]
- 24.Rocca AS, Brubaker PL. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology. 1995;136(12):5593–9. doi: 10.1210/endo.136.12.7588313. [DOI] [PubMed] [Google Scholar]
- 25.Booth CE, Shaw J, Hicks Ga, Kirkup aJ, Winchester W, Grundy D. Influence of the pattern of jejunal distension on mesenteric afferent sensitivity in the anaesthetized rat. Neurogastroenterology and Motility. 2008 Feb;20(2):149–58. doi: 10.1111/j.1365-2982.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 26.Hira T, Muramatsu M, Okuno M, Hara H. GLP-1 secretion in response to oral and luminal palatinose (isomaltulose) in rats. Journal of Nutritional Science and Vitaminology. 2011 Jan;57(1):30–5. doi: 10.3177/jnsv.57.30. [DOI] [PubMed] [Google Scholar]
- 27.Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13(10):1762–72. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 28.Abell TL, Minocha A, Abidi N. Looking to the future: electrical stimulation for obesity. The American Journal of the Medical Sciences. 2006 Apr;331(4):226–32. doi: 10.1097/00000441-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Yin J, Ouyang H, Chen JDZ. Potential of intestinal electrical stimulation for obesity: a preliminary canine study. Obesity (Silver Spring, Md) 2007 May;15(5):1133–8. doi: 10.1038/oby.2007.615. [DOI] [PubMed] [Google Scholar]
- 30.Ouyang H, Yin J, Chen JDZ. Gastric or intestinal electrical stimulation-induced increase in gastric volume is correlated with reduced food intake. Scandinavian journal of gastroenterology [Internet] 2006 Nov;41(11):1261–6. doi: 10.1080/00365520600708008. [cited 2012 Nov 29] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17060118. [DOI] [PubMed] [Google Scholar]
- 31.Yin J, Zhang J, Chen JDZ. Inhibitory effects of intestinal electrical stimulation on food intake, weight loss and gastric emptying in rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2007 Jul;293(1):R78–82. doi: 10.1152/ajpregu.00318.2006. [DOI] [PubMed] [Google Scholar]


