Abstract
Background
Men who have sex with men (MSM) are at elevated risk of having anal cancer. However, the prevalence and incidence among MSM of high-grade anal intraepithelial neoplasia (HGAIN), the putative precursor of anal cancer, is understudied, particularly in Asians.
Methods
A total of 123 HIV-positive and 123 HIV-negative MSM were enrolled at the Thai Red Cross AIDS Research Centre in Bangkok, Thailand, and followed for 12 months. Anal sample collection for human papillomavirus (HPV) genotyping and high-resolution anoscopy (HRA) with biopsies were performed at every visit.
Results
Mean age at enrollment was 28.9 years. HIV-positive MSM were more commonly infected with high-risk HPV types in the anus than HIV-negative MSM (57.5% vs. 36.6%, p=0.001). The prevalence of HGAIN was 18.9% in HIV-positive and 11.4% in HIV-negative MSM (p=0.1). The incidence of HGAIN at 12 months was 29% in HIV-positive and 8% in HIV-negative MSM (p=0.001). The hazard ratios for incident HGAIN in multivariate models were 5.16 (95% CI 1.89–14.08, p<0.001) in MSM with persistent HPV 16 and/or 18 infection and 2.62 (95% CI 1.04–6.61, p=0.042) in HIV-positive MSM.
Conclusions
Approximately one-third of HIV-positive MSM developed incident HGAIN within 12 months. Given the relative increased prevalence of HIV among MSM worldwide, local HGAIN data are needed to guide practitioners, policy makers, and communities in planning for strategies to screen for and treat HGAIN in this population.
Keywords: high-grade anal intraepithelial neoplasia, human papillomavirus, men who have sex with men, HIV
Introduction
The incidence of anal cancer among HIV-positive men who have sex with men (MSM) has continued to increase during the era of highly active antiretroviral therapy (HAART), ranging from 75 to 137 per 100,000 person-years.1-4 The risk of anal cancer among HIV-positive MSM is 5 times higher than that in HIV-negative MSM.3 Human papillomavirus (HPV) has been detected in up to 90% of invasive anal cancers.5,6 Anal HPV infection, particularly with high-risk HPV genotypes, is an important risk factor for anal intraepithelial neoplasia (AIN).7,8
High-grade AIN (HGAIN) is the putative precursor of anal cancer.9-11 AIN has a dynamic picture of temporal progression and regression, but HGAIN is much less likely to regress than low-grade AIN (LGAIN).8,12 A recent systematic review and meta-analysis showed the pooled prevalence of HGAIN to be 29.1% in HIV-positive MSM and 21.5% in HIV-negative MSM.13 HGAIN incidences ranged from 8.5-15.4% per year in HIV-positive MSM and 3.3-6.0% per year in HIV-negative MSM. Even in settings with widespread use of HAART, HGAIN remains common among HIV-positive MSM.14-17 Although data are limited, previous reports have shown a 9-15% progression rate from HGAIN to anal cancer during a median follow-up of 3-5 years.9-11 Based on the prevalence of HGAIN and the incidence of invasive anal cancer from the systematic review, however, the calculated theoretical rates of progression from HGAIN to anal cancer were reported to be 1 in 377 patients per year in HIV-positive men in the HAART era and 1 in 4,196 patients per year in HIV-negative MSM.13
Similar to developed countries, many low- and middle-income countries in Asia are in the midst of expanding epidemics of HIV among MSM.18 The overall HIV prevalence among MSM in Bangkok increased from 17.3% in 2003 to 28.3% in 2005 and then to 30.8% in 2007.19 There are few data on the development and progression of AIN among MSM in Asia. We studied HGAIN prevalence, incidence and associated predictors in a cohort of Thai MSM with and without HIV infection.
Methods
Enrollment and follow-up of study participants
Thai men aged 18 years or older with a history of anal sex with men, who had documented positive or negative (within the previous 30 days) HIV status and who visited Men’s Health Clinic at the Thai Red Cross AIDS Research Centre in Bangkok, Thailand, were consecutively enrolled over a 12-month period into a prospective monitoring study. MSM were excluded if they had 1) prior treatment for anal cancer, 2) anal cytology or high-resolution anoscopy (HRA) or infrared coagulation within 12 months prior to enrollment, 3) trichloroacetic acid or podophyllin application of the intraanal area in the past month, or 4) evidence of active concurrent intraanal or perianal bacterial or herpes simplex virus infection.
All participants gave informed consent. The study was approved by the institutional review board of Chulalongkorn University in Bangkok, Thailand (clinicaltrials.gov identification NCT01637298). Participants were followed at 12 months after baseline except for the first 120 participants who were also followed at 6 months. Demographic data, cancer history, smoking history, sexually transmitted infection (STI) history, HIV risk factors, HIV test results, age at sexual debut, lifetime number and sex of partners, lifetime sexual behaviors, sexual behaviors in the past three months including condom use, and data on urogenital and anal examinations were collected at these visits along with anal sample collection and HRA with biopsy. For HIV-positive participants, data on nadir CD4 count, current CD4 count, plasma HIV RNA, clinical staging, and use of HAART were also collected.
Anal sample collection for anal cytology
All participants had anal cytology samples collected at baseline, month 6 (for the first 120 participants) and month 12 by the same study physician (NT). A moistened, non-lubricated flocked swab (Rovers® EndoCervex-Brush®, Rovers Medical Devices B.V., The Netherlands or FLOQSwabs™, Copan Italia S.p.A., Italy) was gently inserted approximately 2-3 inches into the anal canal. The swab was then removed with a twirling motion and gentle pressure on the walls of the anal canal to maximize cellular yield. The swab was then put in a liquid-based cytology fluid (Liqui-PREP™, LGM International, Inc., Florida, USA), which was stored at 4°C until processing within one week after sample collection. Anal cytology was performed prior to HRA at each visit, so that the study clinician was unaware of the results of cytology at the time of HRA. Anal cytology results were classified using the 2001 Bethesda system20 as normal, atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), or carcinoma.
HRA and biopsy
All participants had HRA at baseline, month 6 (for the first 120 participants) and month 12, regardless of anal cytology results, by the same study physician (NT). Acetic acid solution and Lugol’s solution were used to aid visualization of abnormal anal tissue, which was then biopsied. Histologic diagnoses for each sample were made by three different pathologists from the Department of Pathology and Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University; each was blinded to the diagnoses of the other two. Anal histologic diagnoses were classified as normal, AIN 1, AIN 2, or AIN 3. Discrepancies were resolved by re-evaluation, discussion, and concurrence by at least two pathologists. Participants with histologically confirmed AIN 2 or AIN 3 were referred for infrared coagulation treatment, which was provided at no cost at the study clinic.
HPV genotyping
HPV genotyping was performed on liquid-based anal cytology fluid collected at baseline, month 6 (for the first 120 participants) and month 12 visits and stored at −80°C. HPV typing was done using the LINEAR ARRAY® HPV Genotyping Test (Roche Molecular Systems, Inc., New Jersey, USA). The tests amplified target DNA within the polymorphic L1 region of the HPV genome that is approximately 450 base pairs long by polymerase chain reaction (PCR). It then utilizes nucleic acid hybridization to independently identify 37 anogenital HPV DNA genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39 and CP6108) in cells. These genotypes include the 13 high-risk genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). Primers for human β-globin gene were used to ensure proper extraction, amplification, and cell adequacy. Specimens that were negative for β-globin amplification were excluded from analysis.
Statistical Analysis
Statistical analysis was performed using Stata 12.1 (Statacorp, College Station, TX, USA). Characteristics of HIV-positive and HIV-negative participants were compared using a chi-square or Fisher’s exact test, or student t-test as appropriate; logistic regression was used to identify predictors of MSM who failed to return for 12-month visits or were missing 6-month visits. A composite anal diagnosis was used for analyses based on cytology and histology results. LGAIN included LSIL on cytology and AIN 1 on histology. HGAIN included HSIL on cytology and AIN 2 and AIN 3 on histology. For MSM who had more than one biopsy, the highest histologic grade reported were used. Results with a higher-grade classification were used when both cytology and histology results were available. Cytology results were used for MSM with normal HRA findings, and for MSM with abnormal cytology who refused a biopsy.
HGAIN prevalence was calculated, together with 95% confidence intervals (95% CI). The 12-month HGAIN incidence in individuals without HGAIN at baseline was calculated per person time at risk using the actual visit date; 95% CIs around the incidence rates were calculated assuming a Poisson distribution. A time to first event approach was used, so MSM with prevalent HGAIN were left-censored and those with incidence HGAIN were censored once a diagnosis of HGAIN was made. Kaplan-Meier curves were used to estimate the probability of incident HGAIN at 12 months in HIV-positive vs. HIV-negative MSM. Cox proportional hazards regression with robust estimates of the variance was used to identify potential predictors of incident HGAIN among baseline covariates. Assumptions about linearity of continuous covariates were checked by breaking the variable into quartiles and examining the hazard ratio (HR) and 95% CI for each quartile. When these assumptions were not met, adjacent quartiles were collapsed together if appropriate. We subsequently identified MSM with persistent high-risk HPV genotypes, defined as high-risk HPV genotype(s) which presented at any two or more consecutive visits, the first visit occurring before the HGAIN diagnosis. Cox regression was then used to assess the relative probability of developing HGAIN in the presence of persistent high-risk HPV infection. Multivariate Cox models were built up separately for baseline and persistent HPV infections, including covariates which were significant in univariate models at p<0.2, and adjusting for potential confounders; models were developed for all MSM. A sensitivity analysis using a mid-point assumption for the time to event was also conducted.
Results
Participant characteristics
A total of 123 HIV-positive and 123 HIV-negative MSM were enrolled between December 11, 2009 - December 27, 2010 (Table 1). Among the first 120 MSM scheduled for month 6 follow-up, 92 MSM attended the clinic. MSM who had a month 6 visit were more likely to be HIV-positive than the study population overall, but otherwise had similar baseline characteristics and prevalence of AIN to those who did not have a month 6 visit. Of 246 MSM enrolled, 167 (89 HIV-positive and 78 HIV-negative MSM) completed a month 12 visit. Month 12 visit occurred at a median of 11.1 (interquartile range, IQR 11.0-11.6) months after the baseline visit. Among baseline characteristics, being younger than 30 years was the only factor significantly associated with loss to follow-up at 12 months (OR 2.2, 95% CI 1.2 - 3.9, p=0.006). Those lost to follow-up had similar baseline LGAIN and HGAIN as those who remained in the study at 12 months (LGAIN 28% vs. 34%, p=0.3; HGAIN 15% vs. 14%, p=0.8).
Table 1.
Characteristics of 123 HIV-negative MSM and 123 HIV-positive MSM participants at study enrollment.
| Characteristic | HIV-Negative | HIV-Positive | All MSM | P | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | % | N | % | N | % | ||
| Male sex | 123 | 100 | 123 | 100 | 246 | 100 | 1.0 |
| Thai ethnicity | 123 | 100 | 123 | 100 | 246 | 100 | 1.0 |
| Median age (IQR), years | 28 (23-34) | 28 (24-33) | 28 (23-33) | 0.9 | |||
| Marital Status | 0.62 | ||||||
| Single | 114 | 93 | 113 | 92 | 227 | 92 | |
| Live together | 5 | 4 | 6 | 5 | 11 | 4 | |
| Partner died | 2 | 2 | 2 | 1 | |||
| No response | 4 | 3 | 2 | 2 | 6 | 2 | |
| Education | 0.9 | ||||||
| Secondary school or below | 33 | 27 | 37 | 30 | 70 | 28 | |
| Vocational/college level | 73 | 59 | 68 | 55 | 141 | 57 | |
| Graduate level | 12 | 10 | 14 | 11 | 26 | 11 | |
| No response | 5 | 4 | 4 | 3 | 9 | 4 | |
| Occupation | 0.4 | ||||||
| Unemployed/ Home duties/ retired |
9 | 7 | 13 | 11 | 22 | 9 | |
| Student | 19 | 16 | 15 | 12 | 34 | 14 | |
| Employed | 86 | 70 | 88 | 71 | 174 | 71 | |
| Other | 3 | 2 | 0 | 0 | 3 | 1 | |
| No response | 6 | 5 | 7 | 6 | 13 | 5 | |
|
Monthly income, Thai
Baht |
0.08 | ||||||
| <10,000 | 34 | 28 | 32 | 26 | 66 | 27 | |
| 10-20,000 | 57 | 46 | 41 | 33 | 98 | 40 | |
| 20-50,000 | 18 | 15 | 22 | 18 | 40 | 16 | |
| >50,000 | 1 | 1 | 5 | 4 | 6 | 2 | |
| No response | 13 | 11 | 23 | 19 | 36 | 15 | |
| Sexual preference | 0.54 | ||||||
| Homosexual | 108 | 88 | 111 | 90 | 219 | 89 | |
| Bisexual | 15 | 12 | 12 | 10 | 27 | 11 | |
| Smoking history | 0.4 | ||||||
| Never smoked | 97 | 79 | 87 | 71 | 184 | 75 | |
| Previously smoked | 9 | 7 | 15 | 12 | 24 | 10 | |
| Currently smokes | 15 | 12 | 20 | 16 | 35 | 14 | |
| No response | 2 | 2 | 1 | 1 | 3 | 1 | |
|
Self-reported STI in the
last year |
|||||||
| Syphilis | 4 | 3 | 7 | 6 | 11 | 5 | 0.5 |
| Gonorrhea | 2 | 2 | 14 | 11 | 16 | 7 | 0.003 |
| Non-specific urethritis | 2 | 2 | 4 | 3 | 6 | 2 | 0.7 |
| Herpes simplex infection | 1 | 1 | 2 | 2 | 3 | 1 | 1.0 |
| Warts | 21 | 17 | 11 | 9 | 32 | 13 | 0.09 |
| - Perianal | 18 | 15 | 9 | 7 | 27 | 11 | |
| - Intraanal | 1 | 1 | 2 | 2 | 3 | 1 | |
| - Genitalia | 3 | 2 | 0 | 0 | 3 | 1 | |
|
Syphilis (by VDRL, TPHA,
and treatment history) |
0.01 | ||||||
| No | 100 | 81 | 83 | 67 | 183 | 74 | |
| Yes | 6 | 5 | 4 | 3 | 10 | 4 | |
| Not available | 17 | 14 | 36 | 29 | 53 | 22 | |
| HAART | |||||||
| Naive | 107 | 87 | |||||
| Experienced | 16 | 13 | |||||
|
Median CD4 count, cells/mm3 |
|||||||
| Current CD4 count (IQR) | 343 (248-455) | ||||||
| Nadir CD4 count (IQR) | 295 (206-417) | ||||||
|
Median baseline plasma
HIV RNA |
|||||||
| Baseline log10 copies/mL (IQR) | 4.53 (3.82- 4.94) |
||||||
| N (%) <40 copies/mL | 12 | 10 | |||||
| N (% of those on HAART) <40 copies/mL |
11 | 69 | |||||
IQR, interquartile range; STI, sexually transmitted infection; VDRL, venereal disease research laboratory; TPHA, Treponema pallidum hemagglutination; HAART, highly active antiretroviral therapy.
Percentages may not always add up to 100% because of rounding.
Median (IQR) age at enrollment was 28 (23-33) years. None reported a history of cancer and 14% were current smokers. Syphilis was diagnosed in 4.6% (N=4/87) of HIV-positive and 5.6% (N=6/106) of HIV-negative MSM who had testing at baseline (p=1.0).
Among 123 HIV-positive MSM, median (IQR) baseline CD4 count was 343 (248-455) cells/mm3 and 10% had plasma HIV RNA <40 copies/mL at enrollment. Median (IQR) baseline nadir CD4 count was 295 (206-417) cells/mm3. HAART use was reported by 13% of HIV-positive MSM at baseline, and this increased to 47% at month 12. At month 12, median (IQR) CD4 count was 277 (295-479) cells/mm3 and 33% had plasma HIV RNA <40 copies/mL.
Median (IQR) age at first sex was 18 (16-20) years for HIV-positive and 18 (16-21) years for HIV-negative MSM (p=0.11). Almost all HIV-positive MSM (91.1%) and most HIV-negative MSM (77.2%) reported having >5 lifetime sex partners (p=0.003, Table 2). During the three months prior to study entry, 25.2% of HIV-negative MSM had at least three sexual partners compared with 8.1% of HIV-positive MSM (p=0.005). Among those who practiced receptive anal sex in the past three months, 63.9% of HIV-positive MSM and 59.0% of HIV-negative MSM always used a condom (p=0.82).
Table 2.
Lifetime and recent sexual risk behaviors of 123 HIV-negative MSM and 123 HIV-positive MSM study participants at enrollment.
| Characteristic | HIV-negative | HIV-positive | RR (95% CI) | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | |||
| Lifetime partners | ||||||
| 1 | 4 | 3.3 | 0.003 | |||
| 2-5 | 22 | 17.9 | 9 | 7.3 | ||
| >5 | 95 | 77.2 | 112 | 91.1 | ||
| No response | 2 | 1.6 | 2 | 1.6 | ||
| Lifetime insertive sex | ||||||
| Vaginal | 15 | 12.2 | 12 | 9.8 | 0.8 (0.39-1.60) | 0.68 |
| Anal | 99 | 80.5 | 102 | 82.9 | 1.03 (0.92-1.16) | 0.74 |
| Oral | 118 | 95.9 | 113 | 91.9 | 0.96 (0.90-1.02) | 0.29 |
| Never | 3 | 2.4 | 9 | 7.3 | 3.0 (0.90-1.02) | 0.14 |
| No response | 0 | 0 | 0 | 0 | ||
| Lifetime receptive sex | ||||||
| Anal | 116 | 94.3 | 122 | 99.2 | 1.05 (1.004-1.10) | 0.07 |
| Oral | 114 | 92.7 | 118 | 95.9 | 1.04 (0.97-1.10) | 0.41 |
| Never | 7 | 5.7 | 1 | 0.8 | 0.14 (0.02-1.14) | 0.07 |
| No response | 0 | 0 | 0 | 0 | ||
| Lifetime partner’s sex | ||||||
| Male only | 108 | 87.8 | 111 | 90.2 | 0.54 | |
| Female and Male | 15 | 12.2 | 12 | 9.8 | ||
| Age at sexual debut | ||||||
| ≥22 years | 21 | 17.1 | 17 | 13.8 | 0.69 | |
| 19-21 years | 37 | 30.1 | 33 | 26.8 | ||
| 16-18 years | 37 | 30.1 | 48 | 39.0 | ||
| ≤15 years | 23 | 18.7 | 21 | 17.1 | ||
| Unknown | 5 | 4.1 | 4 | 3.3 | ||
| Median (IQR), years | 18 (16-21) | 18 (16-20) | 0.11 | |||
|
Number of sex partners,
last 3 months |
||||||
| None | 14 | 11.4 | 21 | 17.1 | 0.005 | |
| 1 | 26 | 21.1 | 23 | 18.7 | ||
| 2 | 41 | 33.3 | 49 | 39.8 | ||
| 3-5 | 23 | 18.7 | 9 | 7.3 | ||
| >5 | 8 | 6.5 | 1 | 0.8 | ||
|
Number of sexual acts
per week, last 3 months |
||||||
| None | 13 | 10.6 | 21 | 17.1 | 0.32 | |
| <1 | 51 | 41.5 | 44 | 35.8 | ||
| 1 | 17 | 13.8 | 25 | 20.3 | ||
| 2 | 26 | 21.1 | 21 | 17.1 | ||
| 3 | 15 | 12.2 | 12 | 9.8 | ||
| >3 | 1 | 0.8 | 0 | 0 | ||
| No response | 0 | 0 | 0 | 0 | ||
|
Condom use with
receptive anal sex, last 3 months |
||||||
| Always | 59 | 48.0 | 62 | 50.41 | 0.82 | |
| Sometimes | 32 | 26.0 | 28 | 22.76 | ||
| Never | 9 | 7.3 | 7 | 5.69 | ||
| Not applicable | 22 | 17.9 | 26 | 21.14 | ||
| No response | 1 | 0.8 | 0 | 0 | ||
|
Condom use with the
last receptive anal sex |
||||||
| Yes | 70 | 56.9 | 75 | 60.98 | 0.42 | |
| No | 30 | 24.4 | 22 | 17.89 | ||
| Not applicable | 22 | 17.9 | 26 | 21.14 | ||
| No response | 1 | 0.8 | 0 | 0 | ||
RR, relative risk; CI, confidence interval; IQR, interquartile range.
At month 12, new syphilis diagnoses were made in four MSM with HIV at baseline and in none of HIV-negative MSM (p=0.13). There were four MSM with HIV seroconversion at month 12, giving an HIV incidence rate of 5.1 (95% CI 2.9 – 24.6) per 100 person-years (100 PY). In two of these MSM, anal HPV infection was identified at month 12 but not at baseline.
Anal examination and HRA findings
Anal symptoms reported by the participants (e.g., bleeding, mass, nodule, papule, tag, pain with sex, vesicles, itching, ulcer, pus or other abnormal discharges) and/or signs detected by the examining physician (e.g., anal papilla, mass, warty nodularity, granularity, thickening or induration of the anal wall) were present in 36.2% of MSM with palpable masses being the most common complaint (24.4%). HIV-negative MSM were more likely to report anal bleeding (9.8% vs. 3.3%, p=0.04) than HIV-positive MSM. Condyloma acuminata were identified by urogenital examination in 15.5% of HIV-positive MSM and 13.8% of HIV-negative MSM and the most common location was the perianal area (12.6%).
Anal lesions were identified at HRA in 55% (N=136/246) of participants at baseline, 66% (N=61/92) at month 6, and 56% (N=94/167) at month 12. Abnormal HRA findings were more common in HIV-positive MSM compared with HIV-negative MSM at these visits (67% vs. 43% at baseline, p<0.001, 72% vs. 50% at month 6, p=0.08, and 68% vs. 39% at month 12, p<0.001). The size of the lesions was not recorded. The mean (standard deviation, SD) number of biopsies per participant were 1.2 (0.44) at baseline, 1.2 (0.41) at month 6, and 1.1 (0.48) at month 12. The median (IQR) number of biopsies were 1 (1-1) per participant at all visits.
Prevalence and incidence of HGAIN
Anal cytology identified abnormal results from ASC-US and above in 12.2% of MSM at baseline, 19.6% at month 6, and 31.7% at month 12 (Table 3). HSIL was diagnosed in 1.2% of MSM at baseline, 2.2% at month 6, and 2.4% at month 12. Eight MSM with abnormal HRA findings refused a biopsy (one at baseline, one at month 6, five at month 12, and one at both months 6 and 12). Three biopsies were inadequate samples for histopathological examination (two at month 6 and two at month 12). The histologic diagnoses of the three pathologist concurred in 82% of biopsies. Three MSM who had histologic AIN 1 at baseline had HSIL on cytology. Five MSM without histologic AIN 2 or AIN 3 (one refused biopsy, one had normal histology, three had histologic AIN 1) had HSIL on cytology at month 6 or month 12 visits.
Table 3.
Anal cytology and histology results at baseline, month 6 and month 12 visits.
|
Anal
cytology |
Anal histology
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Normal HRA, No Biopsy |
Abnormal HRA, Refused Biopsy |
Normal | AIN 1 | AIN 2 | AIN 3 | Inadequate | Total | |
| Baseline | ||||||||
| Normal | 104 | 1 | 21 | 55 | 13 | 13 | 0 | 207 |
| ASC-US | 1 | 0 | 0 | 12 | 1 | 4 | 0 | 18 |
| LSIL | 0 | 0 | 0 | 8 | 0 | 1 | 0 | 9 |
| HSIL | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 |
| Missing/ Inadequate |
5 | 0 | 1 | 1 | 1 | 1 | 0 | 9 |
| Total |
110
(45%) |
1
(0.4%) |
22
(9%) |
79
(32%) |
15
(6%) |
19
(8%) |
0 |
246
(100%) |
| Month 6 | ||||||||
| Normal | 29 | 1 | 7 | 19 | 4 | 8 | 1 | 69 |
| ASC-US | 1 | 0 | 1 | 8 | 2 | 0 | 1 | 13 |
| LSIL | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 |
| HSIL | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| Missing/ Inadequate |
1 | 0 | 0 | 1 | 1 | 2 | 0 | 5 |
| Total |
31
(34%) |
1
(1%) |
9
(10%) |
32
(35%) |
7
(8%) |
10
(11%) |
2
(2%) |
92
(100%) |
| Month 12 | ||||||||
| Normal | 64 | 2 | 15 | 20 | 2 | 5 | 0 | 108 |
| ASC-US | 7 | 2 | 1 | 13 | 5 | 6 | 0 | 34 |
| LSIL | 0 | 1 | 0 | 9 | 1 | 3 | 1 | 15 |
| HSIL | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 4 |
| Missing/ Inadequate |
2 | 0 | 2 | 2 | 0 | 0 | 0 | 6 |
| Total |
73
(44%) |
6
(4%) |
18
(11%) |
46
(28%) |
9
(5%) |
14
(8%) |
1
(0.5%) |
167
(100%) |
AIN, anal intraepithelial neoplasia; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Percentages are rounded and may not always add up to 100%.
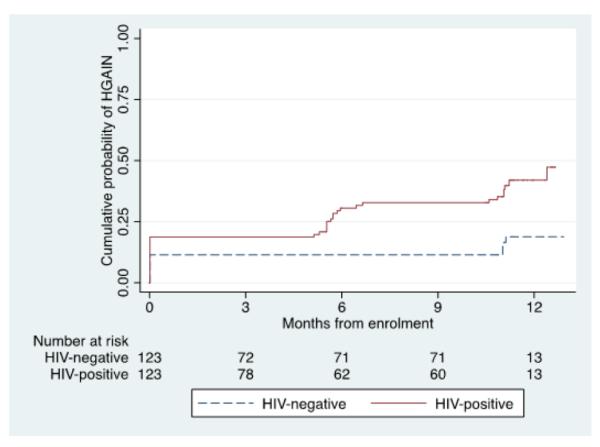
Using a composite diagnosis based on anal cytology and anal histology, the baseline prevalence of HGAIN was 18.9% (95% CI 12.3-26.9) in HIV-positive MSM and 11.4% (95% CI 6.5-18.4) in HIV-negative MSM (p=0.1). During the study period, 26.9% (N=21/78) of HIV-positive MSM and 6.8% (N=5/74) of HIV-negative MSM who did not have HGAIN at baseline developed HGAIN (Figure 1). The probability of incident HGAIN at 12 months in Kaplan-Meier analysis was 29% (95% CI 19-41) in HIV-positive MSM and 8% (95% CI 3-21) in HIV-negative MSM (p=0.001). The cumulative probability of HGAIN (prevalent and incident cases) is shown in Figure 1. There were a total of 1625.3 person-months used in the HGAIN incidence calculations. The 12-month incidence rate of HGAIN was 26.1 per 1000 person-months (95% CI 17.0–40.1) in HIV-positive MSM and 6.1 per 1000 person-months (95% CI 2.5–14.6) in HIV-negative MSM (p=0.001). Among MSM with LGAIN at baseline who had a follow-up visit, 33.3% (N=13/39) of HIV-positive MSM and 14.3% (3/21) of HIV-negative MSM developed HGAIN: incidence rates of 33.0 (95% CI 19.2–56.9) per 1000 person-months in HIV-positive MSM and 13.0 (95% CI 4.2– 40.2) per 1000 person-months in HIV-negative MSM.
Figure 1.
Kaplan-Meier curve showing the cumulative probability of high-grade anal intraepithelial neoplasia (prevalent and incident), by HIV status.
The number of HGAIN cases diagnosed among MSM who had one biopsy performed at each HRA evaluation was lower (24%) than those who had more than one biopsy (39%), p=0.035. Three additional incident HGAIN were diagnosed when a composite endpoint was used, compared to when histology was used alone. If HGAIN was diagnosed based on histology alone, the incidence by the Kaplan-Meier method was 26% (95% CI 17-39) in HIV-positive MSM and 5% (95% CI 1-17) in HIV-negative MSM (p=0.0002). The 12-month HGAIN incidence rate was 24.6 per 1000 person-months (95% CI 15.9 – 38.2) in HIV-positive MSM and 3.5 per 1000 person-months in HIV-negative MSM (p=0.0002)
Anal HPV infection
Anal infection with any HPV type was detected in 85% (95% CI 77-91) of HIV-positive MSM and 58.5% (95% CI 49-67) of HIV-negative MSM (p<0.0001). HIV-positive MSM were more commonly infected with high-risk HPV types in the anus (57.5%, 95% CI 48.5-66.5) than HIV-negative MSM (36.6%, 95% CI 28.0-45.2), p=0.001. HPV 16 was the most common high-risk HPV type in both HIV-positive MSM (22.5%) and HIV-negative MSM (9.8%), p=0.008. In MSM with more than one study visit, persistent infection with any high-risk HPV type was found in 19% (N=15/79) of HIV-negative and 47% (N=45/96) of HIV-positive MSM (p<0.001). HPV 16 persistence was identified in 1% of HIV-negative and 17% of HIV-positive MSM. HPV 18 persistence was found in 4% of HIV-negative and 6% of HIV-positive MSM and persistence of both HPV 16 and HPV 18 in 1% of HIV-negative and 1% of HIV-positive MSM.
Predictors of HGAIN incidence
In univariate analysis performed for all MSM, HIV-positive status, LGAIN at baseline, baseline anal infection with HPV 16 and/or 18, persistent infection with HPV 16 and/or 18, and persistent infection with other high-risk HPV types were significantly associated with incident HGAIN (Table 4). Persistent HPV 16 and/or 18 infection was associated with incident HGAIN in univariate analysis performed separately for HIV-negative MSM. Neither consistent condom use for anal receptive sex, number of sexual partners, whether the participant lived in a rural or urban area, age at sexual debut, nor current smoking were significantly associated with development of HGAIN in univariate models.
Table 4.
Univariate and multivariate analysis of factors associated with risk of incident HGAIN.a
| Covariates | Univariate | Multivariate, baseline HPV | Multivariate, persistent HPV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| HR | Lower CI |
Upper CI |
P | HR | Lower CI |
Upper CI |
P | HR | Lower CI |
Upper CI |
P | |
| All MSM | ||||||||||||
|
Baseline HIV-positive vs. HIV-
negative status |
4.45 | 1.73 | 11.45 | 0.002 | 3.26 | 1.31 | 8.14 | 0.011 | 2.62 | 1.04 | 6.61 | 0.042 |
| LGAIN at baseline | 2.9 | 1.3 | 6.47 | 0.009 | 1.75 | 0.76 | 4.04 | 0.189 | 1.83 | 0.77 | 4.33 | 0.172 |
| Baseline HPV infection | 0.003 | 0.055 | ||||||||||
| No high-risk HPV infection | 1 | 1 | ||||||||||
| HPV 16 and/or 18 infection | 4.81 | 1.92 | 12.04 | 0.001 | 3.12 | 1.19 | 8.21 | 0.021 | ||||
| High-risk HPV infection other than HPV 16 and/or 18 |
1.99 | 0.70 | 5.69 | 0.198 | 1.43 | 0.50 | 4.08 | 0.51 | ||||
| HPV persistence grouping | <0.001 | <0.001 | ||||||||||
| No persistent high-risk HPV infection |
1 | 1 | ||||||||||
| Persistent HPV 16 and/or 18 infection |
8.02 | 3.18 | 20.22 | <0.001 | 5.16 | 1.89 | 14.08 | 0.001 | ||||
| Persistent high-risk HPV infection other than HPV 16 and/or 18 |
3.83 | 1.47 | 9.97 | 0.006 | 2.52 | 0.85 | 7.44 | 0.10 | ||||
| Nadir CD4 count, cells/mm3 b | 0.004 | |||||||||||
| HIV-negative | 1 | |||||||||||
| >350 | 3.51 | 1.13 | 10.9 | 0.03 | ||||||||
| >200-350 | 3.64 | 1.13 | 11.73 | 0.03 | ||||||||
| ≤200 | 7.38 | 2.53 | 21.54 | <0.001 | ||||||||
| Baseline CD4 count, cells/mm3 b | 0.005 | |||||||||||
| HIV-negative | 1 | |||||||||||
| >350 | 3.81 | 1.31 | 11.1 | 0.014 | ||||||||
| >200-350 | 4.12 | 1.3 | 13.04 | 0.016 | ||||||||
| ≤200 | 7.46 | 2.47 | 22.52 | <0.001 | ||||||||
|
Baseline plasma HIV RNA, log10 copies/mL b |
0.016 | |||||||||||
| HIV-negative | 1 | |||||||||||
| <4 | 5.59 | 1.82 | 17.12 | 0.003 | ||||||||
| 4-4.99 | 4.05 | 1.42 | 11.58 | 0.009 | ||||||||
| ≥5 | 4 | 1.08 | 14.84 | 0.04 | ||||||||
HR, hazard ratio; CI, confidence interval; HPV, human papillomavirus; LGAIN, low-grade anal intraepithelial neoplasia.
For categorical covariates, the P on the line with the categorical variable name is the P for heterogeneity. P values for individual categories represent the Wald P value for the category against the reference group.
Nadir CD4 count, baseline CD4 count, and baseline plasma HIV RNA were not included in multivariate analyses for “All MSM”.
In multivariate analysis performed using baseline anal HPV infection for all MSM, HIV-positive status (HR 3.26, 95% CI 1.31-8.14, p=0.011) was independently associated with incident HGAIN after adjusting for baseline LGAIN. There was an association between baseline anal infection with HPV 16 and/or 18 and incident HGAIN (HR 3.12, 95% CI 1.19-8.21, p=0.02 vs. no high-risk HPV infection). When multivariate analysis was performed using persistent anal HPV infection for all MSM, persistent HPV 16 and/or 18 infection was significantly associated with incident HGAIN (HR 5.16, 95% CI 1.89-14.08, p<0.001).
In a sensitivity analysis conducted using a mid-point assumption for the time to event in all MSM (data not shown), HIV-positive status (HR 3.20, 95% CI 1.16-8.84, p=0.025) and baseline anal infection with HPV 16 and/or 18 (HR 2.75, 95% CI 1.21-7.59, p=0.048 vs. no high-risk HPV infection) remained significantly associated with incident HGAIN. Persistent HPV 16 and/or 18 infection also remained a significant risk factor for incident HGAIN (HR 5.23, 95% CI 1.83-15.0, p=0.002).
Discussion
We demonstrated a high incidence of HGAIN in young HIV-positive MSM in Thailand. HGAIN incidence at 12 months was 29% among HIV-positive MSM and 8% among HIV-negative MSM in our study. Before the widespread use of HAART, the incidence of HGAIN was shown to be 15% among HIV-positive MSM compared with 5% among HIV-negative MSM in Seattle during 21 months of follow-up,21 and 34% among HIV-positive MSM and 13% among HIV-negative MSM in San Francisco after four years of follow-up.8 A more recent study in the HAART era from Canada reported HGAIN incidence of 23% at two years and 37% at three years among HIV-positive MSM.16 The HGAIN incidence rate among HIV-positive MSM of 26.1 per 1000 person-months in our study is higher than the 12.8 per 1000 person-months reported from that cohort.22 As up to 15% of persons with HGAIN progress to anal cancer within 3-5 years,9-11 these data highlight the need to address this emerging health issue.
We also found the prevalence of HGAIN to be high at 18.9% among HIV-positive and 11.4% among HIV-negative young Thai MSM. Previously reported HGAIN prevalence among HIV-positive MSM ranged from 6-31% in Germany,23,24 15% in the Netherlands,22 30% in Canada,16 and up to 52% in the US.14,15 These cohorts differ from each other in many aspects including age, sexual risk behaviors, HAART use, and referral patterns to the clinics. Although not statistically significant, HIV-positive MSM tended to have a higher prevalence of HGAIN than HIV-negative MSM in our cohort, consistent with findings from other studies conducted before and after the HAART era.15,25,26
MSM with HIV infection in our study had approximately 2-3 times higher relative risk for incident HGAIN, indicating a role for immunodeficiency and/or HIV-HPV virus interactions in the development of HGAIN. We did not observe a significant association with CD4 counts or HAART use with HGAIN development among HIV-positive MSM. This may be related to the relatively high CD4 counts and the incremental increases in HAART use over the duration of the study. It could also reflect relatively low number of participants and power in this study. HAART use, when initiated at a higher nadir CD4 count and taken for a longer period of time, has been shown to be associated with a lower risk for HGAIN development in previous studies.16,17,22 However, the benefit of HAART use on HGAIN development has not been further confirmed by other studies.14,27 Furthermore, HIV-positive MSM with high current CD4 count were still at higher risk for HGAIN compared with HIV-negative MSM.25
We found MSM with persistent HPV types 16 and/or 18 infection to have a 5.2-fold increased risk of HGAIN incidence, after adjusting for HIV status and baseline LGAIN. A previous longitudinal study in the US also identified MSM who had persistent infection with one or more HPV types to be more likely to develop HGAIN.8 With persistent anal HPV infection being a key risk factor for HGAIN development, and the increased risk of this seen among HIV-positive MSM,28,29 persistent HPV may explain the higher HGAIN incidence in this group. Older age and higher number of recent sex partners were previously reported to be associated with HGAIN development.16 We did not identify age, sexual risk behaviors (including condom use and number of sex partners), or smoking to be predictors of HGAIN.
Our study had a 68% follow-up rate of participants over a 12-month period, which might bias the estimates. However, we did not see any difference in baseline characteristics, except for age, and baseline AIN rates between those who lost to follow-up and those who were retained in the study. Low retention of clients in AIN screening program has previously been reported8,30 and has been attributed to the relative novelty of the screening and lack of consistent guidelines for both the testing component and clinical management of abnormal results.31 The high incidence of HGAIN seen in our study could reflect HGAIN lesions that were present at baseline but were missed. Although it is possible that anoscopist’s acumen to diagnose HGAIN improved over time, this effect is mitigated by the 12-month enrollment period. Some participants had their initial visits while others had their month 12 visits. In addition, although HGAIN incidence seemed to be higher than the prevalence when looked by HIV status, the overall incidence (18.9%) was not much different from the overall prevalence (14.9%). It should be noted that MSM who had month 6 and month 12 visits were more likely to have HGAIN detected than those who only had month 12 visit. The inclusion of month 6 data from a subset of participants who were more likely to be HIV-positive MSM, therefore, could potentially lead to the overestimation of HGAIN incidence among HIV-positive MSM. Finally, the small number of incident HGAIN cases limited our ability to evaluate the contributions of various factors on incident HGAIN. As additional follow-up visits occur in our cohort, our estimates should become more accurate. Our study is strengthened by the performance of HRA in all cases regardless of anal cytology results, and the use of a composite anal diagnosis, which allowed for improved identification of HGAIN.
The high prevalence and incidence of HGAIN in our young MSM cohort is of great concern. Anal cancer is a non-AIDS-defining cancer with increasing incidence in the HAART era.32 Improved survival with HAART, continued high prevalence of HGAIN, and lack of routine screening for HGAIN may explain the increased incidence of anal cancer.33 There remains uncertainty around whether or not to treat HGAIN. All histologically confirmed HGAIN cases are offered treatment by infrared coagulation in our center,34 whereas some prefer to observe HGAIN and wait for the results of large prospective studies which are expected to inform our understanding of rates of progression from HGAIN to anal cancer.13 Until the results from these studies are available, practitioners, policy makers, and communities will need to plan for strategies to screen for and treat AIN using data available from their own settings.
ACKNOWLEDGMENTS
The study team is grateful to the individuals who volunteered to participate in this study. We would like to thank, staff at the Thai Red Cross AIDS Research Centre, Chulalongkorn University, Nitiya Chomchey, and Teresa Darragh for their expert assistance, and Annette Sohn for her review of this manuscript.
The project was supported through a grant from amfAR, The Foundation for AIDS Research through the US National Institutes of Health’s International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907): National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute Of Child Health and Human Development (NICHD), and National Cancer Institute (NCI). The content of this presentation is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Footnotes
AUTHOR CONTRIBUTIONS NP, JP and JA designed the study. NP and JA obtained funding. SJK and NP were responsible for data analysis and interpretation. NT performed anal sample collection, HRA and biopsy. AS collected all data. ST and SN provided anal cytology results. SK and PT provided anal histology results. TP oversaw sample storage and performed HPV genotyping. NP and SJK drafted the report. PP, JP and JA critically revised the report.
REFERENCES
- 1.Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012 Apr;54(7):1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piketty C, Selinger-Leneman H, Grabar S, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008 Jun 19;22(10):1203–1211. doi: 10.1097/QAD.0b013e3283023f78. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008 Aug 1;48(4):491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008 May 20;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 5.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004 Jul 15;101(2):270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 6.Frisch M, Fenger C, van den Brule AJ, et al. Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res. 1999 Feb 1;59(3):753–757. [PubMed] [Google Scholar]
- 7.Palefsky JM, Holly EA, Gonzales J, Berline J, Ahn DK, Greenspan JS. Detection of human papillomavirus DNA in anal intraepithelial neoplasia and anal cancer. Cancer Res. 1991 Feb 1;51(3):1014–1019. [PubMed] [Google Scholar]
- 8.Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS. 1998 Mar 26;12(5):495–503. doi: 10.1097/00002030-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Watson AJ, Smith BB, Whitehead MR, Sykes PH, Frizelle FA. Malignant progression of anal intra-epithelial neoplasia. ANZ J Surg. 2006 Aug;76(8):715–717. doi: 10.1111/j.1445-2197.2006.03837.x. [DOI] [PubMed] [Google Scholar]
- 10.Devaraj B, Cosman BC. Expectant management of anal squamous dysplasia in patients with HIV. Dis Colon Rectum. 2006 Jan;49(1):36–40. doi: 10.1007/s10350-005-0229-z. [DOI] [PubMed] [Google Scholar]
- 11.Scholefield JH, Castle MT, Watson NF. Malignant transformation of high-grade anal intraepithelial neoplasia. Br J Surg. 2005 Sep;92(9):1133–1136. doi: 10.1002/bjs.4994. [DOI] [PubMed] [Google Scholar]
- 12.Palefsky JM, Holly EA, Hogeboom CJ, et al. Virologic, immunologic, and clinical parameters in the incidence and progression of anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual men. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Apr 1;17(4):314–319. doi: 10.1097/00042560-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012 May;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 14.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005 Sep 2;19(13):1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 15.Chin-Hong PV, Berry JM, Cheng SC, et al. Comparison of patient- and clinician-collected anal cytology samples to screen for human papillomavirus-associated anal intraepithelial neoplasia in men who have sex with men. Ann Intern Med. 2008 Sep 2;149(5):300–306. doi: 10.7326/0003-4819-149-5-200809020-00004. [DOI] [PubMed] [Google Scholar]
- 16.de Pokomandy A, Rouleau D, Ghattas G, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis. 2011 May;52(9):1174–1181. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 17.Wilkin TJ, Palmer S, Brudney KF, Chiasson MA, Wright TC. Anal intraepithelial neoplasia in heterosexual and homosexual HIV-positive men with access to antiretroviral therapy. J Infect Dis. 2004 Nov 1;190(9):1685–1691. doi: 10.1086/424599. [DOI] [PubMed] [Google Scholar]
- 18.Beyrer C, Baral SD, Walker D, Wirtz AL, Johns B, Sifakis F. The expanding epidemics of HIV type 1 among men who have sex with men in low- and middle-income countries: diversity and consistency. Epidemiol Rev. 2010 Apr;32(1):137–151. doi: 10.1093/epirev/mxq011. [DOI] [PubMed] [Google Scholar]
- 19.van Griensven F, Varangrat A, Wimonsate W, et al. Trends in HIV Prevalence, Estimated HIV Incidence, and Risk Behavior Among Men Who Have Sex With Men in Bangkok, Thailand, 2003-2007. J Acquir Immune Defic Syndr. 2010 Nov 5;53:234–239. doi: 10.1097/QAI.0b013e3181c2fc86. [DOI] [PubMed] [Google Scholar]
- 20.Darragh TM, Birdsong G, Luff R, Davey D. In: The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes. 2nd ed Solomon D, Nayar R, editors. Springer-Verlag; New York: 2004. [Google Scholar]
- 21.Critchlow CW, Surawicz CM, Holmes KK, et al. Prospective study of high grade anal squamous intraepithelial neoplasia in a cohort of homosexual men: influence of HIV infection, immunosuppression and human papillomavirus infection. AIDS. 1995 Nov;9(11):1255–1262. doi: 10.1097/00002030-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 22.van der Snoek EM, van der Ende ME, den Hollander JC, Schutten M, Neumann HA, van Doornum GJ. Use of highly active antiretroviral therapy is associated with lower prevalence of anal intraepithelial neoplastic lesions and lower prevalence of human papillomavirus in HIV-infected men who have sex with men. Sex Transm Dis. 2012 Jul;39(7):495–500. doi: 10.1097/OLQ.0b013e31825aa764. [DOI] [PubMed] [Google Scholar]
- 23.Kreuter A, Brockmeyer NH, Hochdorfer B, et al. Clinical spectrum and virologic characteristics of anal intraepithelial neoplasia in HIV infection. J Am Acad Dermatol. 2005 Apr;52(4):603–608. doi: 10.1016/j.jaad.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Kreuter A, Brockmeyer NH, Weissenborn SJ, et al. Penile intraepithelial neoplasia is frequent in HIV-positive men with anal dysplasia. J Invest Dermatol. 2008 Sep;128(9):2316–2324. doi: 10.1038/jid.2008.72. [DOI] [PubMed] [Google Scholar]
- 25.Palefsky JM, Holly EA, Ralston ML, et al. Anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual and bisexual men: prevalence and risk factors. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Apr 1;17(4):320–326. doi: 10.1097/00042560-199804010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Kiviat NB, Critchlow CW, Holmes KK, et al. Association of anal dysplasia and human papillomavirus with immunosuppression and HIV infection among homosexual men. AIDS. 1993 Jan;7(1):43–49. doi: 10.1097/00002030-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Fox P, Stebbing J, Portsmouth S, et al. Lack of response of anal intra-epithelial neoplasia to highly active antiretroviral therapy. AIDS. 2003 Jan 24;17(2):279–280. doi: 10.1097/00002030-200301240-00028. [DOI] [PubMed] [Google Scholar]
- 28.Critchlow CW, Hawes SE, Kuypers JM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998 Jul 9;12(10):1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 29.de Pokomandy A, Rouleau D, Ghattas G, et al. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. J Infect Dis. 2009 Apr 1;199(7):965–973. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 30.Mathews WC, Sitapati A, Caperna JC, Barber RE, Tugend A, Go U. Measurement characteristics of anal cytology, histopathology, and high-resolution anoscopic visual impression in an anal dysplasia screening program. J Acquir Immune Defic Syndr. 2004 Dec 15;37(5):1610–1615. doi: 10.1097/00126334-200412150-00014. [DOI] [PubMed] [Google Scholar]
- 31.Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006 Jul 15;43(2):223–233. doi: 10.1086/505219. [DOI] [PubMed] [Google Scholar]
- 32.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009 Aug 19;101(16):1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palefsky JM. Antiretroviral therapy and anal cancer: the good, the bad, and the unknown. Sex Transm Dis. 2012 Jul;39(7):501–503. doi: 10.1097/OLQ.0b013e31825f7921. [DOI] [PubMed] [Google Scholar]
- 34.Palefsky JM. Anal cancer prevention in HIV-positive men and women. Curr Opin Oncol. 2009 Sep;21(5):433–438. doi: 10.1097/CCO.0b013e32832f511a. [DOI] [PMC free article] [PubMed] [Google Scholar]