Abstract
The UNC-45 family of molecular chaperones is expressed in metazoan organisms from Caenorhabditis elegans to humans. The UNC-45 protein is essential in C. elegans for early body-wall muscle cell development and A-band assembly. We show that the myosin-binding UCS domain of UNC-45 alone is sufficient to rescue lethal unc-45 null mutants arrested in embryonic muscle development and temperature-sensitive loss-of-function unc-45 mutants defective in worm A-band assembly. Removal of the Hsp90-binding TPR domain of UNC-45 does not affect rescue. Similar results were obtained with overexpression of the same fragments in wild-type nematodes when assayed for diminution of myosin accumulation and assembly. Titration experiments show that, on a per molecule basis, UCS has greater activity in C. elegans muscle in vivo than full-length UNC-45 protein, suggesting that UNC-45 is inhibited by either the TPR domain or its interaction with the general chaperone Hsp90. In vitro experiments with purified recombinant C. elegans Hsp90 and UNC-45 proteins show that they compete for binding to C. elegans myosin. Our in vivo genetic and in vitro biochemical experiments are consistent with a novel inhibitory role for Hsp90 with respect to UNC-45 action.
Key words: UNC-45, Hsp90, Myosin, Assembly, Accumulation
Introduction
The myosin superfamily of protein motors has essential roles in actin-based cell motility. At least 24 classes of myosins have been identified in multiple eukaryotic species on the basis of their conserved motor domains, which exhibit their own specialized structures and related functions (Foth et al., 2006). Class II, or conventional, myosins function in a broad spectrum of essential cellular processes, including cytokinesis during cell division and muscle contraction (Sellers, 2000). Generally, all myosins have three regions: a motor domain (myosin head), for the binding and hydrolysis of ATP and actin binding; a neck domain, where myosin light chains bind; and a tail domain to position motor domains.
Several studies indicate that proper myosin folding and assembly require the function of additional proteins. Recombinant myosin motor domains cannot be expressed in bacteria to produce soluble and functional proteins (McNally et al., 1988), but, when expressed in cultured C2C12 myogenic cells, are functional (Chow et al., 2002; Resnicow et al., 2010). The chaperones UNC-45 and heat shock protein 90 (Hsp90) have been implicated in proper myosin folding and thick filament assembly (Du et al., 2008). Chaperone deficiency or chaperone excess can lead to decreased myosin assembly and accumulation in C. elegans; lower or over-expression of UNC-45 in C. elegans results in defective myofibril organization (Barral et al., 1998; Landsverk et al., 2007) (Fig. 1A).
Fig. 1.
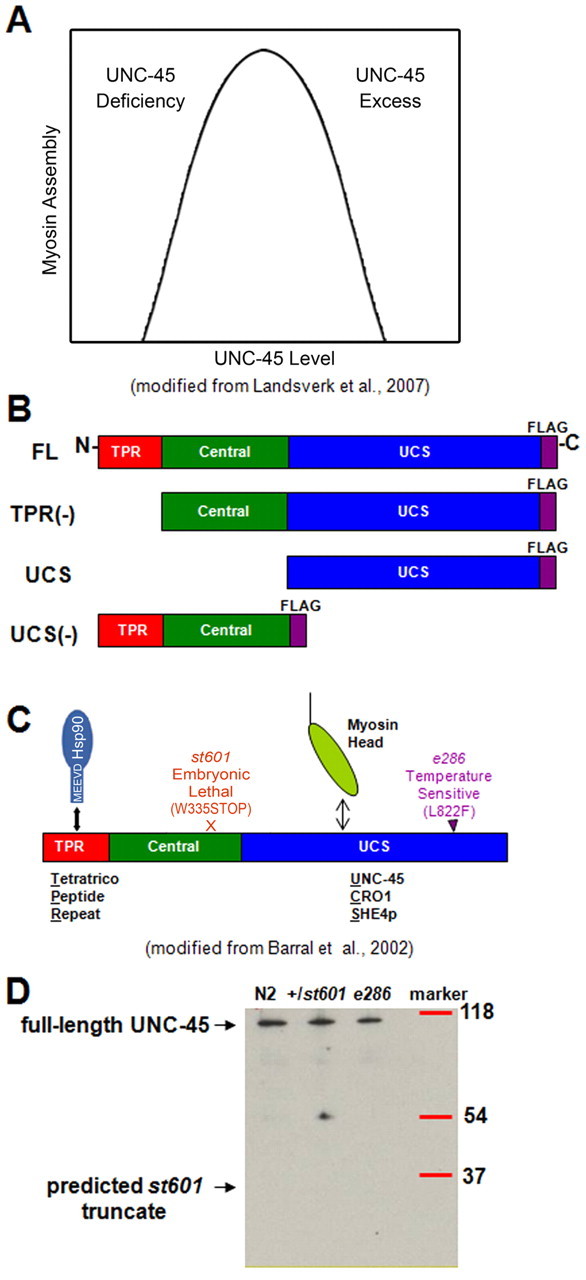
UNC-45 is a multifunctional protein consisting of three identifiable regions. (A) Model of myosin assembly dependence on UNC-45 protein levels in C. elegans body-wall muscle cells. Myosin heavy chain accumulation and its consequent assembly are controlled by protein degradation on either side of the optimal UNC-45 concentration range (Hoppe et al., 2004; Landsverk et al., 2007). (B) Fragments cloned for injection under control of the body-wall muscle-specific unc-54 promoter with the FLAG tag on the C-terminus. (C) UNC-45 contains the N-terminal Hsp90-binding TPR domain, the central region and the C-terminal myosin-binding UCS domain (Barral et al., 1998; Barral et al., 2002). The arrows show the location of the embryonic lethal mutation st601 and the temperature-sensitive mutation e286. (D) The st601 mutation does not produce the predicted truncated protein fragment. st601 UNC-45 protein was examined in heterozygous st601, N2 and e286 worms by immunoblots with rabbit polyclonal anti-C. elegans UNC-45 antibody.
UNC-45 contains three regions: an N-terminal tetratricopeptide repeat (TPR) domain, a central region and a C-terminal UCS domain (Barral et al., 1998). The TPR domain has been shown to interact with Hsp90 (Barral et al., 2002; Russell et al., 1999; Scheufler et al., 2000). The function of the central region has not been determined as yet in C. elegans. It has been reported that the central region mediates Z line association and interacts with Apo2a (the cytidine deaminase Apobec2a) from zebrafish (Etard et al., 2008; Etard et al., 2010). The UCS domain is named for three identified proteins (UNC-45 from C. elegans, Cro1 from Podospora anserina and She4 from Saccharomyces cerevisiae) and interacts with myosin motor domains (Barral et al., 1998; Barral et al., 2002; Lord and Pollard, 2004; Toi et al., 2003). The fungal homologs show similarity to UNC-45 only in the C-terminal UCS domains.
UNC-45 homologs from C. elegans to humans might function as putative Hsp90 co-chaperones (Barral et al., 1998; Barral et al., 2002; Price et al., 2002; Young et al., 2003). In vitro myosin folding experiments suggest that Hsp90-dependent folding of the myosin motor domain is activated by the muscle-specific isoform UNC-45b; activation is blocked by the Hsp90 inhibitor geldanamycin (Liu et al., 2008). Furthermore, both UNC-45b and Hsp90 colocalize in cytoplasmic complexes with sarcomeric myosin during myofibril assembly in C2C12 myogenic cultures (Liu et al., 2008; Mishra et al., 2005; Srikakulam et al., 2008). These results suggest that UNC-45 might serve to modulate Hsp90 function.
Hsp90 has also been reported to inactivate glucocorticoid and estrogen receptors by blocking the access to their DNA-binding domains (Eilers et al., 1989; Picard et al., 1988). Moreover, Hsp90 inhibits promoter-dependent transcription by glucocorticoid receptors (Kang et al., 1999). Hsp90 is also involved in the disruption of transcriptional regulatory complexes (Freeman and Yamamoto, 2002). Therefore, Hsp90 is not only necessary for the folding and assembly of active client proteins, but might also be important for the inactivation of client proteins.
Chadli et al. (Chadli et al., 2006) show that UNC-45A might inhibit the Hsp90-dependent maturation of the human progesterone receptor. Multiple studies indicate that certain functions of UNC-45 and its fungal UCS domain homologs might be independent of Hsp90 in vivo and in vitro (Toi et al., 2003). The structure of She4p from S. cerevisiae has been determined by X-ray crystallography (Shi and Blobel, 2010), proposing that She4p is a dimer of L-shaped molecules. The X-ray crystal structure of Drosophila UNC-45 has recently been published and is an L-shaped monomer with a contiguous series of stacked armadillo repeats (Lee et al., 2011). Recombinant expression of yeast UCS proteins or their UCS domains alone can rescue loss-of-function temperature-sensitive mutants of She4p in the budding yeast S. cerevisiae and Rng3p in the fission yeast S. pombe (Lord and Pollard, 2004; Lord et al., 2008). The Rng3 UCS domain restores myosin II motor Myo2 motility in vitro. In zebrafish, deletion of the N-terminal TPR domain has no effect on the disruptive activity of UNC-45b on myosin thick filament organization, whereas deletion of the C-terminal UCS domain abolishes the disruptive effect of UNC-45b overexpression (Bernick et al., 2010). UNC-45b and Apo2 proteins act in an Hsp90-independent pathway that is required for integrity of the myosepta and myofiber attachment (Etard et al., 2010). Alternatively, Hsp90 might function in inhibitory as well as activating functions with respect to UNC-45.
Here, we test whether the UCS domain of UNC-45 exhibits intrinsic biological activity and examine the effects of the interactions of Hsp90 and UNC-45 in C. elegans body-wall muscle in vivo and with C. elegans proteins in vitro. We cloned various UNC-45 constructs for generation of transgenic worms: FL contains all three regions; TPR(−) consists of the central region and UCS domain, and cannot bind Hsp90; UCS is the UCS domain and can bind myosin; UCS(−) is composed of the Hsp90-binding TPR domain and central region, and cannot bind myosin motor domains (Barral et al., 2002) (Fig. 1B). The relationship between UNC-45 levels and myosin assembly in Fig. 1A, based on our earlier work, serves as the basis for the experimental design of this paper (Landsverk et al., 2007). Both overexpression and rescue of loss-of-function mutants by transgenic UNC-45 and its fragments were tested. The st601 pat (paralyzed at two-fold) stop-codon mutation in the central region prevents egg development around the two-fold stage, and the amino acid substitution mutation e286 in the UCS domain results in severely paralyzed worms with pronounced disorganization of the sarcomere at the 25°C restrictive temperature (Epstein and Thomson, 1974; Venolia and Waterston, 1990) (Fig. 1C,D). Our in vivo and in vitro results suggest a novel inhibitory role of Hsp90 with respect to UNC-45 in C. elegans body-wall muscle.
Results
Transgenic expression of the myosin-binding UCS domain shows the greatest reduction in wild-type nematode motility, A-band assembly and body-wall muscle myosin accumulation
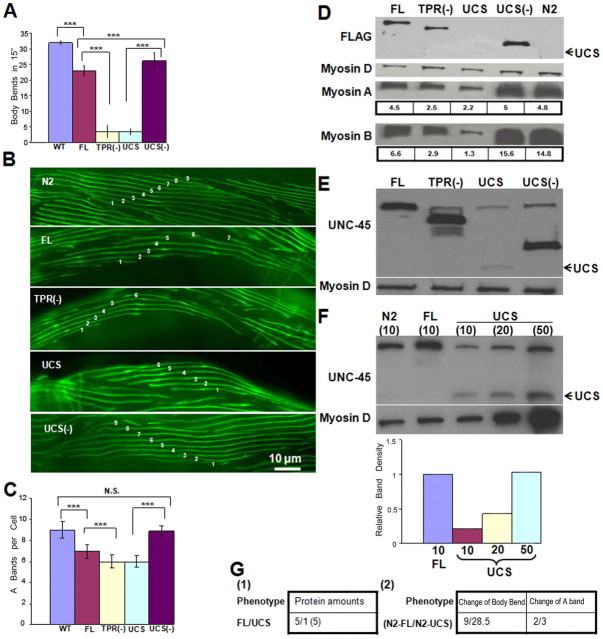
The overexpression of UNC-45 in the N2 wild-type background results in decreased motility and reduced A-band assembly (the assemblage of myosin-containing thick filaments) (Landsverk et al., 2007) (Fig. 1A). To investigate the consequences of expressing different UNC-45 fragments in wild-type worms, we generated specific transgenic lines in N2 by microinjection. These worms carried extrachromosomal arrays to express different UNC-45 fragments under the control of the strong unc-54 promoter that localizes expression to body-wall muscles (Fig. 1B). We found that expression of FL, TPR(−) and UCS, but not UCS(−), markedly decreased the motility of wild-type worms (Fig. 2A). The expression of FL as a positive control decreased the motility of wild type, as previously described (Landsverk et al., 2007). TPR(−) and UCS were able to further decrease the motility of wild type. UCS(−) expression affected the motility of wild-type worms less than other UNC-45 fragments. The UCS domain and TPR(−) fragments, which can bind myosin but not Hsp90, were most active in decreasing motility. Expression of UCS(−), which can bind Hsp90 but not myosin, clearly affected motility less than the other UCS-containing proteins (Fig. 2A).
Fig. 2.
Reduction in wild-type nematode motility, thick filament assembly and body-wall muscle myosin accumulation by transgenic UCS-containing proteins. (A) The UCS domain and TPR(−) constructs significantly decrease wild-type nematode motility. Error bars indicate mean ± s.d. ***P<0.001. (B) The UCS domain and TPR(−) construct significantly diminish A-band assembly in wild-type nematode. (C) Quantification of A-band assembly diminution of transgenic wild type. Error bars indicate mean ± s.d. ***P<0.001. N.S., not significant. (D) The UCS domain is expressed at low levels compared with other transgenic proteins and the myosin-binding truncates show reduction in body-wall muscle myosin accumulation similar to FL UNC-45 in wild-type nematodes. Transgenic expression of UNC-45 fragments was detected by immunoblots with anti-FLAG antibody. UCS was almost invisible by anti-FLAG antibody. Body-wall muscle-specific myosin heavy chains A and B were detected and quantified by immunoblots with mAb 5–6 and mAb 28.2. Pharyngeal myosin heavy chain D, detected by mAb 5–17, was used as loading control. (E) UNC-45 truncate expression in wild type was examined by immunoblots with rabbit polyclonal anti-C. elegans UNC-45 antibody. UCS and other fragments were detectable on the blots. Endogenous UNC-45 and FLAG-tagged full-length UNC-45 overlapped on the top of the immunoblot. Pharyngeal myosin heavy chain D was used as loading control. (F) A titration experiment of transgenic UCS worms in wild type was performed to semi-quantitatively compare the level of UCS expression with that of FL in FL transgenic wild type by rabbit polyclonal anti-C. elegans UNC-45 antibody. Endogenous UNC-45 and FLAG-tagged full-length UNC-45 overlapped on the top of the immunoblot. UCS became detectable by polyclonal anti-C. elegans UNC-45 antibody. Pharyngeal myosin heavy chain D was used as loading control. (G) Table 1 shows the ratio of protein amounts. Table 2 shows ratios of the decreased body bends and the diminished A-bands between transgenic FL and UCS worms compared with those of wild type.
Sarcomere assembly in transgenic wild-type worms was investigated by labeling A-bands with FITC-conjugated monoclonal antibody (mAb) 5–6 (anti-MHC A). The reduction in A-band assembly was detected in transgenic FL, TPR(−) and UCS, but not in the UCS(−) or control parental wild-type lines (Fig. 2B,C). Wild-type worms had normal thick filament assembly. The FL protein was able to reduce A-band assembly. The TPR(−) and UCS fragments were able to further reduce A-band assembly. By contrast, the UCS(−) fragment did not seem to reduce thick filament assembly. In summary, the UCS domain and TPR(−) fragment reduce A-band assembly significantly more than either FL or UCS(−) in wild-type.
The different extents of reduction in motility and myosin assembly in transgenic wild-type worms expressing various UNC-45 fragments could be due to distinct levels of expression of the fragments or differences in their intrinsic activities. The accumulation of these transgenically expressed fragments in wild-type worms was verified by immunoblots with the anti-FLAG antibody. The accumulation of the UCS fragment in wild type was discovered to be significantly lower (almost undetectable) than that of the other transgenic products (Fig. 2D). Body-wall muscle-specific myosins A and B were determined by immunoblots at the same time. Pharyngeal myosin D was used as loading control. Densitometric ratios of body-wall myosins A and B compared to myosin D show that UCS and TPR(−), but not UCS(−), reduced myosin accumulation similarly to FL (Fig. 2D). These results demonstrate that the UCS domain, but not the TPR domain, modifies myosin accumulation and its consequent assembly in this assay.
Using rabbit polyclonal anti-C. elegans UNC-45 antibody (gift of Thorsten Hoppe, University of Cologne, Germany) that reacts with all three UNC-45 regions, UCS was confirmed to be expressed in lower amounts (Fig. 2E). Endogenous UNC-45 and FLAG-tagged full-length UNC-45 overlapped on the top of the immunoblot. The number of transgenic UCS worms per lane on immunoblots was varied in order to semi-quantitatively compare UCS expression levels relative to FL. Rabbit polyclonal anti-C. elegans UNC-45 antibody was used to enhance the detection of the UCS fragment (Fig. 2F). Quantification of the immunoreacted proteins showed that the UCS fragment was expressed at about one-fifth of the FL protein level. With this quantification, ratios of FL versus UCS activities were calculated (Fig. 2G). UCS was strikingly more active on a per molecule basis in reducing A-band assembly and motility of myosin in wild-type worms than full-length protein.
The myosin-interacting UCS domain, but not the Hsp90-binding TPR domain, is necessary for rescue of the embryonic lethal st601 and the temperature-sensitive e286 UNC-45 mutants
To test the possible dominant-negative effects of TPR(−) and UCS fragments on myosin, we studied the effects of expression of these fragments by rescue of unc-45 loss-of-function mutants in vivo. In many cases, particularly in the body-wall muscle cells of C. elegans, expression of transgenes is mosaic (Fire, 1986; Fire and Waterston, 1989; Krause et al., 1994; Okkema et al., 1993). This condition results from the fact that body-wall muscle cells arise from multiple embryonic cell lineages, with the number of cell divisions from zygote to terminally differentiated muscle cells varying from 7 to 13 (Sulston et al., 1983). The opportunity for losing or silencing extrachromosomal constructs or even integrated transgenes exists (Landsverk et al., 2007). Partial rescue is commonly the level of rescue in transgenic C. elegans nematodes. Therefore, the term ‘rescue’ should be taken to mean ‘partial rescue’ as the result of mosaicism.
The st601 mutation results in severely arrested embryonic development (Venolia and Waterston, 1990) (Fig. 1C). st601 appears to be a null mutation, as there is no truncated protein fragment at the expected molecular weight produced in +/st601 heterozygous worms detected by western blotting with the polyclonal antibody, which reacts with UCS, TPR(−) and UCS(−) recombinant fragments and full-length UNC-45 (Fig. 1D). Furthermore, there is significant published literature on the nonsense-mediated mRNA decay pathway in C. elegans and, in particular, its muscle cells (Baker and Parker, 2004; Conti and Izaurrlade, 2005; Hodgkin et al., 1989; Pulak and Anderson, 1993). To investigate the consequences of expressing different UNC-45 fragments to rescue st601 (Fig. 1B), cloned UNC-45 constructs under control of the unc-54 promoter were injected into the parental heterozygous st601 hermaphrodite. The dead egg percentage produced by the parental heterozygous st601 hermaphrodite was 25%, as predicted based on segregation of embryonic lethal homozygotes (Venolia and Waterston, 1990). After injection of the FL construct, the dead egg percentage decreased. The injection of TPR(−) and UCS constructs also decreased the average st601 dead egg percentages, but injection of the UCS(−) construct did not change the percentage of st601 dead eggs (Fig. 3A). Therefore, the presence of the UCS domain, but not the TPR domain, in FL, TPR(−) and UCS is necessary for significant rescue of st601 lethality. This result eliminates the dominant-negative hypothesis.
Fig. 3.
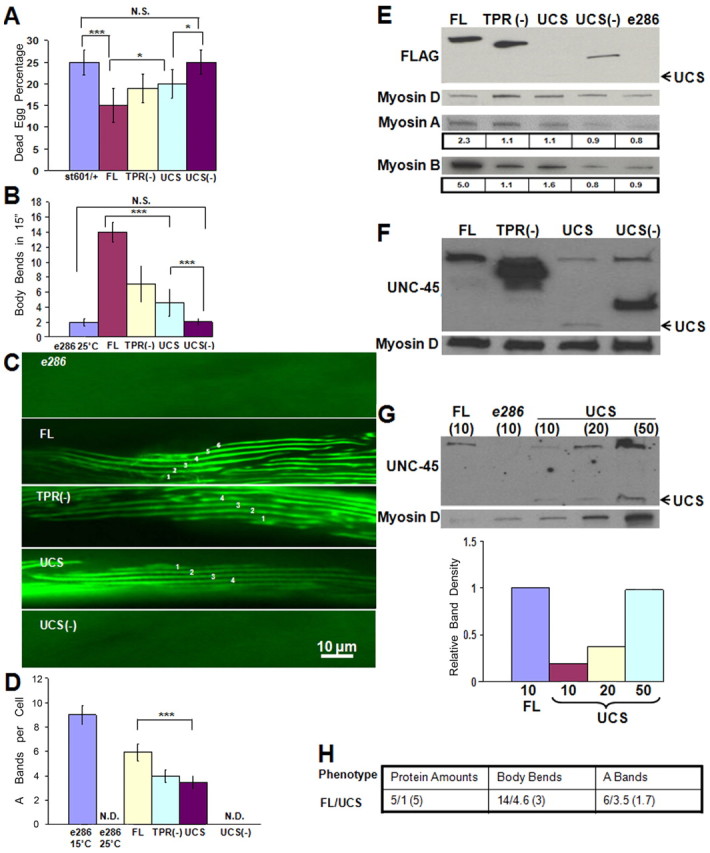
Rescue of st601 and e286 unc-45 mutants by transgenic UCS-containing proteins. (A) The UCS domain and TPR(−) construct are sufficient for significant rescue of st601 lethality. The percentage of dead eggs of the total progeny was measured after injection of the parental hermaphrodite with the specific constructs. Error bars indicate mean ± s.d. *P≤0.05; ***P<0.001. N. S., not significant. (B) The UCS domain and TPR(−) construct are sufficient to significantly rescue e286 motility at 25°C. Error bars indicate mean ± s.d. ***P<0.001. (C) The UCS domain and TPR(−) construct rescue A-band assembly in e286 at 25°C. The outline of worm muscle cell was faint in the UCS(−) or parental e286 mutant lines. (D) Quantification of A-band assembly rescue of transgenic e286 at 25°C. Error bars indicate mean ±s.d. ***P<0.001. (E) The UCS domain is expressed at lower levels compared with the other transgenic proteins, and the myosin-binding truncates rescue body-wall muscle myosin accumulation as does FL in e286. Transgenic expression of UNC-45 fragments was detected by immunoblots with anti-FLAG antibody. UCS band was quite weak with anti-FLAG antibody staining. Body-wall muscle-specific myosin heavy chains A and B were detected and quantified by immunoblots. Pharyngeal myosin heavy chain D was used as loading control. (F) The detection of UNC-45 truncates in e286 by rabbit polyclonal anti-C. elegans UNC-45 antibody. UCS and other fragments were detectable on the blots. Endogenous UNC-45 and FLAG-tagged full-length UNC-45 overlapped on the top of the immunoblot. Pharyngeal myosin heavy chain D was used as loading control. (G) A titration experiment of transgenic UCS worms in e286 was performed to semi-quantitatively compare the level of UCS expression with that of FL in FL transgenic e286 by reaction with rabbit polyclonal anti-C. elegans UNC-45 antibody. Endogenous UNC-45 and FLAG-tagged full-length UNC-45 overlapped on the top of the immunoblot. UCS was visible by polyclonal anti-C. elegans UNC-45 antibody. Pharyngeal myosin heavy chain D was used as loading control. (H) Table showing the ratios of protein amounts, body bends and A-bands between transgenic FL and UCS e286.
As it is very difficult to evaluate the actual expression of the full-length and UCS fragments in the embryos, we cannot rule out that UCS is less effective than FL in the rescue of embryonic lethality of st601 (Fig. 3A). An alternative interpretation is that the expression of the UCS fragment is low, as in the transgenic experiment with adult nematodes. The loss-of-function temperature-sensitive e286 mutation permits the further analysis of the potential effects of transgenic expression of UNC-45 fragments on myosin assembly and consequent motility (Barral et al., 1998; Epstein and Thomson, 1974) (Fig. 1C). Specific transgenic lines, carrying extrachromosomal arrays to express UNC-45 fragments under control of the strong body-wall muscle-specific unc-54 promoter, were generated by microinjection into the e286 background. All transgenically expressed proteins have a C-terminal FLAG tag. The results demonstrate that FL, TPR(−) and UCS, but not UCS(−), were able to rescue the motility defect of e286 at the 25°C restrictive temperature (Fig. 3B). e286 worms grown at 25°C moved very slowly. The expression of FL as a positive control rescued the e286 motility. TPR(−) and UCS were also able to restore the e286 motility. Transformation with UCS(−) did not change the motility of e286 worms. Thus, the UCS domain and TPR(−) fragments most significantly rescued e286 motility at 25°C.
Sarcomere assembly in the transgenic e286 worms grown at 25°C was monitored by labeling A-bands or their absence with FITC-conjugated mAb 5–6 (anti-MHC A). A-bands were detected in transgenic FL, TPR(−) and UCS, but not in the UCS(−) or parental e286 mutant lines (Fig. 3C,D). e286 worms grown at 25°C had abnormal thick filament assembly with no detectable myosin organization (Barral et al., 1998). The FL protein was able to rescue A-band assembly. The TPR(−) and UCS fragments showed similar rescue of A-band assembly. However, the UCS(−) fragment did not rescue thick filament assembly. In summary, FL, UCS and TPR(−) fragments, but not the UCS(−) fragment, can rescue A-band assembly in e286 at the restrictive temperature 25°C.
Differences in the extent of rescue of motility and myosin assembly in transgenic e286 worms could be caused by their differential expression of the fragments or their differential intrinsic activity. The accumulation of these transgenically expressed fragments in e286 was evaluated by immunoblots with anti-FLAG antibody. Similar to the results presented above, UCS accumulation was significantly lower than that of the other transgenic products. Densitometric ratios of myosins A and B compared to the loading control pharyngeal myosin D show that accumulation of the body-wall muscle-specific myosins A and B in the e286 background was increased by UCS, TPR(−) and FL, but not UCS(−) (Fig. 3E).
We also used rabbit polyclonal anti-C. elegans UNC-45 antibody for independent detection of these UNC-45 fragments in transgenic e286 worms, which further confirmed that UCS protein was expressed in lower amounts (Fig. 3F). Endogenous UNC-45 and FLAG-tagged full-length UNC-45 overlapped on the top of the immunoblot. The number of transgenic UCS e286 per lane on immunoblots was modified to semi-quantitatively compare its expression levels with that of FL. Rabbit polyclonal anti-C. elegans UNC-45 antibody was employed to provide recognition of all three UNC-45 regions in vivo and better detection of the UCS domain (Fig. 3G). After quantification of the immunoreacted proteins, UCS fragment was found to be expressed at about one-fifth of the FL protein level in e286 worms. The ratios of FL versus UCS activities based on this quantification were calculated (Fig. 3H). We quantitatively compared e286 transformed by either FL or UCS according to the expressed protein levels, number of body bends for 15 second intervals and the A-bands assembled per body-wall muscle cell. Although FL is expressed at a five times greater level than UCS in the transformed e286, its motility in terms of body bend rate is only threefold higher and its A-band assembly is only 1.7-fold higher. These results suggest that FL had about half of the activity compared to UCS on a per molecule basis when expressed in e286 mutant body-wall muscle. Therefore, UCS is more active in rescuing loss-of-function assembly mutants than the full-length protein. Because FL, but not UCS, can bind Hsp90, these e286 results further suggest that actual Hsp90 interaction or that the TPR domain itself might reduce the myosin chaperone activity of the UCS domain in relation to myosin accumulation and its consequent assembly within C. elegans muscle.
UNC-45 and Hsp90 compete for binding to myosin
Direct binding experiments were performed to distinguish between the effects of Hsp90 and those of the TPR domain upon UNC-45–myosin interaction. In order to test the in vivo binding of Hsp90 to UNC-45, suIs2 (integrated lines overexpressing UNC-45) worms were used in the FLAG pull-down experiment. suIs2 worms overexpress more UNC-45 in body-wall muscle than transgenic FL wild-type worms carrying extrachromsomal arrays (Landsverk et al., 2007). The FLAG IP experiment indicates that FLAG-tagged UNC-45 can bind Hsp90 in worm lysates, which is consistent with the previously published in vitro insect cell lysate pull-down result (Barral et al., 2002) (Fig. 4A). Thus, Hsp90 and UNC-45 have the potential to form complexes in vivo.
Fig. 4.
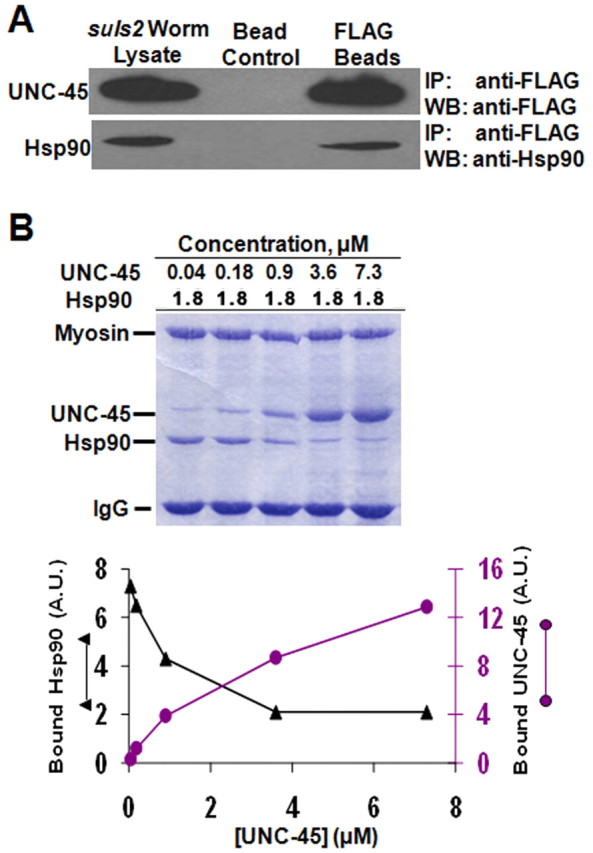
Hsp90 and UNC-45 compete for interaction with myosin. (A) UNC-45 interacts with Hsp90. FLAG beads were incubated with suIs2 worm lysate and myosin-containing complex eluted with FLAG peptide. Hsp90 and UNC-45 were determined by immunoblotting with anti-FLAG antibody and anti-Hsp90 antibody. (B) Titration of UNC-45 to a fixed amount of Hsp90 at a fixed concentration of myosin. The purified proteins were detected by Coomassie Blue staining.
Titration of either UNC-45 or Hsp90 binding to a fixed amount of myosin was performed to establish apparent half-maximal binding of UNC-45 or Hsp90 to myosin (supplementary material Fig. S1). The apparent 50% binding of Hsp90 to myosin was at approximately 1.8 μM, whereas that for UNC-45 was at approximately 1.3 μM, based on the quantification accompanying supplementary material Fig. S1. The titration of recombinant UNC-45 protein with 1.8 μM recombinant Hsp90 protein at a fixed concentration of purified C. elegans myosin was then performed in order to investigate the potential effect of the interaction between Hsp90 and the TPR domain on UNC-45 (Fig. 4B). SDS-PAGE of the titration of these recombinant C. elegans proteins showed that increasing the concentration of UNC-45 prevented the binding of Hsp90 to myosin. Quantification of the Coomassie Blue staining further showed that the decreases in Hsp90 occur concomitantly with increases in UNC-45, consistent with the two proteins competing with one another for binding to myosin.
Discussion
Previous studies have demonstrated that UNC-45 functions as a myosin chaperone that regulates myosin accumulation and assembly by linkage to the ubiquitin proteasome system for degradation (Landsverk et al., 2007). In this report, we have shown that the UCS domain exhibits greater chaperone activity for myosin accumulation and assembly in vivo compared with full-length UNC-45 protein. Full-length UNC-45 can bind the well-known molecular chaperone Hsp90 through its TPR domain, whereas the UCS domain can bind myosin but not Hsp90. Therefore, we propose that, in C. elegans body-wall muscle, Hsp90 might inhibit rather than activate the myosin chaperoning activity of UNC-45. In vitro binding experiments also suggest that UNC-45 and Hsp90 compete for binding to myosin.
Transgenic expression of full-length and fragments of UNC-45 in a wild-type background, UCS and TPR(−) led to decreased worm motility, A-band assembly and body-wall muscle myosin accumulation, as did full-length UNC-45 (Fig. 2A–D). Low levels of UCS could drastically alter the wild-type phenotype compared with that of FL (Fig. 2D–G). The wild-type experiment with the UCS(−) fragment further verified that, without the highly conserved UCS domain, the TPR domain plus central region show the least reductions. This result could be explained by three possibilities: dominant-negative effects of the UCS domain; the inhibition of the UCS domain by the interaction between Hsp90 and the TPR domain; and the inhibition of the activity of the UCS domain due to the existence of the TPR domain itself. In order to clarify the effects of the UCS domain on myosin, mutant worms overexpressing these UNC-45 fragments were analyzed.
In the st601 experiments, injection of FL and the UCS and TPR(−) fragments rescued the embryonic development of mutant worms. However, UCS(−), the truncated protein missing the UCS domain, did not rescue homozygous st601 development at the twofold stage (Fig. 3A). Therefore, the myosin-binding UCS domain, but not the Hsp90-binding TPR domain, is necessary for normal myosin function at this early developmental stage. This finding rules out the dominant-negative possibility. The explanation for the enhanced activity of the UCS domain in the wild-type background might be explained through inhibition through the TPR domain itself or the binding of Hsp90 to the TPR domain.
In the e286 experiments, the expression of UCS and TPR(−) restored worm motility, A-band assembly and body-wall muscle myosin accumulation of e286 to an extent approaching rescue by full-length UNC-45, whereas UCS(−) did not appreciably rescue the temperature-sensitive mutant (Fig. 3B–E). The distinct extents of rescue by UNC-45 protein truncates in e286 worms could be explained by their differential accumulation (Fig. 3E,F). FL levels are fivefold greater than those of UCS by the titration experiment (Fig. 3G). If UCS were expressed at the same level as FL, its rescue of e286 would be predicted to be twofold greater than that by FL (Fig. 3H). Therefore, the UCS domain not only shows intrinsic biological activity in UNC-45-deficient backgrounds, but also is more active on a per molecule basis than full-length UNC-45, which can interact with Hsp90.
UNC-45 is monomeric in solution (Barral et al., 2002; Liu et al., 2008; Srikakulam et al., 2008). Recently, it has been shown that Drosophila UNC-45 is a monomer in the crystal and in solution (Lee et al., 2011). This result is more relevant to our work because the worm and fly proteins show about 40% identity across all three regions of UNC-45, whereas She4p or Rng3p from S. pombe share more limited identity only in the C-terminal UCS domains. Although the S. cerevisiae UCS protein She4p in the crystal led to dimerization through multiple alanine substitutions in the N-terminal helix, wild-type She4p was still more than 90% monomeric in solution (Shi and Blobel, 2010). Mouse UNC-45 proteins are also monomeric in solution (Liu et al., 2008). These proteins (UNC-45a and UNC-45b) are also more closely related to C. elegans UNC-45 than the fungal proteins.
The pull-down experiment confirms that Hsp90 and UNC-45 interact in C. elegans lysates (Fig. 4A). The binding experiment with purified recombinant C. elegans proteins demonstrates that UNC-45 and Hsp90 compete for myosin. Increasing UNC-45 concentration blocks the binding of Hsp90 to myosin (Fig. 4B). The interaction between UNC-45 and Hsp90 but not the existence of the TPR domain itself might have an inhibitory effect on myosin binding to UNC-45.
If the N-terminal TPR domain could bind Hsp90 and the C-terminal UCS domain could bind myosin motor simultaneously, UNC-45 might function to target Hsp90 to myosin motor domains as a Hsp90 co-chaperone, as in model II of Fig. 5 (Barral et al., 2002; Liu et al., 2008; Srikakulam et al., 2008). However, our results are not consistent with this model. Alternatively, Hsp90 and the UCS domain might not be able to bind myosin simultaneously because of steric constraints between them when bound to the TPR and UCS domains, respectively. It is possible that Hsp90 and UNC-45 might bind to the same non-native region of the myosin motor domain. The proposed L-shaped structure of the yeast UCS domain protein She4p, which is comparable to the central UCS fragment that we studied as TPR(−), would be consistent with such constraints (Shi and Blobel, 2010). Drosophila UNC-45 also possesses a similar structure that might underlie these constraints (Lee et al., 2011). Therefore, the interaction between Hsp90 and UNC-45 might inhibit binding to myosin, as in model I of Fig. 5.
Fig. 5.
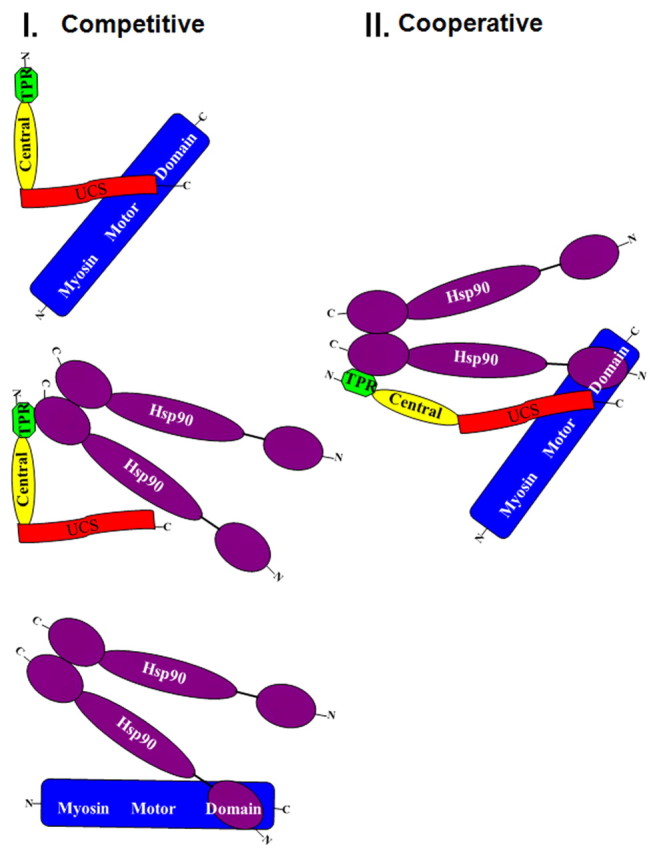
Alternative models of UNC-45, Hsp90 and myosin motor interactions. In model I, UNC-45 and Hsp90 compete for myosin motor binding; binding of Hsp90 to the TPR domain and of myosin to the UCS domain would be sterically incompatible with one another. In this model, only binary complexes of the combinations of the three proteins would be significant. In model II, UNC-45 and Hsp90 cooperate for myosin motor binding in a ternary complex. Model II would require a major conformational change from the crystallographically based L-shaped structure (Lee et al., 2011; Shi and Blobel, 2010) to accommodate binding, because the Hsp90 dimer and myosin motor region have molecular weights of 182,000 and 115,000, respectively, significantly greater than the 107,000 of UNC-45 (Barral et al., 1998; Barral et al., 2002). Hsp90 assumes the open structure because the in vitro binding experiments in Figs 4B and supplementary material Fig. S1 were performed in the absence of ATP. Previous experiments (A. H. H. and H. F. E., unpublished results) showed that myosin did not bind UNC-45 in the presence of ATP.
The binding of FL and UCS to myosin would be predicted to be very similar. Hsp90 inhibition of FL versus UCS is proposed to be responsible for the differences in per mole activity of FL and UCS in vivo. UCS cannot bind Hsp90 and therefore its interaction with myosin is not inhibited by that mechanism. In the presence of Hsp90, the predicted difference would be the result of the formation of binary complexes of Hsp90 and FL in addition to the competition of both myosin binding, whereas UCS would only potentially compete for myosin.
Similar inhibition has been shown in the Hsp90-mediated dissociation of transcriptional regulatory complexes from DNA, diminishing specific transcriptional activity (Freeman and Yamamoto, 2002). In addition, Hsp90 inactivates steroid receptors such as glucocorticoid receptor by masking the DNA-binding domain, and the binding of Hsp90 to the hormone-binding domain of the glucocorticoid receptor causes the unfolding of its polypeptide (Eilers et al., 1989; Picard et al., 1988; Yamamoto et al., 1988). The interaction of Hsp90 with glucocorticoid receptor prevents its activation by making the DNA-binding domain, receptor dimerization sites and nuclear localization sequences of the glucocorticoid receptor inaccessible (Hsu et al., 1992; Picard and Yamamoto, 1987; Qi et al., 1989). Hsp90 also can negatively regulate the activity of this receptor with respect to promoter-dependent transcription (Kang et al., 1999). Interestingly, UNC-45A inhibits the Hsp90–progesterone receptor complex in the maturation pathway (Chadli et al., 2006). The results of our experiments in C. elegans are similar to those with these steroid receptors.
The hypothesis that Hsp90 activates the UNC-45b muscle isoform is based on several correlative lines of evidence. That the two proteins colocalize in C2C12 mouse myogenic cells (Liu et al., 2008; Srikakulam et al., 2008) does not necessarily imply that they physically interact and could be consistent with either an activating or inhibiting role for Hsp90. The effects of the Hsp90 inhibitor geldanamycin upon myofibril assembly in C2C12 cells could be the result of any one of a number of interactions within the myoblasts, dependent on or independent of UNC-45. The interactome of Hsp90 in a variety of cell types is quite large (Falsone et al., 2005; Zhao et al., 2005; Zhao and Houry, 2007). To our knowledge, there is no physical evidence for the existence of a ternary complex of UNC-45, Hsp90 and myosin.
Our study shows that the UCS domain possesses intrinsic biological activity in vivo and the interaction between the TPR domain and Hsp90 inhibits the function of the UCS domain in C. elegans body-wall muscle. Our results support the novel finding that Hsp90 might function as an inhibitory regulator of the UNC-45 chaperone, compared to its more usual function as an activating chaperone. Hsp90 might modulate UNC-45 activity to ensure functional levels of myosin accumulation and proper myosin assembly given that overexpression can be as deleterious as underexpression (Barral et al., 1998; Landsverk et al., 2007). Hsp90 activity can be regulated by phosphorylation (Mollapour et al., 2011; Zhao et al., 2001), which might switch on and off its inhibition of UNC-45–myosin interactions for optimal control of myosin accumulation and assembly.
Materials and Methods
General C. elegans methods
N2 (wild type), CB286 (e286) and LV15 (st601) were obtained from the CGC (Caenorhabditis Genetic Center). suIs2 (integrated lines overexpressing UNC-45) worms were as described previously (Landsverk et al., 2007). N2, CB286 and LV15 were grown on NGM plates for analysis of phenotypes (Brenner, 1974; Epstein and Thomson, 1974; Venolia and Waterston, 1990). suIs2 worms were grown on 8P plates for biochemical studies (Sulston and Brenner, 1974). Transgenic worms with body-wall muscle-specific extrachromosomal arrays of the Punc-54::unc-45 fragmentFLAG were generated by microinjection as previously described (Hoppe et al., 2004; Mello and Fire, 1995; Stinchcomb et al., 1985).
Transgene construction
The cDNA sequences of C. elegans unc-45 fragments with a 39 FLAG tag sequence were subcloned into pPD30.38 (Andrew Fire Lab, Stanford, CA). FL contains all 961 amino acids, amplified with primers 5′-CGGGGTACCCCGATGGTTGCTCGAGTACAGACT-3′ and 5′-CGGGGTACCCCGTCACTTGTCATCGTCGTCCTTGTAGTCGGATCCTTCCTGAATGGTGCTCAT-3′. TPR(−) is amino acids 135–961, cloned by primers 5′-CGGGGTACCCCGATGACCACTTCACTGGCTAAT-3′ and 5′-CGGGGTACCCCGTCACTTGTCATCGTCGTCCTTGTAGTCGGATCCTTCCTGAATGGTGCTCAT-3′. UCS is amplified from amino acids 524–961 with primers 5′-CGGGGTACCCCGATGGCAGTGATCAGTTTGGCG-3′ and 5′-CGGGGTACCCCGTCACTTGTCATCGTCGTCCTTGTAGTCGGATCCTTCCTGAATGGTGCTCAT-3′. UCS(−) is cloned from amino acids 1–523 using primers 5′-CGGGGTACCCCGATGGTTGCTCGAGTACAGACT-3′ and 5′-CGGGGTACCCCGTCACTTGTCATCGTCGTCCTTGTAGTCGGATCCTTCTTCTTTCATCGTTGC-3′.
Immunoblotting
Young-adult worms were hand picked, placed in SDS sample buffer, heated at 95°C for 10 minutes and, after brief cooling, run in 7.5% SDS-PAGE to minimize protein degradation (Miller et al., 1983; Zengel and Epstein, 1980). Mouse monoclonal anti-FLAG M2-peroxidase (HRP) antibody (Sigma), Hsp90 mouse monoclonal antibody AC88 (Stressgen), rabbit polyclonal anti-C. elegans UNC-45 antibody (gift of Thorsten Hoppe, University of Cologne, Germany), mouse monoclonal anti-C. elegans MHC A (myosin heavy chain A) antibody (mAb 5–6), mouse monoclonal anti-C. elegans MHC B (myosin heavy chain B) antibody (mAb 28.2) and mouse monoclonal anti-C. elegans MHC D (myosin heavy chain D) antibody (mAb 5–17) (Miller et al., 1983) were employed for specific immunoblotting (Barral et al., 1998; Landsverk et al., 2007). Densitometry of the exposed films was performed using AlphaEaseFC software (Alpha Innotech). A.U. means arbitrary unit.
Microscopy
Immunofluorescence microscopy on whole mounts of young-adult nematodes was performed according to Finney and Ruvkun (Finney and Ruvkun, 1990). Worms were reacted with FITC-conjugated mAb 5–6 (Miller et al., 1983) and imaged using an Axioplan 2 microscope with a 40 × /0.75 plan-NEOFLUAR objective, equipped with an AxioCam MRc5 digital camera and processed through AxioVision 3.0 software (Carl Zeiss MicroImaging) at room temperature (Barral et al., 1998; Landsverk et al., 2007). The number of A-bands per body-wall muscle cell in region III of transgenic worms was counted.
Motility assays
Individual young-adult nematodes were placed in M9 buffer and body bends were counted for 15-second intervals (Epstein and Thomson, 1974).
Pull-down assays
In Fig. 4A, suIs2 worms were lysed and incubated with anti-FLAG beads (Sigma) for 2 hours at 4°C. After washing beads with lysis buffer three times, proteins were eluted by FLAG peptide (Sigma) three times and run in 7.5% SDS-PAGE for immunoblotting (Barral et al., 2002). In Fig. 4B, C. elegans myosins from worm lysate were immobilized on beads coupled to mouse monoclonal anti-C. elegans mAb 28.2 (Miller et al., 1983). 1.8 μM recombinant C. elegans Hsp90 purified from E. coli and different amounts of recombinant C. elegans UNC-45 purified from Sf9 insect cells were then added to the fixed concentration of myosin beads for interaction. After washing, the resulting protein complexes were analyzed by 7.5% SDS-PAGE, Coomassie Blue staining and densitometry using a BioRad Gel Doc 2000 system with Quantity One Quantitation Software (Bio-Rad) (Barral et al., 2002). In supplementary material Fig. S1, different amounts of purified recombinant C. elegans UNC-45 or Hsp90 were added to the fixed concentration of myosin beads for interaction. Apparent 50% binding of Hsp90 or UNC-45 to myosin was estimated by SigmaPlot Software (Systat Software).
Statistics
The two-tailed Student's t-test was used to evaluate the statistical significance of the result at the 95% confidence level; a P-value less than 0.05 was considered to indicate statistical significance.
Acknowledgments
We thank Jose Barral and Darren Boehning for helpful suggestions and criticisms during the course of this work. We greatly appreciate the gift of polyclonal anti-C. elegans UNC-45 from Torsten Hoppe. The research was supported in part by grants from the Muscular Dystrophy Association and the National Institute of Arthritis, Musculoskeletal and Skin Diseases. Generous support has come from the Cecil H. and Ida M. Green Endowment at UTMB. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.087320/-/DC1
References
- Baker K. E., Parker R. (2004). Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr. Opin. Cell Biol. 16, 293-299 [DOI] [PubMed] [Google Scholar]
- Barral J. M., Bauer C. C., Ortiz I., Epstein H. F. (1998). Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J. Cell Biol. 143, 1215-1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral J. M., Hutagalung A. H., Brinker A., Hartl F. U., Epstein H. F. (2002). Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295, 669-671 [DOI] [PubMed] [Google Scholar]
- Bernick E. P., Zhang P. J., Du S. (2010). Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos. BMC Cell Biol. 11, 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadli A., Graham J. D., Abel M. G., Jackson T. A., Gordon D. F., Wood W. M., Felts S. J., Horwitz K. B., Toft D. (2006). GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Mol. Cell. Biol. 26, 1722-1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow D., Srikakulam R., Chen Y., Winkelmann D. A. (2002). Folding of the striated muscle myosin motor domain. J. Biol. Chem. 277, 36799-36807 [DOI] [PubMed] [Google Scholar]
- Conti E., Izaurralde E. (2005). Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 17, 316-325 [DOI] [PubMed] [Google Scholar]
- Du S. J., Li H., Bian Y., Zhong Y. (2008). Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc. Natl. Acad. Sci. USA 105, 554-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Picard D., Yamamoto K. R., Bishop J. M. (1989). Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature 340, 66-68 [DOI] [PubMed] [Google Scholar]
- Epstein H. F., Thomson J. N. (1974). Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature 250, 579-580 [DOI] [PubMed] [Google Scholar]
- Etard C., Roostalu U., Strähle U. (2008). Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J. Cell Biol. 180, 1163-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard C., Roostalu U., Strähle U. (2010). Lack of Apobec2-related proteins causes a dystrophic muscle phenotype in zebrafish embryos. J. Cell Biol. 189, 527-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsone S. F., Gesslbauer B., Tirk F., Piccinini A. M., Kungl A. J. (2005). A proteomic snapshot of the human heat shock protein 90 interactome. FEBS Lett. 579, 6350-6354 [DOI] [PubMed] [Google Scholar]
- Finney M., Ruvkun G. (1990). The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 63, 895-905 [DOI] [PubMed] [Google Scholar]
- Fire A. (1986). Integrative transformation of Caenorhabditis elegans. EMBO J. 5, 2673-2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Waterston R. H. (1989). Proper expression of myosin genes in transgenic nematodes. EMBO J. 8, 3419-3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth B. J., Goedecke M. C., Soldati D. (2006). New insights into myosin evolution and classification. Proc. Natl. Acad. Sci. USA 103, 3681-3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. C., Yamamoto K. R. (2002). Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296, 2232-2235 [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Papp A., Pulak R., Ambros V., Anderson P. (1989). A new kind of informational suppression in the nematode Caenorhabditis elegans. Genetics 123, 301-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe T., Cassata G., Barral J. M., Springer W., Hutagalung A. H., Epstein H. F., Baumeister R. (2004). Regulation of the myosin-directed chaperone UNC-45 by a novel E3/E4-multiubiquitylation complex in C. elegans. Cell 118, 337-349 [DOI] [PubMed] [Google Scholar]
- Hsu S. C., Qi M., DeFranco D. B. (1992). Cell cycle regulation of glucocorticoid receptor function. EMBO J. 11, 3457-3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K. I., Meng X., Devin-Leclerc J., Bouhouche I., Chadli A., Cadepond F., Baulieu E. E., Catelli M. G. (1999). The molecular chaperone Hsp90 can regulate the activity of a glucocorticosteroid-dependent promoter. Proc. Natl. Acad. Sci. USA 96, 1439-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Harrison S. W., Xu S. Q., Chen L., Fire A. (1994). Elements regulating cell- and stage-specific expression of the C. elegans MyoD family homolog hlh-1. Dev. Biol. 166, 133-148 [DOI] [PubMed] [Google Scholar]
- Landsverk M. L., Li S., Hutagalung A. H., Najafov A., Hoppe T., Barral J. M., Epstein H. F. (2007). The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J. Cell Biol. 177, 205-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. F., Hauenstein A. V., Fleming J. K., Gasper W. C., Engelke V., Sankaran B., Bernstein S. I., Huxford T. (2011). X-ray crystal structure of the UCS domain-containing UNC-45 myosin chaperone from Drosophila melanogaster. Structure 19, 397-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Srikakulam R., Winkelmann D. A. (2008). Unc45 activates Hsp90-dependent folding of the myosin motor domain. J. Biol. Chem. 283, 13185-13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M., Pollard T. D. (2004). UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J. Cell Biol. 167, 315-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M., Sladewski T. E., Pollard T. D. (2008). Yeast UCS proteins promote actomyosin interactions and limit myosin turnover in cells. Proc. Natl. Acad. Sci. USA 105, 8014-8019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally E. M., Goodwin E. B., Spudich J. A., Leinwand L. A. (1988). Coexpression and assembly of myosin heavy chain and myosin light chain in Escherichia coli. Proc. Natl. Acad. Sci. USA 85, 7270-7273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C., Fire A. (1995). DNA transformation. Methods Cell Biol. 48, 451-482 [PubMed] [Google Scholar]
- Miller D. M., Ortiz I., Berliner G. C., Epstein H. F. (1983). Differential localization of two myosins within nematode thick filaments. Cell 34, 477-490 [DOI] [PubMed] [Google Scholar]
- Mishra M., D'Souza V. M., Chang K. C., Huang Y., Balasubramanian M. K. (2005). Hsp90 protein in fission yeast Swo1p and UCS protein Rng3p facilitate myosin II assembly and function. Eukaryot. Cell 4, 567-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollapour M., Tsutsumi S., Truman A. W., Xu W., Vaughan C. K., Beebe K., Konstantinova A., Vourganti S., Panaretou B., Piper P. W., et al. (2011). Threonine 22 phosphorylation attenuates hsp90 interaction with cochaperones and affects its chaperone activity. Mol. Cell 41, 672-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkema P. G., Harrison S. W., Plunger V., Aryana A., Fire A. (1993). Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135, 385-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. (1987). Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 6, 3333-3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. (1988). A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell 54, 1073-1080 [DOI] [PubMed] [Google Scholar]
- Price M. G., Landsverk M. L., Barral J. M., Epstein H. F. (2002). Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci. 115, 4013-4023 [DOI] [PubMed] [Google Scholar]
- Pulak R., Anderson P. (1993). mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 7, 1885-1897 [DOI] [PubMed] [Google Scholar]
- Qi M., Hamilton B. J., DeFranco D. (1989). v-mos oncoproteins affect the nuclear retention and reutilization of glucocorticoid receptors. Mol. Endocrinol. 3, 1279-1288 [DOI] [PubMed] [Google Scholar]
- Resnicow D. I., Deacon J. C., Warrick H. M., Spudich J. A., Leinwand L. A. (2010). Functional diversity among a family of human skeletal muscle myosin motors. Proc. Natl. Acad. Sci. USA 107, 1053-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. C., Whitt S. R., Chen M.-S., Chinkers M. (1999). Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J. Biol. Chem. 274, 20060-20063 [DOI] [PubMed] [Google Scholar]
- Scheufler C., Brinker A., Bourenkov G., Pegoraro S., Moroder L., Bartunik H., Hartl F. U., Moarefi I. (2000). Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101, 199-210 [DOI] [PubMed] [Google Scholar]
- Sellers J. R. (2000). Myosins: a diverse superfamily. Biochim. Biophys. Acta 1496, 3-22 [DOI] [PubMed] [Google Scholar]
- Shi H., Blobel G. (2010). UNC-45/CRO1/She4p (UCS) protein forms elongated dimer and joins two myosin heads near their actin binding region. Proc. Natl. Acad. Sci. USA 107, 21382-21387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikakulam R., Liu L., Winkelmann D. A. (2008). Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain. PLoS ONE 3, e2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Shaw J. E., Carr S. H., Hirsh D. (1985). Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol. Cell. Biol. 5, 3484-3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Brenner S. (1974). The DNA of Caenorhabditis elegans. Genetics 77, 95-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119 [DOI] [PubMed] [Google Scholar]
- Toi H., Fujimura-Kamada K., Irie K., Takai Y., Todo S., Tanaka K. (2003). She4p/Dim1p interacts with the motor domain of unconventional myosins in the budding yeast, Saccharomyces cerevisiae. Mol. Biol. Cell 14, 2237-2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia L., Waterston R. H. (1990). The unc-45 gene of Caenorhabditis elegans is an essential muscle-affecting gene with maternal expression. Genetics 126, 345-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Godowski P. J., Picard D. (1988). Ligand-regulated nonspecific inactivation of receptor function: a versatile mechanism for signal transduction. Cold Spring Harb. Symp. Quant. Biol. 53, 803-811 [DOI] [PubMed] [Google Scholar]
- Young J. C., Barral J. M., Hartl F. U. (2003). More than folding: localized functions of cytosolic chaperones. Trends Biochem. Sci. 28, 541-547 [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Epstein H. F. (1980). Mutants altering coordinate synthesis of specific myosins during nematode muscle development. Proc. Natl. Acad. Sci. USA 77, 852-856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Houry W. A. (2007). Molecular interaction network of the Hsp90 chaperone system. Adv. Exp. Med. Biol. 594, 27-36 [DOI] [PubMed] [Google Scholar]
- Zhao R., Davey M., Hsu Y. C., Kaplanek P., Tong A., Parsons A. B., Krogan N., Cagney G., Mai D., Greenblatt J., et al. (2005). Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120, 715-727 [DOI] [PubMed] [Google Scholar]
- Zhao Y. G., Gilmore R., Leone G., Coffey M. C., Weber B., Lee P. W. (2001). Hsp90 phosphorylation is linked to its chaperoning function. Assembly of the retrovirus cell attachment protein. J. Biol. Chem. 276, 32822-32827 [DOI] [PubMed] [Google Scholar]