Abstract
Alveolar bone grafting is an important part of the reconstructive journey for many cleft lip and palate patients. The reconstruction of the alveolar cleft can provide both aesthetic and functional benefits to the patient. To be able to effectively treat alveolar clefts, it is essential to possess an understanding of several aspects of the problem. Acquiring this knowledge will allow the provider to treat the different variants of the cleft alveolus. In this article, the author will discuss anatomy, history, techniques, controversies, and new technologies to provide the reader with new insight into treating this challenging condition.
Keywords: alveolar cleft, primary alveolar cleft grafting, secondary alveolar bone grafting, bone morphogenic protein, bone graft sources, cleft lip, cleft palate
Anatomy and Embryology
The alveolus is a component of the primary palate and is formed by the fusion of the maxillary prominences around the fifth to sixth weeks of gestation. The structures that arise from the primary palate are the nose, lip, prolabium, and the premaxilla; all of these structures are anterior to the incisive foramen.1
The term alveolus is derived from Latin and refers to a small cavity. The dental alveolus is the tooth cavity or socket. The alveolar process of the maxilla is where the tooth sockets are located: This is the primary functional concern related to clefting of the alveolus. The alveolar bone is connected to the teeth via periodontal ligaments. The permanent teeth above the alveolar cleft are not sustainable if there is not adequate bone stock placed in the alveolar cleft into which they can erupt. The maxillary canine/cuspid tooth typically erupts into the alveolar cleft space. The lateral incisor tooth erupts either adjacent to the cleft or into the cleft. These teeth typically erupt between 7 to 12 years of age. The alveolar bone grafting is ideally performed prior to the eruption of involved teeth. It is our practice to obtain dental x-rays relatively early (e.g., age 5) to be able to prevent loss of a tooth due to eruption into an ungrafted cleft alveolus (see Figs. 1, 2).
Fig. 1.
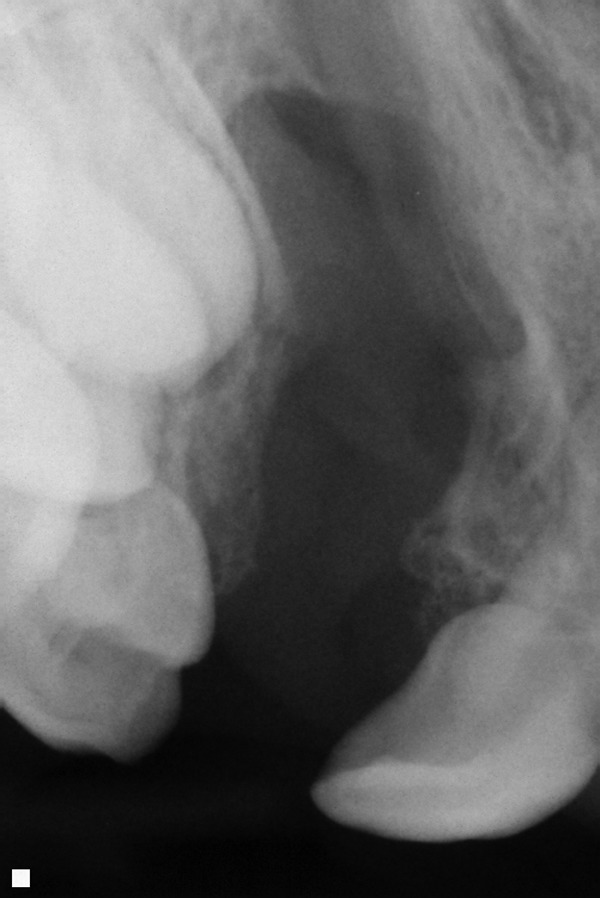
Dental x-ray that shows unerupted permanent teeth above an unoperated cleft.
Fig. 2.
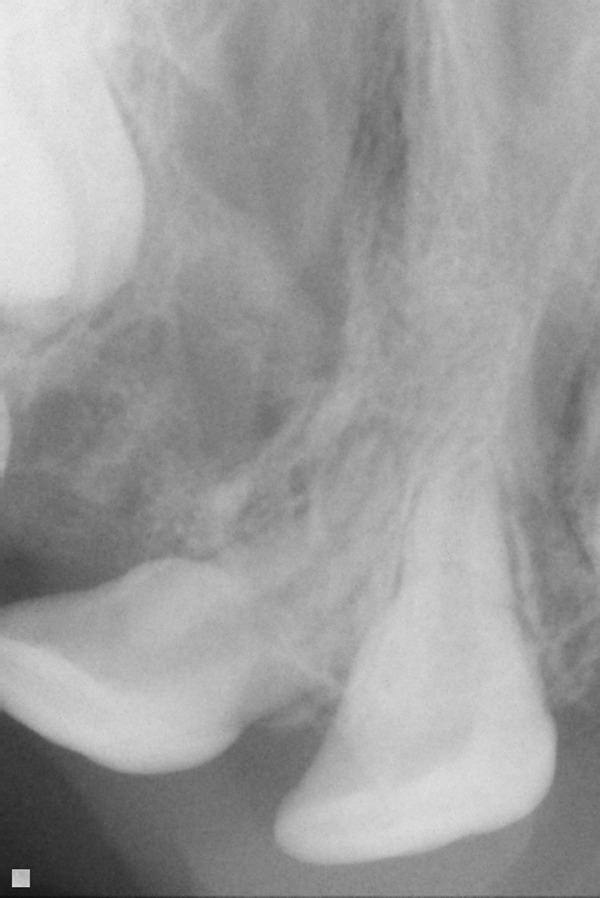
Dental x-ray of teeth erupting into an unoperated cleft.
Historical Perspective
There is no hiding the stigmata of the unrepaired cleft lip. It is likely, for this reason, that the first cleft lip repair dates back to the times of Hippocrates in 400 bc and Galen 150 ad.2 Improvements in technique and outcomes have continued throughout the years. There are also early reports of surgical intervention for cleft palate for similar reasons. In 1764, Le Monnier, a French dentist, repaired a cleft velum.3 Clefting of the alveolus is a less conspicuous component of the cleft continuum; this and the necessity of bone grafting have resulted in a paucity of ancient historical references to alveolar cleft repair. It has been only in the not so distant past that pioneers have attempted and had success with alveolar cleft repair.
The first reports of bone grafting to the alveolus were reported in 1901 by Von Eiselsberg. It is reported that he used a pedicled osteocutaneous flap to reconstruct a palatal defect.1 The first successful bone graft to an alveolar defect was by Drachter in 1914; he utilized a tibial bone graft, including periosteum.4 Veau reportedly was unsuccessful in 1931 in his attempt to graft the alveolar cleft with tibial chips. Millard wrote that there was a relative lack of enthusiasm for alveolar grafting until the 1950s. During the 1950s and 1960s, many surgeons began employing primary bone grafting of the alveolus during the early months of life. The following describes the three-stage operation for primary bone grafting by Johanson and Ake Ohlsson in 1961.5
“The initial operation is performed at the age of three to four weeks without special prior treatment. The nasal floor of the hard palate, in one layer, is closed with a vomerine mucosal flap and anterior to the alveolar process by direct adaptation of the labial soft tissues in two layers. The vomerine flap, which on the oral side is first covered with granulation tissue, has after some few weeks a stable covering of secondary epithelium. At this junction, the orthopaedic correction of the jaw is started and continued up to the age of six months. Special expansion plates are used, fixed to a head cap by means of extra-oral shafts. At the second operation, the components of the upper jaw should be ideally positioned in relation to each other and in correct occlusion. Careful repair of the lip is now combined with transplantation of autografts, chips and marrow, in the cleft in the hard palate and alveolar process. The donor site is tibia…..A continuous orthodontic control is subsequently kept until the permanent bite is fully developed. At the third operation, which is usually performed at one year, the posterior palate is closed….The treatment has been completed in 27 primary and 31 secondary cases. The graft united and a stable homogenized upper jaw was secured in every instance.”5
To assess the utility and value of an operative intervention, one must ask what the particular intervention is trying to accomplish. A relatively early article by Brauer, Cronin, and Reaves in 1962 provides valuable insight into what one group of surgeons and orthodontists were trying to accomplish with “early” alveolar bone grafting. The authors stated that in the unilateral cleft, the “absence of bone and soft tissue” on the cleft side, as well as the pull of the repaired lip on the noncleft side, causes flattening and retrusion of the central face.6 It was their belief that early repair of the unilateral alveolar cleft provided a bridge between the retruded cleft side and the maxillary growth center of the cartilaginous septum that was attached to the noncleft side. This early bridging via bone grafting would allow the retruded cleft-sided maxilla to experience more normal growth and result in less maxillary hypoplasia. With regard to the bilateral deformity, the authors felt that the premaxillary segment being bridged to the adjacent maxillary components helped to stabilize and create more predictable/manageable premaxillary/maxillary relationships.6 This same thought process was echoed by Skoog in 1965 when he stated that the alveolar cleft will require “special measures” to prevent or correct collapse of the maxilla; he included bone grafting as one of these “special measures.”7
This approach of early primary bone grafting continued throughout the 1970s in many parts of the developed world.
However, closer examination and longer-term follow-up by some called into question the practice of primary alveolar bone grafting. As described by Millard in Cleft Craft,3 Kenneth L. Pickrell followed 25 patients that received primary alveolar bone grafting and reported the following:
“Primary rib grafts in the maxilla do not increase in size concomitant with facial growth and development.
Teeth do not migrate and erupt spontaneously through a rib bone graft.
Rib bone grafts do not form a true alveolar process; a permanent alveolar notch remains.
The orthopedic effect of the bone graft decreases as its incorporation increases.”3
These exact observations were not echoed by all of the opponents of primary bone grafting, but there were other negative observations regarding primary alveolar bone grafting.
Rehrmann of Germany reported in 1969 that his observations over a 10-year period comparing primary to secondary bone grafting supported the relatively new approach of delaying alveolar bone grafting. He found that permanent stabilization of the maxillary segments did not result from early bone grafting of the alveolar process. The frontal ends of the alveolar processes bridged by the bone graft had their development restricted. He found that the grafted bone bridge shortened over time and resulted in maxillary growth restriction and malocclusion.3 In addition to these detractors of early primary bone grafting, there was a growing contingent that was finding that delayed secondary bone grafting had intrinsic positive value.3 Two of the benefits of secondary bone grafting are bony support for tooth eruption and stabilization of the maxilla.8 Secondary alveolar bone grafting is usually performed during the period of mixed dentition, which is between the ages of 7 and 12 years. Eruption of the lateral incisor and canine tooth occurs approximately at the ages of 7 and 11 years, respectively. Bone grafting prior to eruption of the canine allows the tooth to erupt into solid bone and provides enhanced stabilization of the maxilla. Transverse maxillary growth is also nearly complete by this age, so that there is no inhibition of maxillary growth in this dimension.8
It is not accepted practice in developed countries to forego bone grafting altogether. In Losee and Kirschner's thorough text,9 contributors describe outcomes at an institution where alveolar bone grafting was not practiced. They found that most patients still had noticeable stigmata of the cleft due to the flat and asymmetric support of the lip. Residual oronasal fistulas persisted in most of these patients, even after multiple attempts at soft tissue closure. Many of the patients suffered from periodontal disease due to chronic inflammation from oral nasal reflux through the fistulas. As a result, many of these patients suffered from lost dentition and required bridge work.9 This account underscores the physiologic importance and long-term benefits of alveolar bone reconstruction.
Techniques
A review of the literature regarding techniques for alveolar bone grafting reveals that there is no consensus on the correct method to reconstruct the alveolus. In addition, there are still some that feel there are indications for primary bone grafting. In many studies, the emphasis is not necessarily on what is done during the surgery, but often on what is not to be done. Some proponents of primary alveolar bone grafting have argued that because of a lack of standardized techniques, it was the overly aggressive technique and not the timing that led to the failure of some primary alveolar bone grafting to prevent midface growth restriction. Historical uses of primary alveolar bone grafting have run the gamut from wedging rib graft into the cleft to transplanting resected segments of vomer into the alveolar cleft all the way to wide dissection along the alveolus, vomer, and hard palate for tibial graft placement.10,11,12,13 van Aalst et al13 in 2005 describe a technique for primary alveolar bone grafting that requires minimal dissection so as not to disrupt the vomeromaxillary suture and thus purports to avoid midface hypoplasia. This technique requires that patients with complete unilateral and bilateral cleft lip and palate be fitted with a palatal obturator shortly after they are born, which results in alignment of the greater and lesser maxillary segments. The cleft lip repair that is performed at 10 weeks also acts to help align the maxillary segments. Not all patients are candidates for primary alveolar bone grafting in this model; after the obturator, if the maxillary segments are not abutting or up to 1 mm apart, then primary alveolar bone grafting is thought to be of less benefit. In patients that meet criteria, rib graft is placed into the cleft at between 7 and 9 months of age. The fifth through seventh ribs may be utilized. Unilateral clefts require a 2.5-cm segment of rib and bilateral clefts require a 3.5-cm segment of rib. The authors describe a trapezoidal flap elevation of mucosa to create a pocket into which the graft is placed. There is no dissection extending on the palate or piriform aperture. The authors found that their patients did not have midface growth attenuation.13 It is interesting to note that in cases where the mucosa of the maxillary segments is abutting, a gingivoperiosteoplasty is sometimes performed without bone grafting. This suggests/reinforces that there may be limited utility of this technique and primary bone grafting for most alveolar clefts.
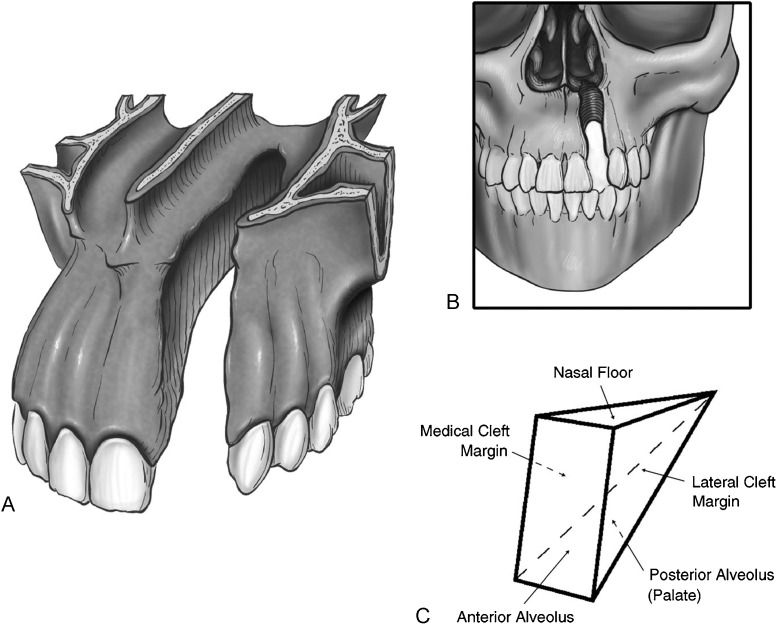
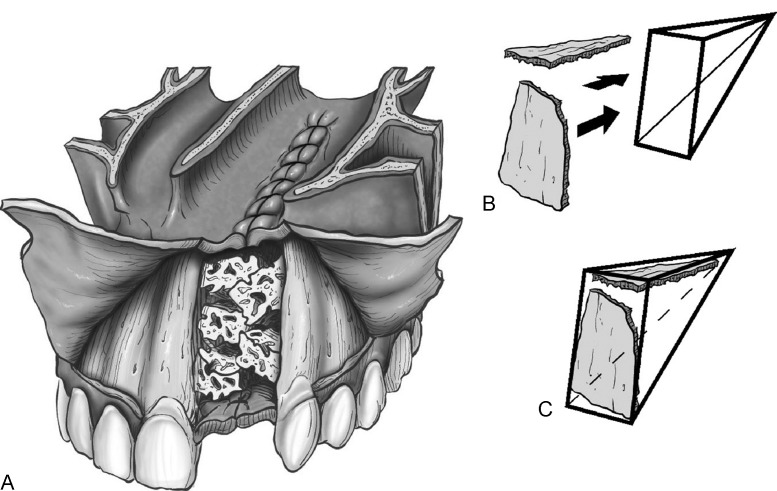
There are many different techniques described for delayed alveolar bone grafting. An elegant, straightforward approach was recently described by Craven et al.14 Conceptualization of the defect in three dimensions is essential to adequately reconstruct the alveolar cleft with a bone graft according to this technique. The surgeon must take into account the need to reconstruct all of the surfaces of the anatomic subunits of the alveolar cleft, which includes the nasal floor, medial cleft margin, lateral cleft margin, anterior alveolus, and the posterior alveolus (palate). If there are any concerns about soft tissue integrity (e.g., oronasal fistula), staged/delayed bone grafting should take place after the soft tissue defect has been reconstructed. This technique requires that labial gingivoperiosteal flaps be elevated mesial and distal to the cleft. The incision is near the teeth adjacent to the cleft and fans out over the more lateral and mesial teeth to leave a 2- to 3-mm gingival cuff. The lateral flap extends to the mesial aspect of the first molar. Immediately beyond the contralateral incisor is the limit of the incision extending toward the midline. Gingivoperiosteal flaps are elevated to close the palatal defect, while the nasal floor is closed with mucoperiosteal flaps elevated off the pyriform aperture. These flaps are sutured for closure of the alveolar cleft. If this maneuver creates an apparently stable soft tissue envelope, then bone grafting can proceed. If the soft tissue integrity does not appear to be adequate, then the soft tissue is completely closed and bone grafting is delayed for 3 to 4 months.14 The authors emphasize an important technical point that involves the type and position of certain types of bone that are placed in the alveolar cleft. The article features an illustration that depicts the placement of the cortical bone on the sides of the cleft, especially the anterior side. The cancellous bone is then placed on the interior part of the defect. The purpose of this juxtaposition is that the more-dense cortical bone acts to resist the contractile nature of the overlying soft tissue as it heals. By resisting these contractile forces, the bone graft has a better chance to maintain its volume and potentially reduces the need for an additional graft (see Figs. 3, 4).
Fig. 3.
(A–C) The alveolar and hard palate cleft should be viewed as a three-dimensional defect resembling a triangle or pyramid. (Reprinted with permission from Craven C, Cole P, Hollier L Jr, Stal S. Ensuring success in alveolar bone grafting: a three-dimensional approach. J Craniofac Surg 2007;18(4):855–859)
Fig. 4.
(A) The alveolar cleft after packing with cortical and cancellous bone. (B,C) Cortical bone reinforcing the roof of the cleft (nasal floor) and the anterior wall of the alveolus. (Reprinted with permission from Craven C, Cole P, Hollier L Jr, Stal S. Ensuring success in alveolar bone grafting: a three-dimensional approach. J Craniofac Surg 2007;18(4):855–859)
Graft Sources
There are several different graft sources for alveolar bone grafting; both cortical and cancellous bone is utilized. Cortical bone takes a longer time to incorporate because it relies on vascular ingrowth via a process called creeping substitution. Cancellous grafts consist of actual cells and the incorporation process is much faster due to osteoinduction and osteoconduction. The cancellous grafts are also found to better enable tooth eruption.8
The iliac crest is one of the most popular alveolar bone graft sources. The cancellous portion is the most commonly used part of this bone, although the cortical portion is sometimes used in conjunction as a buttress or stent to resist soft tissue contractile forces. Some of the drawbacks cited include donor site scarring, postoperative pain resulting in delayed ambulation, and the potential for cutaneous nerve injury.8
Studies have shown that membranous bone has a higher graft survival rate compared with endochondral bone.15 The flat bones of the craniofacial skeleton are made up of membranous bone, whereas the bones of the trunk and extremities are made up endochondral bone. Cranial bone is the alveolar bone graft of choice for some. They cite its hidden scar, limited postoperative pain, ease of harvest, and the intrinsic advantages of membranous bone. Detractors cite longer operative time and the potential for more serious complications (e.g., cerebrospinal fluid leak, dural tear, hemorrhage, epidural hematoma).8
Another source of membranous bone that has been utilized in alveolar cleft grafting is bone from the mandibular symphysis; favorable outcomes have been reported with this source. Other reported advantages include use of the same operative field, no visible scar, and decreased postoperative pain.16
Tibia has been used by some as a source of cancellous bone; trephining is commonly used to harvest this graft. Studies have shown that this technique results in less postoperative pain and shorter hospital stays when compared with alveolar bone grafting.17
Rib has also been utilized to close the alveolar cleft; however, it is considered to be of limited use by many due to its donor site morbidities, including visible scarring and pain.8 Rib grafts have also been criticized for difficulties in orthodontic tooth movement.18
The Future
Autologous bone is not the perfect source of material for alveolar cleft reconstruction. Because of this, there are many ongoing efforts to find an alternative source for graft material. One of these sources is bone morphogenic protein (BMP) delivered on a collagen sponge. Reports have shown some success with the application of this product.19 It is important to note that this product has substantial cost and for some its availability is limited. It should also be known that alveolar bone grafting is considered a Food and Drug Administration “off label” use of this product and has not been approved for this application.
Conclusion
Treatment of the alveolar cleft should not be an afterthought in the care of cleft lip and palate patients. It has functional and aesthetic significance and reconstruction potentially carries a significant benefit to the patient. Most believe that timing of repair is an important consideration. Successful reconstruction relies on several factors. The team approach to care relies on collaboration with other specialists (e.g., pediatric dentists, orthodontists, oral surgeons, and speech pathologists) and provides optimal care. The choice of graft source must take into account the cost of donor site morbidity as well as the intrinsic characteristics of the type of bone selected. New technologies, such as BMP, are being utilized in hopes of finding an alternative to autologous bone graft harvesting. The ideal substitute has not been discovered yet, but the investigative search continues.
References
- 1.van Aalst J A, Kolappa K K, Sadove M. MOC-PSSM CME article: Nonsyndromic cleft palate. Plast Reconstr Surg. 2008;121(1, Suppl):1–14. doi: 10.1097/01.prs.0000294706.05898.f3. [DOI] [PubMed] [Google Scholar]
- 2.Meara J G, Andrews B T, Ridgway E B, Raisolsadat M A, Hiradfar M. Unilateral cleft lip and nasal repair: techniques and principles. Iran J Pediatr. 2011;21:129–138. [PMC free article] [PubMed] [Google Scholar]
- 3.Millard R. Boston: Little, Brown; 1980. Cleft Craft. Vol. 3; pp. 299–301. [Google Scholar]
- 4.Eiselsberg A. Zur technik der uranoplastik. Arch Klin Chir. 1901;64:509–529. [Google Scholar]
- 5.Lilja J. Alveolar bone grafting. Indian J Plast Surg. 2009;42(Suppl):S110–S115. doi: 10.4103/0970-0358.57200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brauer R O, Cronin T D, Reaves E L. Early maxillary orthopedics, orthodontia and alveolar bone grafting in complete clefts of the palate. Plast Reconstr Surg Transplant Bull. 1962;29(6):625–641. doi: 10.1097/00006534-196206000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Skoog T. The management of the bilateral cleft of the primary palate (lip and alveolus) I. General considerations and soft tissue repair. Plast Reconstr Surg. 1965;35(1):34–44. doi: 10.1097/00006534-196501000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj A K, Wongworawat A A, Punjabi A. Management of alveolar clefts. J Craniofac Surg. 2003;14(6):840–846. doi: 10.1097/00001665-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Turvey T, Ruiz R, Tiwana P. New York: McGraw Hill; 2008. Bone graft construction of the cleft maxilla and palate; pp. 836–865. [Google Scholar]
- 10.Rosenstein S W, Jacobson B N, Monroe C W, Griffith B H, McKinney P. A series of cleft lip and palate children five years after undergoing orthopedic and bone grafting procedures. Angle Orthod. 1972;42(1):1–8. doi: 10.1043/0003-3219(1972)042<0001:ASOCLA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Rosenstein S W, Monroe C W, Kernahan D A, Jacobson B N, Griffith B H, Bauer B S. The case of early bone grafting in cleft lip and cleft palate. Plast Reconstr Surg. 1982;70(3):297–309. doi: 10.1097/00006534-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstein S W Dado D V Kernahan D Griffith B H Grasseschi M The case for early bone grafting in cleft lip and palate: a second report Plast Reconstr Surg 1991874644–654., discussion 655–656 [PubMed] [Google Scholar]
- 13.van Aalst J A, Eppley B L, Hathaway R R, Sadove A M. Surgical technique for primary alveolar bone grafting. J Craniofac Surg. 2005;16(4):706–711. doi: 10.1097/01.scs.0000157311.85161.e8. [DOI] [PubMed] [Google Scholar]
- 14.Craven C, Cole P, Hollier L Jr, Stal S. Ensuring success in alveolar bone grafting: a three-dimensional approach. J Craniofac Surg. 2007;18(4):855–859. doi: 10.1097/scs.0b013e31806849fa. [DOI] [PubMed] [Google Scholar]
- 15.Zins J E, Whitaker L A. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72(6):778–785. doi: 10.1097/00006534-198312000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Ilankovan V, Stronczek M, Telfer M, Peterson L J, Stassen L F, Ward-Booth P. A prospective study of trephined bone grafts of the tibial shaft and iliac crest. Br J Oral Maxillofac Surg. 1998;36(6):434–439. doi: 10.1016/s0266-4356(98)90459-4. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg B, Padwa B L, Boyne P, Kaban L. State of the art in oral and maxillofacial surgery: treatment of maxillary hypoplasia and anterior palatal and alveolar clefts. Cleft Palate Craniofac J. 1999;36(4):283–291. doi: 10.1597/1545-1569_1999_036_0284_sotaio_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 18.Kuijpers-Jagtman A M, Stoelinga P J. Letters to the editor. Cleft Palate Craniofacial Online Journal. 2000;37:421. doi: 10.1597/1545-1569_2000_037_0421_ltte_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 19.Fallucco M A, Carstens M H. Primary reconstruction of alveolar clefts using recombinant human bone morphogenic protein-2: clinical and radiographic outcomes. J Craniofac Surg. 2009;20 02:1759–1764. doi: 10.1097/SCS.0b013e3181b5d08e. [DOI] [PubMed] [Google Scholar]