Abstract
Mammalia are so named based on the presence of the mammary gland in the breast. The mammary gland is an epidermal appendage, derived from the apocrine glands. The human breast consists of the parenchyma and stroma, originating from ectodermal and mesodermal elements, respectively. Development of the human breast is distinctive for several reasons. The human breast houses the mammary gland that produces and delivers milk through development of an extensive tree-like network of branched ducts. It is also characterized by cellular plasticity, with extensive remodeling in adulthood, a factor that increases its susceptibility to carcinogenesis. Also, breast development occurs in distinct stages via complex epithelial–mesenchymal interactions, orchestrated by signaling pathways under the regulation of systemic hormones. Congenital and acquired disorders of the breast often have a basis in development, making its study essential to understanding breast pathology.
Keywords: breast embryology, mammary gland development, Tanner staging
Development of the Human Breast
The human breast consists of parenchymal and stromal elements. The parenchyma forms a system of branching ducts eventually leading to secretory acini development and the stroma consists mainly of adipose tissue, providing the environment for development of the parenchyma.1,2,3 These building blocks of the breast are recognized as early as the embryonic stage of human development. The process of development of the ductal system and acini is termed branching morphogenesis and although it commences in the fetus, it halts in early childhood until puberty when hormonal stimulation triggers further differentiation.4 Under the influence of hormones, complex reciprocal interactions between the epithelium and mesenchyme lead to differentiation of the prenatally developed rudimentary structure to form a mature mammary gland.5 Although the precise mechanisms are still unclear, our understanding of branching in the mammary gland is increasing.
Prenatal Development
Prenatal breast development can be classified into two main processes; formation of a primary mammary bud and development of a rudimentary mammary gland.6 The earliest stages of embryogenesis are largely hormone independent4,7; hormones and regulatory factors are important for development in the second trimester.8
Most knowledge of morphological changes in the fetal breast comes from studies on rodents.9,10,11,12 Of note, prenatal human breast development does not differ between genders. The successive, distinct stages of intrauterine breast development described below correlate loosely with gestational age and significant variations at similar stages can be seen.13,14,15
First Trimester
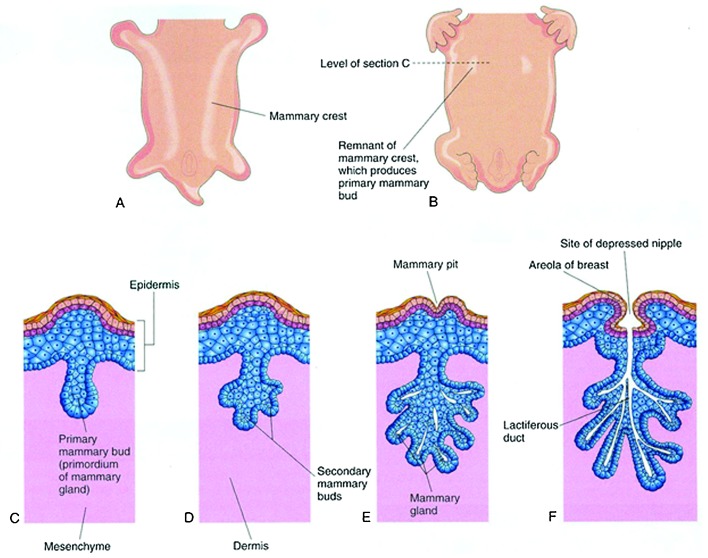
As early as 4 to 6 weeks of gestation, mammary-specific progenitor cells may be seen.1 Around day 35 of gestation, proliferation of paired areas of epithelial cells in the epidermis of the thoracic region occurs. These discrete areas of proliferation extend in a line between the fetal axilla and inguinal region and form two ridges called the mammary crests or milk lines (Fig. 1).
Fig. 1.
Development of the mammary gland. (A) Ventral view of an embryo at 28-days gestation showing mammary crests. (B) Similar view at 6-week gestation showing the remains of the mammary crests. (C) Transverse section of a mammary crest at the site of the developing mammary gland. (D–F) Similar sections showing successive stages of breast development between the 12th week of gestation and birth. (Reprinted with permission from Moore KL, Persaud TVN, Torchia MG, The Developing Human: Clinically Oriented Embryology. 9th ed. 2013 Copyright Elsevier).
Most of the mammary crest atrophies except for paired solid epithelial masses in the pectoral region at the fourth intercostal space, which form the primary mammary buds.6,16 Supernumerary nipples (polythelia) occur in 2 to 5% of humans in a position from the groin to the axilla, supporting the existence of the mammary crest or ridge.13,17,18 These supernumerary nipples can appear similar to pigmented macules or fully developed nipple and areola complexes.19,20 These are rarely functioning but can occasionally be a cosmetic issue.
Toward the end of the first trimester21 the primary mammary buds begin to grow downwards into the underlying mesenchyme, under an inductive influence of regulatory factors secreted by the mesenchyme.10,12,13 Next, the primary mammary bud enlarges14 and moves from a more dorsal to ventral position.6 Indentations along its basolateral margin appear, becoming sites for the future secondary mammary outgrowths.14 This core of cells continues to evaginate into the underlying stroma and becomes surrounded by a more cellular zone of fibroblast like cells within a collagenous mesenchyme.
At the end of the first trimester of pregnancy, a well-defined mammary bud penetrating into the upper dermis can be observed.3 Two distinct populations of epithelial cells (central and basal) can be identified.14 Concomitantly, the mesenchymal cells differentiate to form fibroblasts, smooth muscle cells, capillary endothelial cells, and adipocytes.
Second Trimester
Secondary epithelial buds appear from the indentations on the main mammary bud.3,13,14 Each secondary epithelial bud grows vertically into the mesenchyme surrounding the primary bud and has a slender stalk and bulbous end.14 The secondary epithelial sprouts canalize and coalesce forming secondary buds that give rise to lactiferous ducts (Fig. 1).13 The epithelial cells lining the lactiferous ducts are arranged in two layers, with the layer adjacent to the lumen gaining secretory function while the basal layer differentiates into myoepithelial cells.3
By 6 months of gestational age, the basic framework of the gland is established. A well-defined tubular architecture in a bed of dense fibroconnective tissue stroma is noted at this stage.4 This is around the time breast tissue in both boys and girls can be apparent.22
Third Trimester
Repeated branching of the secondary epithelial buds and canalization occur in the third trimester.3,6,14,23 Disagreement exists over the final morphology of the breast at birth. Although most sources agree these secondary processes end in rudimentary lobular structures or end buds,3,6,23 some argue that the breast at birth does not contain any evidence of lobules, only ductal structures with surrounding stroma.24
The epidermis in the region of the future nipple becomes depressed, forming the mammary pit during the third trimester (Fig. 1). 25 The lactiferous ducts drain into retroareolar ampullae that converge into this pit on the overlying skin.13 The nipple is further delineated by proliferation of the mesoderm stimulated by the invagination of ectoderm in this region. The nipple is created with smooth muscle fibers aligned in a circular and longitudinal fashion.13 The surrounding areola is formed by the ectoderm during the fifth month of gestation.
During the final weeks of gestation, the loose fibroconnective tissue stroma increases in vascularity. Due to a complex interplay between fetal, placental, and maternal hormones that has not yet been elucidated,14 limited secretory activity in the late-term fetus and newborn infant may occur.4 The failure of preterm infants to develop breast nodules or secrete milk after birth indicates that the intrauterine environment is essential for breast development.20,26 Preterm infants do not develop breast nodules or secrete milk after birth, further lending evidence to the fact that the intrauterine environment is essential for breast development.
At term, approximately 15 to 20 lobes of glandular tissue have formed, each containing a lactiferous duct that opens onto the breast surface through the mammary pit. Both the surrounding skin and the fibrous suspensory ligaments of Cooper that anchor the breast to the pectoralis major fascia provide support to the breast.
Infant Breast
The first 2 years of life are a critical period for some aspects of breast maturation as well as involution.24,27 The normal gland remains quiescent from 2 years of age to puberty.24,28 At birth, the breast is usually palpable in the newborn with varying amounts of tissue and no significant difference between the genders.29 Falling levels of maternal estrogens in the neonate stimulate the neonatal pituitary gland to produce prolactin, which results in unilateral or bilateral breast enlargement and/or transient secretion of milk in as many as 70% of term neonates.13,26,27 It has been speculated that the infant breast undergoes stimulation at approximately 3 to 4 months postnatally through a surge of the infant's own reproductive hormones, including estradiol.30 Breast tissue in female infants persists longer than in male infants29,30 due to higher estradiol levels in infancy in girls.30
Soon after birth, the nipples become everted from proliferation of the underlying mesoderm,13 and the areolae increase in pigmentation. Development of erectile tissue in the nipple areolar complex increases response of the nipple to stimulation. Nipples that remain inverted until puberty are not uncommon. An increase in vascularity of the gland stroma soon after birth causes a visible difference between the light periductal connective tissue and the denser supporting stroma.31
The most well-accepted morphological and functional maturation stages from birth to 2 years have been described by Anzbagahan et al.27 The morphological changes of the breast are depicted by the degree of glandular differentiation (branching and formation of acini) and functional maturation is characterized by the secretory capacity of the lining epithelium (Table 1).13,27
Table 1. Morphological and functional changes in the infant breast.
| Morphological type I | Branching ductal system with no or less than two dichotomous branchings |
| Morphological type II | Branching ductal system with more than two dichotomous branchings, but no terminal lobular units |
| Morphological type III | Branching ductal system with number of branchings and well developed lobular system |
| Table 1 | Summary of functional changes |
| Functional type I | All ducts and ductules are lined by secretory type of epithelium |
| Functional type II | Mixture of ducts lined by secretory and apocrine type epithelium |
| Functional type III | Almost all ducts lined by apocrine type of epithelium |
| Functional type IV | Mixture of ducts lined by apocrine type of epithelium and involuting ducts lined by multilayered epithelium |
Source: Reprinted with permission from Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia 2000;5(2):119–137. 2000 Copyright Springer.
The morphological changes begin in the immediate postnatal period and do not follow a linear progression.13 In fact, three different morphological types (I–III) can occur. The functional changes from birth to 2 years follow a more linear progression than do the morphological changes.13,27 There is a period of apocrine metaplasia (functional stage II–III) prior to involution (functional stage IV).
Many combinations of morphological type and functional stage can occur due to the wide variations in infant breast development.13 By 2 years of age, small ductal structures in a fibroblastic stroma are all that remain and the infant breast is relatively quiescent. The time taken for the glands to regress to this stage of quiescence varies.29
Development of the Mammary Gland at Puberty
Sexually dimorphic development of the breast first begins at puberty and unlike the preceding stages of development, pubertal changes are heavily under the influence of sex hormones, in particular estrogen.32 Whereas the gross anatomic changes that occur at puberty are well described,33 events on an ultrastructural level are less well defined.34
Pubertal Female Breast Development
Gross Anatomic Changes
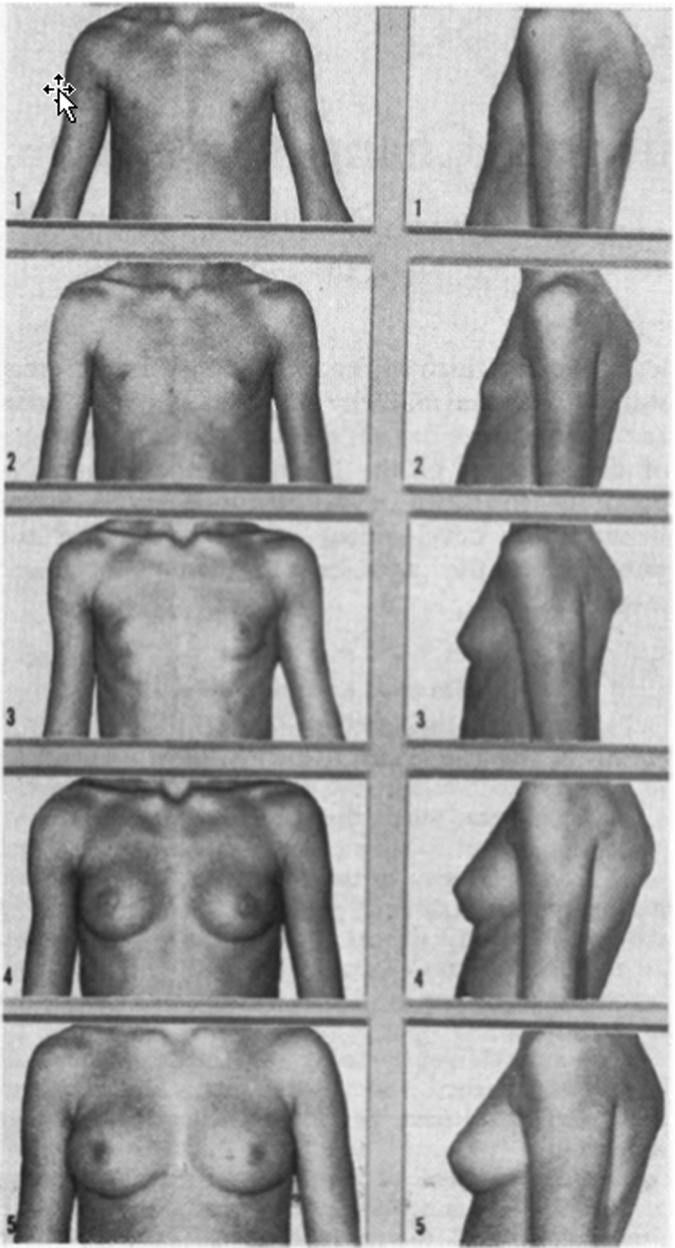
Tanner described the most well-accepted macroscopic stages of development in the breast at puberty (Fig. 2). 35 These gross anatomic changes begin with stage 1, the preadolescent phase with only elevation of the papilla. At this point, there is no additional development of the stroma or parenchyma beyond what has occurred in infancy. Breast development is generally the first secondary sexual characteristic to develop, preceding pubic hair development by about 6 months.19,36 Although the pubertal surge of estrogen is the immediate stimulus to mammary development, the action of estrogen depends upon the presence of pituitary growth hormone and the ability of growth hormone to stimulate production of insulin-like growth factor-1 (IGF-I) in the mammary gland.37 The age range in which this can occur is 8½ to 13½ years. No breast development by 14 years of age in girls should prompt further investigation.20
Fig. 2.
Tanner stages of breast development. (Reprinted with permission from Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. Copyright BMJ Publishing).
Tanner stage 2 involves formation of the breast bud with elevation of the nipple as well as a small mound of breast tissue along with enlargement of the diameter of the areola.35 The average age of girls at this stage is 11 years in a British cohort and has been shown to occur 6 months earlier in the United States.38,39 There is recent literature pointing toward an earlier age at onset of breast development in girls in the United States (average age 9.8 vs. 10.8 years over approximately a decade).38,40 The normal range of thelarche is from 8½ to 13 years.35
Significant variations in breast development occur in individuals at the same age based on level of pubertal maturation, ethnicity,36 and hormonal concentrations. Clinically, Tanner stage 2 of breast development correlates with the entity of thelarche. Tanner stage 3, attained at an average age of 12.5 years, is characterized by further enlargement of the breast and areola. No separation of the contours is noted at this time.35,36 Between Tanner stages 2 and 3, discrepancy in size between the breasts of a pubertal girl is commonly seen and tends to become less noticeable by Tanner stage 4 and 5.20 If marked breast asymmetry is persistent, reconstructive surgery may be a consideration, generally when Tanner 5 breast maturity is reached.19,20,35 Marked discrepancy between breast size in puberty, particularly if persistent, is presumed to be due to poor mammary bud development in the smaller breast.20
During Tanner stage 4, at the average age of 13 to 14 years, there is enlargement of the nipple and areola, leading to the formation of a secondary mound on the breast. Menarche tends to occur between Tanner stage 3 and Tanner stage 4.38 Some girls may progress from Tanner stage 3 to 5 without a transitory stage 4.20
Tanner stage 5 is characterized by the recession of the areola on to the breast with resulting loss of the separation of contours. This stage is attained by an average age of 15 years.35
The average time spent between Tanner stages 2 and 5 is 4 to 4½ years.13,36,38 There are inherent variations in this estimate and the duration of time spent progressing through Tanner stages of breast development can range from 1.5 to 6 years. Also, the breast bud stage can persist from 6 months to 2 years before advancing to Tanner stage 3.35
After or during these stages of development, breast shrinkage may occur if there is weight loss due to decrease in adipose tissue.19,20 This is particularly relevant in the pubertal girl when eating disorders such as anorexia nervosa are most commonly encountered. The loss of fatty tissue gives a wrinkled appearance to the skin of the breast, leading Capraro and Dewhurst to coin the term “instant senility” to describe this phenomenon in adolescents.41 Unilateral or bilateral pathologic enlargement of the breast at puberty is termed juvenile hypertrophy, and it is histologically similar to gynecomastia in males and distinct from lactational hypertrophy.19
Significant development of the nipple also occurs during puberty.42 The most marked increase in size and diameter of the nipple is seen between Tanner stages 3 and 5, particularly soon after menarche.43 The average increase in diameter between Tanner stages 1 to 5 is 5 to 6 mm.43 It is difficult to form measurable criteria of nipple diameter at each Tanner stage due to extensive variations found in increments of nipple size based on hormonal status, race, nutrition, and genetics.44
Cellular Changes
Underlying the extensive tissue remodeling that occurs at puberty is a mammary cell hierarchy composed of multipotent stem and lineage-restricted progenitor cells.45,46,47,48 At the cellular level, both stromal and parenchymal changes are occurring during pubertal development,13 but increase in fibrous and fatty tissue of the stroma precedes further ductal changes. Following a period of stromal changes, ductal elongation and dichotomous branching occurs, with both these events being under the influence of estrogen.13,49
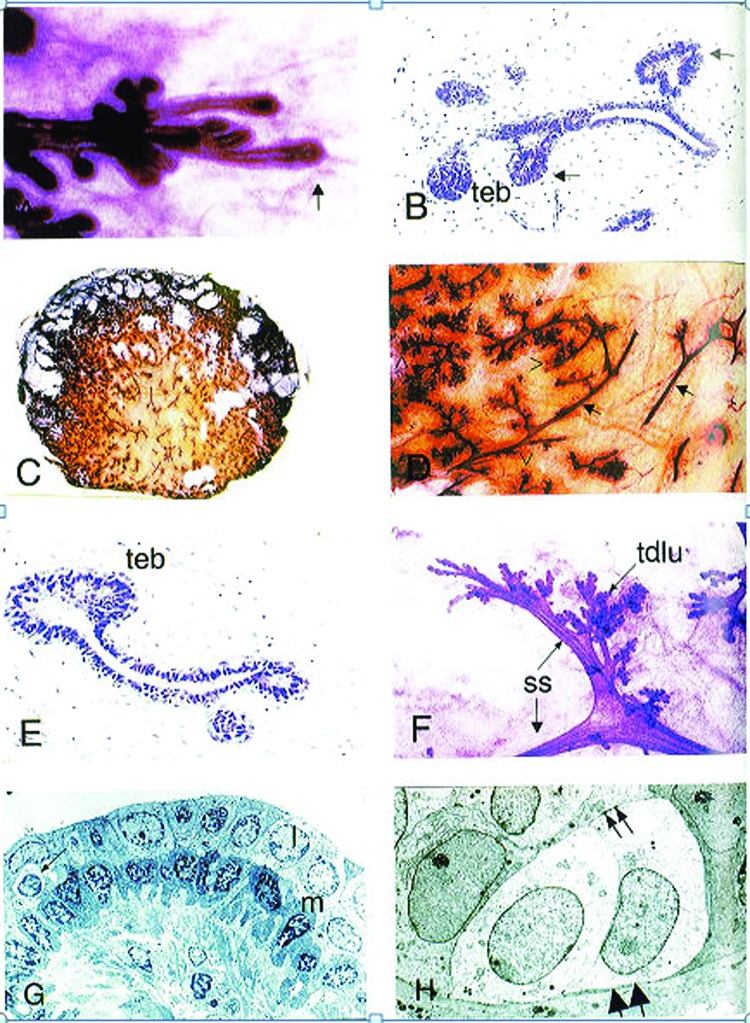
During puberty, the epithelium forms into a branching, bilayered ductal structure, consisting of an outer basal myoepithelial layer of cells and an inner luminal cell layer that can be divided further into ductal luminal cells, lining the inside of the ducts, and alveolar luminal cells, which secrete milk during lactation (Fig. 3). 5 More alveoli are laid during each menstrual cycle, but the degree of alveolar expansion is only significant once pregnancy occurs.34,49
Fig. 3.
Pubertal breast development. (A) Carmine-stained whole-mount preparation of the advancing edge (arrow) of the parenchyma from a 13-year-old girl. (B) Hematoxylin- and eosin-stained developing breast of 13-year-old girl showing solid end bud-like structures (denoted teb) and lateral buds (arrows). (C) Coronal section of breast of 15-year-old girl. (D) Higher power view of panel C, arrows indicate ducts and unfilled arrowheads indicate duct termini. (E) Histology section of the peripheral region of parenchyma seen in (C). teb denotes terminal end bud. (F) Carmine-stained whole mount preparation of breast from 18-year-old nulliparous woman. A segmental duct divides into two subsegmental ducts (ss), which then lead to the terminal duct lobular units (tdlu). (G) Electron micrograph of a normal adult subsegmental duct. The bilayered histology with paler luminal cells (l), darker basal (myoepithelial) cells (m) is evident. An intraepithelial lymphocyte (arrow) is also seen. (H) Electron micrograph of a terminal duct lobular unit showing two basal clear cells. These have microfilaments in the basal part of the cell (large arrows) and desmosome attachments with the luminal cells (small arrows). (Reprinted with permission from Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia 2000;5(2):119–137. 2000 Copyright Springer).
Ductal elongation and complex branching originates at the site of the terminal end bud, specifically at the site of the mammary stem cells in the cap cell layer of the terminal end bud.13,28,47,48 The primary ducts that reach the nipple form a complex of subsidiary ducts. The primary ducts branch into segmental and subsegmental ducts.5,13,34 The subsegmental ducts lead to terminal duct formation, which further subdivides to form several terminal ductules or acini.13,38 A collection of acini arising from one terminal duct along with the surrounding intralobular stroma is termed a terminal duct lobular unit (TDLU), which is the functional unit of the breast.13
As ductal elongation continues, the remainder of the space in the breast is taken up by adipose tissue, along with a mixture of blood vessels, immune cells, and fibroblasts.50 Estrogen and progesterone are thought to be responsible for ductal elongation and side branching, respectively.51
As for lobular development, four types of lobules, from 1 to 4, are well recognized in the human female breast.13 Lobule type 1 consists of a short terminal duct ending in a cluster of secretory cells called alveoli. Lobule types 2, 3, and 4 consist of a terminal duct branching into several ductules and an increasing number of alveoli.13 Lobule type 4 is attained in adult women having gone through pregnancy and lactation.52,53 The adult nulliparous breast is complete in ductal and stromal maturation by 18 to 20 years of age and the lobules it contains are mainly type 1. The mammary glands remain in this mature, but inactive state until pregnancy, which brings about the next major change in the hormonal environment.2
Pubertal Male Breast Development
At puberty, no further development of the breast occurs in the male due to rising testosterone concentrations. During puberty, up to 40% of boys may develop transient gynecomastia, presumably due to relative estrogen dominance.19 Gynecomastia is secondary to ductal and stromal, but not lobular hypertrophy.13 Although this is transient in most cases, gynecomastia can be a distressing physical anomaly for a young male. Rarely, pubertal gynecomastia may persist and this appears to be due to either end-organ idiosyncrasy or a particularly abnormal estrogen–androgen ratio at the onset of puberty.
Boys also undergo nipple diameter increase during puberty. Until pubic hair stage 3, boys and girls do not differ in nipple diameter.54 After this, marked enlargement of the female nipple occurs. Boys with gynecomastia have larger nipple size than boys who have none.54
Regulation of Breast Development
Mutual and reciprocal interactions between epithelial components and mesenchymal or stromal cells are responsible for prenatal, infant, and pubertal breast development.10,12,55,56 Evidence suggests the mesenchyme has inductive properties that lead to the local migration and changes in cell adhesion of epithelial cells. Hormonal influences on this paracrine interaction between the mesenchyme and parenchyma exist at all stages of development. The formation of lactiferous ducts is induced by placental hormones entering the fetal circulation. Other hormones implicated, but not completely elucidated in prenatal and pubertal breast development are progesterone, growth hormone, insulin-like growth factors, estrogen, prolactin, adrenal corticoids, and triiodothyronine.57,58,59 Epidermal growth factor receptors, ubiquitously expressed in prenatal breast, were shown to be significant in mammogenesis in rodent studies.60,61 Some regulators, such as ErbB2, seem to influence both ductal morphology and branching.62 Much attention has also been focused on bcl-2, which is an inhibitor of apoptosis in the fetal and infant breast.24
The mammary stem cells and progenitors do not express receptors for hormones and hormone receptor-positive cells generally do not proliferate.47 This is why hormones elicit morphological changes by acting on a complex regulatory network of paracrine signals and transcription factors to modulate the activity of mammary stem cells.63,64
Conclusion
The development of the human breast is distinctive due to the extensive remodeling it undergoes into adulthood. This occurs in distinct stages under the influence of several hormonal signals. Study of human breast development is essential to understanding pathology, in particular congenital and acquired disorders that often have a basis in development.
References
- 1.Medina D. The mammary gland: a unique organ for the study of development and tumorigenesis. J Mammary Gland Biol Neoplasia. 1996;1(1):5–19. doi: 10.1007/BF02096299. [DOI] [PubMed] [Google Scholar]
- 2.Forsyth I A. The mammary gland. Baillieres Clin Endocrinol Metab. 1991;5(4):809–832. doi: 10.1016/s0950-351x(10)80016-3. [DOI] [PubMed] [Google Scholar]
- 3.Tobon H, Salazar H. Ultrastructure of the human mammary gland. I. Development of the fetal gland throughout gestation. J Clin Endocrinol Metab. 1974;39(3):443–456. doi: 10.1210/jcem-39-3-443. [DOI] [PubMed] [Google Scholar]
- 4.Sternlicht M D. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8(1):201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiede B, Kang Y. From milk to malignancy: the role of mammary stem cells in development, pregnancy and breast cancer. Cell Res. 2011;21(2):245–257. doi: 10.1038/cr.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes E SR. The development of the mammary gland. Ann R Coll Surg Eng. 1949;6:99–119. [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson G W, Karpf A B, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4(1):9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- 8.Turashvili G BJ, Bouchal J, Burkadze G, Kolar Z. Mammary gland development and cancer. Cesk Patol. 2005;41(3):94–101. [PubMed] [Google Scholar]
- 9.Hens J R, Wysolmerski J J. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005;7(5):220–224. doi: 10.1186/bcr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson G W, Karpf A B, Kratochwil K. Regulation of mammary gland development by tissue interaction. J Mammary Gland Biol Neoplasia. 1999;4(1):9–19. doi: 10.1023/a:1018748418447. [DOI] [PubMed] [Google Scholar]
- 11.Plante I SM. L.D., Evaluation of mammary gland development and function in mouse models. J Vis Exp. 2011;21:2828. doi: 10.3791/2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakakura T. New York: Plenum Press; 1987. Mammary embryogenesis; pp. 37–66. [Google Scholar]
- 13.Howard B A, Gusterson B A. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5(2):119–137. doi: 10.1023/a:1026487120779. [DOI] [PubMed] [Google Scholar]
- 14.Jolicoeur F. Intrauterine breast development and the mammary myoepithelial lineage. J Mammary Gland Biol Neoplasia. 2005;10(3):199–210. doi: 10.1007/s10911-005-9581-9. [DOI] [PubMed] [Google Scholar]
- 15.Osin P P, Anbazhagan R, Bartkova J, Nathan B, Gusterson B A. Breast development gives insights into breast disease. Histopathology. 1998;33(3):275–283. doi: 10.1046/j.1365-2559.1998.00479.x. [DOI] [PubMed] [Google Scholar]
- 16.Seltzer V. The breast: embryology, development, and anatomy. Clin Obstet Gynecol. 1994;37(4):879–880. [PubMed] [Google Scholar]
- 17.Dixon J. London: BMJ; 1995. ABC of Breast Diseases. [Google Scholar]
- 18.Oftedal O T. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia. 2002;7(3):253–266. doi: 10.1023/a:1022848632125. [DOI] [PubMed] [Google Scholar]
- 19.Simmons P S. Diagnostic considerations in breast disorders of children and adolescents. Obstet Gynecol Clin North Am. 1992;19(1):91–102. [PubMed] [Google Scholar]
- 20.Dewhurst J. Breast disorders in children and adolescents. Pediatr Clin North Am. 1981;28(2):287–308. doi: 10.1016/s0031-3955(16)33997-9. [DOI] [PubMed] [Google Scholar]
- 21.Jolicoeur F. Intrauterine breast development and the mammary myoepithelial lineage. J Mammary Gland Biol Neoplasia. 2005;10(3):199–210. doi: 10.1007/s10911-005-9581-9. [DOI] [PubMed] [Google Scholar]
- 22.Jolicoeur F GL, Gaboury L A, Oligny L L. Basal cells of second trimester fetal breasts: immunohistochemical study of myoepithelial precursors. Pediatr Dev Pathol. 2003;6(5):398–413. doi: 10.1007/s10024-003-1125-y. [DOI] [PubMed] [Google Scholar]
- 23.Osin P P, Anbazhagan R, Bartkova J, Nathan B, Gusterson B A. Breast development gives insights into breast disease. Histopathology. 1998;33(3):275–283. doi: 10.1046/j.1365-2559.1998.00479.x. [DOI] [PubMed] [Google Scholar]
- 24.Naccarato A GVP, Viacava P, Vignati S. et al. Bio-morphological events in the development of the human female mammary gland from fetal age to puberty. Virchows Arch. 2000;436(5):431–438. doi: 10.1007/s004280050470. [DOI] [PubMed] [Google Scholar]
- 25.Moore K L, Persod T VN, Torchia M G. Philadelphia, PA: Elsevier Saunders; 2013. The Developing Human. Clinically Oriented Embryology. 9th ed. [Google Scholar]
- 26.McKiernan J F, Hull D. Breast development in the newborn. Arch Dis Child. 1981;56(7):525–529. doi: 10.1136/adc.56.7.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anbazhagan R, Bartek J, Monaghan P, Gusterson B A. Growth and development of the human infant breast. Am J Anat. 1991;192(4):407–417. doi: 10.1002/aja.1001920408. [DOI] [PubMed] [Google Scholar]
- 28.McNally S, Martin F. Molecular regulators of pubertal mammary gland development. Ann Med. 2011;43(3):212–234. doi: 10.3109/07853890.2011.554425. [DOI] [PubMed] [Google Scholar]
- 29.Jayasinghe Y CR, Cha R, Horn-Ommen J, O'Brien P, Simmons P S. Establishment of normative data for the amount of breast tissue present in healthy children up to two years of age. J Pediatr Adolesc Gynecol. 2010;23(5):305–311. doi: 10.1016/j.jpag.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt I MCM, Chellakooty M, Haavisto A M. et al. Gender difference in breast tissue size in infancy: correlation with serum estradiol. Pediatr Res. 2002;52(5):682–686. doi: 10.1203/00006450-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Milchdruse A DD. Heidelberg, Germany: Springer-Verlag; 1957. Handbuch der Mikroskopischen Anatomie des Menschen. [Google Scholar]
- 32.Stingl J. Estrogen and progesterone in normal mammary gland development and in cancer. Horm Cancer. 2011;2(2):85–90. doi: 10.1007/s12672-010-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurence D J, Monaghan P, Gusterson B A. The development of the normal human breast. Oxf Rev Reprod Biol. 1991;13:149–174. [PubMed] [Google Scholar]
- 34.Monaghan P, Perusinghe N P, Cowen P, Gusterson B A. Peripubertal human breast development. Anat Rec. 1990;226(4):501–508. doi: 10.1002/ar.1092260412. [DOI] [PubMed] [Google Scholar]
- 35.Marshall W A, Tanner J M. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Susman E J, Houts R M, Steinberg L. et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 91/2 and 151/2 years. Arch Pediatr Adolesc Med. 2010;16:166–173. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinberg D L, Ruan W. IGF-I, GH, and sex steroid effects in normal mammary gland development. J Mammary Gland Biol Neoplasia. 2008;13(4):353–360. doi: 10.1007/s10911-008-9103-7. [DOI] [PubMed] [Google Scholar]
- 38.Drife J O. Breast development in puberty. Ann NY Acad Sci. 1986;464:58–65. doi: 10.1111/j.1749-6632.1986.tb15993.x. [DOI] [PubMed] [Google Scholar]
- 39.Schlummer A P, Beller F K, Borsos A, Csoknyay I, Kieback D. Central European study of the development of secondary sex characteristics in girls. I. Axillary hair as the 4th secondary sex characteristic [In German] Geburtshilfe Frauenheilkd. 1988;48(11):768–775. doi: 10.1055/s-2008-1026624. [DOI] [PubMed] [Google Scholar]
- 40.Aksglaede L, Sørensen K, Petersen J H, Skakkebaek N E, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123(5):e932–e939. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 41.Capraro V J, Dewhurst C J. Breast disorders in childhood and adolescence. Clin Obstet Gynecol. 1975;18:25–50. doi: 10.1097/00003081-197506000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Rohn R D. Nipple (papilla) development in puberty: longitudinal observations in girls. Pediatrics. 1987;79(5):745–747. [PubMed] [Google Scholar]
- 43.Rohn R D. Papilla (nipple) development during female puberty. J Adolesc Health Care. 1982;2(3):217–220. doi: 10.1016/s0197-0070(82)80044-2. [DOI] [PubMed] [Google Scholar]
- 44.Büyükgebiz A, Kinik E. Nipple development in female puberty. Turk J Pediatr. 1989;31(4):275–279. [PubMed] [Google Scholar]
- 45.Van Keymeulen A, Rocha A S, Ousset M. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nat Rev Mol Cell Biol. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 46.Lee H J, Ormandy C J. Interplay between progesterone and prolactin in mammary development and implications for breast cancer. Mol Cell Endocrinol. 2012;357(1-2):101–107. doi: 10.1016/j.mce.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Shackleton M VF, Vaillant F, Simpson K J. et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 48.Stingl J RA, Raouf A, Emerman J T, Eaves C J. Epithelial progenitors in the normal human mammary gland. J Mammary Gland Biol Neoplasia. 2005;10(1):49–59. doi: 10.1007/s10911-005-2540-7. [DOI] [PubMed] [Google Scholar]
- 49.Russo J, Russo I H. New York: Plenum Press; 1987. Development of the human mammary gland; pp. 67–93. [Google Scholar]
- 50.Wiseman B S, Werb Z. Stromal effects on mammary gland development and breast cancer. Sci Total Environ. 2002;296(5570):1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brisken C PS, Park S, Vass T, Lydon J P, O'Malley B W, Weinberg R A. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95(9):5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo J, Balogh G A, Chen J. et al. The concept of stem cell in the mammary gland and its implication in morphogenesis, cancer and prevention. Front Biosci. 2006;1:151–172. doi: 10.2741/1788. [DOI] [PubMed] [Google Scholar]
- 53.Russo J, Russo I H. Development of the human breast. Maturitas. 2004;49(1):2–15. doi: 10.1016/j.maturitas.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Rohn R D. Papilla (nipple) development in puberty. The adolescent male. J Adolesc Health Care. 1985;6(6):429–432. doi: 10.1016/s0197-0070(85)80047-4. [DOI] [PubMed] [Google Scholar]
- 55.Propper A Gomot L; GL. Tissue interactions during organogenesis of the mammary gland in the rabbit embryo [in French] C R Acad Sci Hebd Seances Acad Sci D 1967264222573–2575. [PubMed] [Google Scholar]
- 56.Cunha G RYP, Young P, Christov K. et al. Mammary phenotypic expression induced in epidermal cells by embryonic mammary mesenchyme. Acta Anat (Basel) 1995;152(3):195–204. doi: 10.1159/000147698. [DOI] [PubMed] [Google Scholar]
- 57.Ceriani R L. Fetal mammary gland differentiation in vitro in response to hormones I. Morphological findings. Dev Biol. 1970;21(4):506–529. doi: 10.1016/0012-1606(70)90075-8. [DOI] [PubMed] [Google Scholar]
- 58.Ceriani R L. Fetal mammary gland differentiation in vitro in response to hormones II. Biochemical findings. Dev Biol. 1970;21(4):530–546. doi: 10.1016/0012-1606(70)90076-x. [DOI] [PubMed] [Google Scholar]
- 59.Flint D JTE, Tonner E, Beattie J, Allan G J. Role of insulin-like growth factor binding proteins in mammary gland development. J Mammary Gland Biol Neoplasia. 2008;13(4):443–453. doi: 10.1007/s10911-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 60.Howard B, Ashworth A. Signalling pathways implicated in early mammary gland morphogenesis and breast cancer. PLoS Genet. 2006;2(8):e112. doi: 10.1371/journal.pgen.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sternlicht M D, Sunnarborg S W, Kouros-Mehr H, Yu Y, Lee D C, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132(17):3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson-Fisher A JBG, Bellinger G, Ramabhadran R, Morris J K, Lee K F, Stern D F. ErbB2 is required for ductal morphogenesis of the mammary gland. Proc Natl Acad Sci U S A. 2004;101(49):17138–17143. doi: 10.1073/pnas.0407057101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asselin-Labat M L Vaillant F Sheridan J M et al. Control of mammary stem cell function by steroid hormone signalling Nature 2010. 10;4657299798–802. [DOI] [PubMed] [Google Scholar]
- 64.Joshi P A Jackson H W Beristain A G et al. Progesterone induces adult mammary stem cell expansion Nature 2010. 10;4657299803–807. [DOI] [PubMed] [Google Scholar]