Abstract
Parkinson’s disease (PD) is a movement disorder caused by the loss of dopaminergic neurons in the substantia nigra pars compacta, leading to nigrostriatal degeneration. The inhibition of mitochondrial respiratory chain complex I and oxidative stress-induced damage have been implicated in the pathogenesis of PD. The present study used an in vitro animal model of PD induced by specific mitochondrial complex I inhibitors: rotenone or 1-methyl-4- phenylpyridinium (MPP+). The goal was to test our hypothesis that pretreatment with nearinfrared light (NIR) via light-emitting diode (LED) had a greater beneficial effect on primary neurons grown in media with rotenone or MPP+ than those with or without LED treatment during exposure to poisons. Striatal and visual cortical neurons from newborn rats were cultured in a media with or without 200 nM of rotenone or 250 μM of MPP+ for 48 hrs. They were treated with NIR-LED twice a day before, during, and both before and during the exposure to the poison. Results indicate that pretreatment with NIR-LED significantly suppressed rotenone- or MPP+-induced apoptosis in both striatal and cortical neurons (P < 0.001), and that pretreatment plus LED treatment during neurotoxin exposure was significantly better than LED treatment alone during exposure to neurotoxins. In addition, MPP+ induced a decrease in neuronal ATP levels (to 48% of control level) that was reversed significantly to 70% of control by NIR-LED pretreatment. These data suggest that LED pretreatment is an effective therapy for neurons damaged by neurotoxins linked to PD.
Keywords: ATP content, MPTP, near-infrared light, neurotoxicity, Parkinson’s disease, Rotenone
1. Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by progressive motor dysfunction and variable cognitive impairment (Dauer and Przedborski, 2003; Gandhi and Wood, 2005). The key neuropathological feature of PD is the loss of striatal dopamine content, which leads to bradykinesia, tremors, and postural instability (Gandhi and Wood, 2005). The etiology of PD remains largely unknown, as no study has demonstrated thus far that the majority of PD cases are due to a single factor. Present evidence suggests that PD is a multifactorial disorder, probably caused by a combination of age, genetics, and environmental factors (Veldman et al., 1998). Epidemiological studies have suggested that environmental factors play an important role in the pathogenesis of PD and other related disorders. These include trace metals, pesticides, herbicides, and industrial chemicals (Gorell et al., 1998; Veldman et al., 1998). In particular, the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and the pesticide rotenone have been found to produce, at least in experimental animals, a Parkinsonian syndrome characterized by highly selective nigrostriatal dopaminergic degeneration just as in idiopathic PD. Biochemical studies have implicated mitochondrial complex I dysfunction in both the pathogenesis of PD as well as in MPTP- or rotenone-induced neurotoxicity. MPTP is highly lipophilic and easily crosses the blood-brain barrier. In the brain, MPTP is converted to MPP+ in glial cells and then incorporated into neurons via the dopamine transporter (DAT) (Gainetdiniv et al., 1997; Cappelletti et al., 2005). MPP+ is believed to be concentrated in mitochondria and to inhibit the multi-subunit complex I (Ramsay et al., 1986) of the electron transport chain, leading to the depletion of ATP and the production of reactive oxygen species (ROS) (Dauer and Przedborski, 2003). MPP+ causes selective damage to dopaminergic neurons and has been widely used to generate models of Parkinson’s disease (Gainetdiniv et al., 1997; Cappelletti et al., 2005).
Rotenone has recently been shown to reproduce the same progressive, selective, nigrostriatal dopaminergic degeneration seen in PD (Betarbet et al., 2000). This rotenone model of PD has reinforced the notion that complex I inhibition may be a key factor involved in the death of dopaminergic neurons and the development of Parkinsonism, mainly by increasing oxidative stress.
Near-infrared light (NIR) via light-emitting diode (LED) is a well-accepted therapeutic tool in the treatment of infected, ischemic, and hypoxic wounds and other soft tissue injuries in humans and animals (Whelan et al., 2001, 2003). The mechanism of NIR-LED action is the upregulation of cytochrome c oxidase activity and the production of adenosine triphosphate (ATP), as shown in primary cultures of rat visual cortical neurons functionally inactivated by tetrodotoxin, potassium cyanide (KCN), or sodium azide (N3Na) (Wong-Riley et al., 2001, 2005). NIR-LED treatment effectively rescues poisoned neurons from apoptotic cell death (Wong-Riley et al., 2005; Liang et al., 2006). Moreover, such treatment has recently been shown to partially rescue neurons from both rotenone- and MPP+-induced neurotoxicity (Liang et al., 2008). The goal of the present study was to test our hypothesis that pretreatment with NIR-LED enhances this protective effect.
2. Results
2.1. LED treatment rescued neurons from rotenone-induced apoptotic cell death
In the normal rat primary neuronal cultures (control), very few striatal neurons or cortical neurons underwent cell death (Figs. 1A and 1E, 2A and 2E). However, exposure to 200 nM of rotenone for 48 h induced a 29.81% of striatal neurons to undergo apoptosis (P < 0.001) (Figs. 1B and 1E). Similar exposure to rotenone rendered higher numbers of cortical neurons to undergo cell death (42.96%; P < 0.001; Figs. 2B and 2E). LED treatments twice a day for 2 days during toxin exposure (LED & rotenone group) effectively decreased the number of apoptotic neurons induced by 200 nM of rotenone to 19.78% in striatal neurons and to 25.16% in cortical neurons, representing a 33.65% and 41.43% reduction, respectively (P < 0.001 for all) (Figs. 1C and 1E, 2C and 2E). The percentage of apoptotic neurons in the LED pretreatment group (twice a day for 2 days before exposure to rotenone plus LED treatments during rotenone exposure) (pre- LED/rotenone & LED group) was only 13.07% in striatal neurons and 14.12% in cortical neurons, representing a 56.16% and 66.43% reduction, respectively, as compared to rotenone only (P < 0.001), and an additional 33.92% and 42.68% reduction as compared to rotenone plus LED (P < 0.01 and P < 0.001, respectively) (Fig. 1D and 1E, 2D and 2E).
Fig. 1.
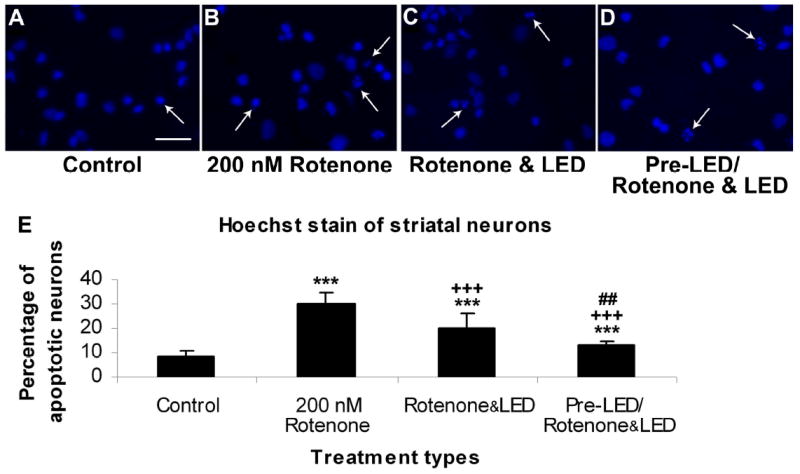
Hoechst staining of cultured striatal neurons in control (A), rotenone exposed (B), rotenone plus LED treated (C), and LED pretreatment for 2 days before rotenone exposure plus LED treatment during rotenone exposure (D). The arrows show apoptotic neurons with nuclear condensation or decreased nuclear size, with or without nuclear fragmentation. Quantitative assays of percent apoptotic neurons under various conditions are shown in E. Rotenone exposure significantly increased the number of apoptotic neurons (P < 0.001), and LED treatment markedly reduced this percentage (P < 0.001). However, LED pretreatment further reduced this percentage (P < 0.01).
All “* P” values were compared to controls: ** P < 0.01, *** P < 0.001. All “+P” values were compared to MPP+ or rotenone alone: ++ P < 0.01, +++ P < 0.001. All “#P” values compared “LED pretreatment plus LED treatment during toxin exposure group” to “LED during toxin exposure only group”. Scale bar: 25 μm for all.
Fig. 2.
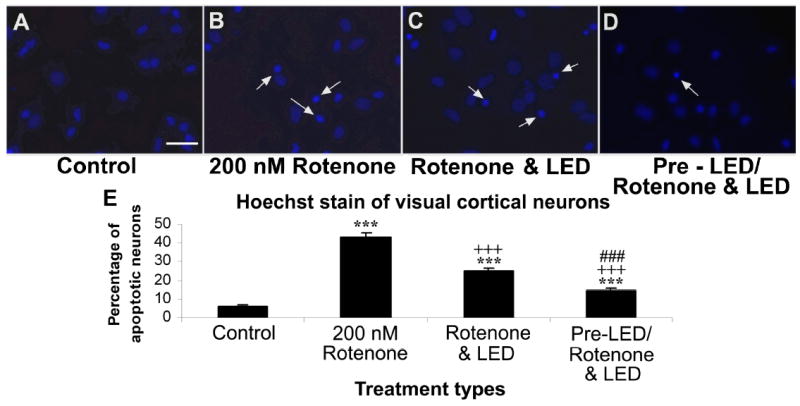
Hoechst staining of cultured visual cortical neurons in control (A), rotenone exposed (B), rotenone plus LED treated (C), and LED pretreatment for 2 days before rotenone exposure plus LED treatment during rotenone exposure (D). The arrows show apoptotic neurons with nuclear condensation or decreased nuclear size, with or without nuclear fragmentation. Quantitative assays of percent apoptotic neurons under various conditions are shown in E. Rotenone exposure significantly increased the number of apoptotic neurons (P < 0.001), and LED treatment markedly reduced this percentage (P < 0.001). However, LED pretreatment further reduced this percentage (P < 0.01).
All “* P” values were compared to controls: ** P < 0.01, *** P < 0.001. All “+P” values were compared to MPP+ or rotenone alone: ++ P < 0.01, +++ P < 0.001. All “#P” values compared “LED pretreatment plus LED treatment during toxin exposure group” to “LED during toxin exposure only group”. Scale bar: 25 μm for all.
2.2. LED treatment rescued neurons from MPP+-induced apoptotic cell death
Exposure to 250 μM of MPP+ for 48 h induced 35.10% of striatal neurons and 38.59% of visual cortical neurons to undergo apoptosis (P < 0.001 for both) (Figs. 3B and 3F, 4B and 4F). The percentage of apoptotic neurons in the LED pretreatment group (twice a day for 2 days before exposure to MPP+) (pre-LED/ MPP+ & LED group) was 26.56% for striatal neurons and 29.81% for cortical neurons, representing 24.33% and 22.75% reduction, respectively, as compared to MPP+ alone (P < 0.001 for both). (Figs. 3C and 3F, 4C and 4F). LED treatments twice a day for 2 days during MPP+ exposure (MPP+ & LED group) effectively decreased the number of apoptotic neurons to 26.32% in striatal neurons and to 31.07% in cortical neurons, representing a 25.01% and 19.48% reduction, respectively, as compared to MPP+ alone (P < 0.001 for all) (Figs. 3D and 3F, 4D and 4F). Pretreatment with LED plus LED treatment during MPP+ exposure (pre-LED/ MPP+ & LED group) reduced it further to only 21.95% in striatal neurons and 20.35% in cortical neurons, representing a reduction of 37.46% and 47.26%, respectively, as compared to MPP+ alone (P < 0.001 for both), and a further reduction of 17.36% and 31.73%, respectively, as compared to LED treatment only during MPP+ exposure. It also represented a 16.60% further reduction in striatal neurons and a 34.50% further reduction in cortical neurons as compared to LED pretreatment only group (P < 0.001 for all) ( Fig. 3E and 3F, 4E and 4F). There was no statistically significant difference between pretreatment alone and LED treatment alone during neurotoxin exposure in either striatal or cortical neurons.
Fig. 3.
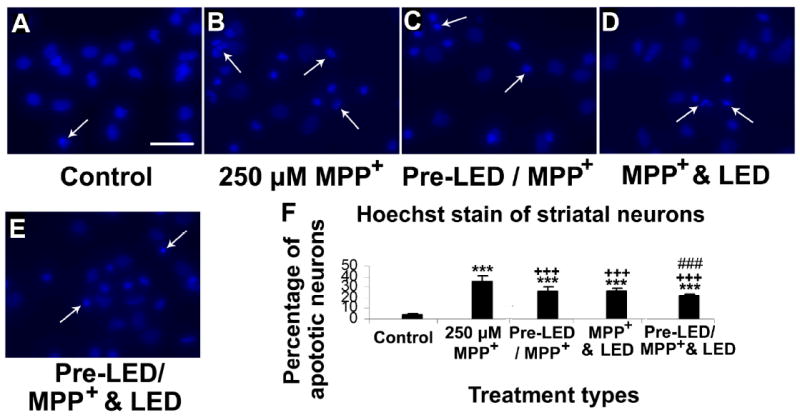
Hoechst staining of cultured striatal neurons in control (A), MPP+ exposed (B), LED pretreatment for 2 days before MPP+ exposure (C), MPP+ plus LED treated (D), and LED pretreatment for 2 days before MPP+ exposure plus LED treatment during MPP+ exposure (E). The arrows show apoptotic neurons with nuclear condensation or decreased nuclear size, with or without nuclear fragmentation. Quantitative assays of percent apoptotic neurons under various conditions are shown in F. MPP+ exposure significantly increased the number of apoptotic neurons (P < 0.001). LED treatment markedly reduced this percentage (P < 0.001), and pretreatment with LED showed comparable results (P < 0.001) with no significant difference between the two paradigms. However, LED pretreatment plus LED during MPP+ exposure further reduced this percentage (P < 0.001).
All “* P” values were compared to controls: ** P < 0.01, *** P < 0.001. All “+P” values were compared to MPP+ or rotenone alone: ++ P < 0.01, +++ P < 0.001. All “#P” values compared “LED pretreatment plus LED treatment during toxin exposure group” to “LED during toxin exposure only group”. Scale bar: 25 μm for all.
Fig. 4.
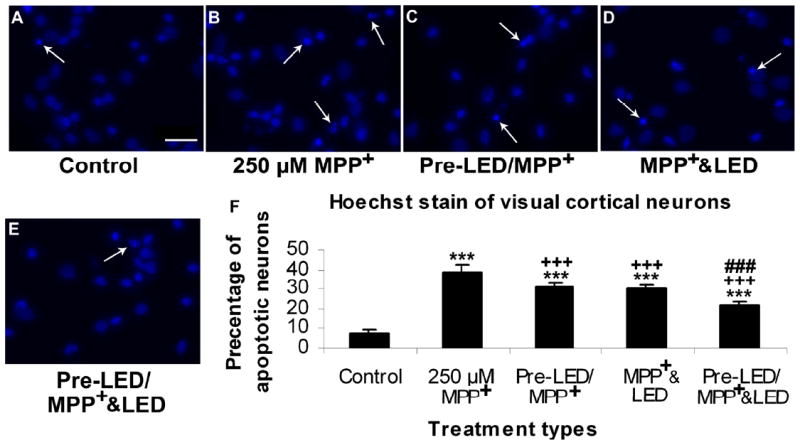
Hoechst staining of cultured visual cortical neurons in control (A), MPP+ exposed (B), LED pretreatment for 2 days before MPP+ exposure (C), MPP+ plus LED treated (D), and LED pretreatment for 2 days before MPP+ exposure plus LED treatment during MPP+ exposure (E). The arrows show apoptotic neurons with nuclear condensation or decreased nuclear size, with or without nuclear fragmentation. Quantitative assays of percent apoptotic neurons under various conditions are shown in F. MPP+ exposure significantly increased the number of apoptotic neurons (P < 0.001). LED treatment markedly reduced this percentage (P < 0.001), and pretreatment with LED showed comparable results (P < 0.001) with no significant difference between the two paradigms. However, LED pretreatment plus LED during treatment further reduced this percentage (P < 0.001).
All “* P” values were compared to controls: ** P < 0.01, *** P < 0.001. All “+P” values were compared to MPP+ or rotenone alone: ++ P < 0.01, +++ P < 0.001. All “#P” values compared “LED pretreatment plus LED treatment during toxin exposure group” to “LED during toxin exposure only group”. Scale bar: 25 μm for all.
2.3. Pre-LED treatment increased cellular ATP content on striatal neurons after MPP+ exposure
Cellular ATP content in striatal neurons was significantly decreased by exposure to 250 μM MPP+ for 48 h (P < 0.001; Fig. 5). Both LED treatment or pretreatment increased cellular ATP content in comparison to MPP+ exposure alone (++P < 0.01 for both). Pretreatment plus treatment during MPP+ exposure further increased ATP content (+++P < 0.001 as compared to MPP+ alone, ##: P < 0.01 as compared to Pre-LED/MPP+, and xxx P < 0.001 as compared to MPP+ & LED; Fig. 5). There was no significant difference between pretreatment alone and LED treatment alone during MPP+ exposure.
Fig. 5.
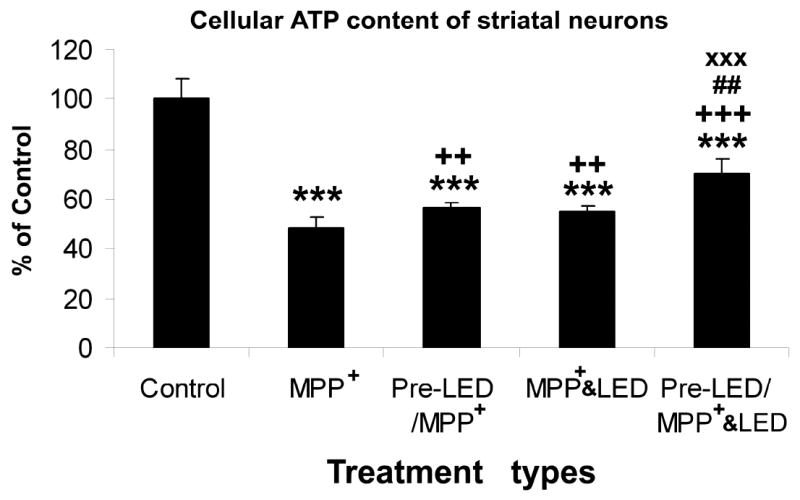
Effect of LED treatment and pre-treatment on cellular ATP content in striatal neurons exposed to 250 μM MPP+. MPP+ severely reduced ATP content from control levels (P < 0.001). Either LED treatment or pretreatment was effective in increasing ATP content above that of MPP+ alone (P < 0.01), and there was no significant difference between the two groups. However, LED pretreatment plus LED treatment during MPP+ exposure was most beneficial in increasing ATP content of striatal neurons (## P < 0.01 in comparison to pre-LED/ MPP+, and XXX P < 0.001 in comparison to MPP+ & LED).
All “* P” values were compared to controls: ** P < 0.01, *** P < 0.001. All “+P” values were compared to MPP+ or rotenone alone: ++ P < 0.01, +++ P < 0.001. All “#P” values compared “LED pretreatment plus LED treatment during toxin exposure group” to “LED during toxin exposure only group”. Scale bar: 25 μm for all.
3. Discussion
Parkinson’s disease is one of the most common neurodegenerative disorders affecting people over 60 years of age (Van Den Eeden et al., 2003). This disease is characterized by the selective loss of dopaminergic neurons in the substantia nigra, pars compacta and selective nigrostriatal dopaminergic degeneration (Dauer and Przedborski, 2003; Moore et al., 2005).
Two mitochondrial inhibitors, MPP+ and rotenone, are known to inhibit complex I of the electron transport chain. They have been widely used to induce dopaminergic neuronal death in both in vitro and in vivo models of PD (Denton et al., 1987, Storch et al., 2000). Complex I inhibition has two major consequences: the depletion of ATP (hence the impairment of all ATP-dependent cellular processes) and the generation of free radicals that cause oxidative stress (Gerlach et al,.1991; Gandhi and Wood, 2005; Watababe et al., 2005), resulting in a damage to human dopaminergic neurons (Langston et al., 2003).
Near-infrared light via laser has long been known to promote wound healing, but the detailed molecular mechanisms have only recently been studied (Lane, 2006). Karu’s group proposed that NIR works through cytochrome c oxidase, as copper A and B centers in this enzyme act as photoacceptors in the NIR range (Karu, 1999). Our group has documented that NIR via LED indeed up-regulated cytochrome c oxidase activity and ATP content in neurons poisoned by tetrodotoxin or cyanide, and that it rescued neurons from apoptotic cell death induced by cyanide (Wong-Riley et al., 2001, 2005). In vivo, NIR-LED was therapeutic to retinal neurons poisoned by methanol that accumulated formate, an inhibitor of cytochrome c oxidase (Eells et al., 2003). NIR can further protect cells against NO-induced cell death (Karu et al., 2005). LED treatment (and most likely pretreatment) may trigger a cascade of events that changed the expressions of a number of genes, such as the up-regulation of genes involved in energy generation and antioxidant production (Eells et al., 2004).
Recently, we have shown that NIR-LED treatment increased ATP production and cytochrome c oxidase activity, and reduced the generation of reactive oxygen species (ROS), reactive nitrogen species (RNS), and apoptosis in neurons poisoned by cyanide, MPP+, or rotenone (Liang et al., 2006, 2008). We have further shown that twice a day of LED treatment is most therapeutic as compared to once, three times, or four times a day of treatment (Liang et al., 2008).
The present study confirmed and extended our previous findings to document that LED pretreatment for only 80 seconds per day for only two days significantly rescued more striatal and cortical neurons from apoptotic cell death induced by either rotenone or MPP+. LED pretreatment also substantially increase cellular content of ATP in primary neurons grown in rotenone or MPP+. Such increases were markedly greater than those of LED treatment alone during exposure to neurotoxins. This suggests that increasing neurons’ oxidative capacity and ATP generation better prepares neurons to combat the detrimental effects of neurotoxins. These findings have substantial relevance to animal models of PD, as therapeutic treatment may be prescribed to animals in their early stages of Parkinsonian induction, before overt manifestations of Parkinsonian symptoms commence. The implication for future clinical trials of PD treatment is significant.
In summary, the present study demonstrates that LED pretreatment for 80 seconds, 2 times/day for two days (at a total energy density of 4 J/cm2 per treatment significantly rescued striatal and cortical neurons from MPP+- and rotenone- induced apoptosis, most likely via a mechanism that increases cellular ATP production and decreases ROS generation. It indicates that LED treatment plus pretreatment have a better therapeutic effect in protecting neurons from both MPP+- or rotenone-induced neurotoxicity than LED treatment alone during exposure to neurotoxins. These findings lay a solid foundation for future therapeutic considerations in animal models of PD and conceivably for future treatment of pre-symptomatic PD patients.
4. Experimental procedure
4.1. Primary culture of neurons
Sprague Dawley rats (1 or 2-day-old) were used for primary cultures of neurons derived from the striatum or visual cortex as previously described (Liang et al., 2006). Briefly, rats were anesthetized with CO2, and brains were removed, placed in balanced salt solution (Invitrogen, Carlsbad, CA, USA), and carefully dissected. Meninges and surface blood vessels were stripped, brain tissues were minced into 1mm3 pieces and digested with 0.75% trypsin at 37°C for 15 min. Cells were mechanically dissociated, and plated on poly-L-lysine- (Sigma, St. Louis, MO, USA) coated 35 mm dishes or 20 mm coverslips at a density of 5×105 cells/ml. Neuronal cultures were maintained at 37°C with 5% CO2 in a humidified incubator, with half of the medium replaced every five days. In this culture system, 95% of the cell population were neurons (Zhang and Wong-Riley, 1999; Liang et al., 2006).
4.2. Culture treatments
Experiments were carried out on 7–10 day old cultures of visual cortical or striatal neurons, with or without 670nm LED pretreatment for 80 sec at a power intensity of 50 mW/cm2, giving a total energy density of 4 J/cm2. An LED array (25 cm×10 cm) with a peak wavelength at 670nm (Quantum Devices, Inc. Barnaveld, WI, USA) was placed beneath the covered culture dish (35mm or 60mm diameter), with the room light turned off and irradiated accordingly. Visual cortical and striatal neurons were each subdivided into 9 groups: 1) normal cells without exposure to MPP+ and without treatment with LED (Control); 2) cells exposed to 250 μM MPP+ for 48 h without LED treatment (MPP+); 3) cells pretreated with LED 2 times/d for 2 days and then exposed to 250 μM MPP+ for 48 h (Pre-LED/MPP+); 4) cells exposed to 250 μM MPP+ for 48 h, during which they were treated with LED 2 times/d (MPP+ & LED); and 5) cells pretreated with LED 2 times/d for 2 days and then exposed to 250 μM MPP+ for 48 h, during which they were treated with LED 2 times/d (Pre-LED/MPP+ & LED). The other 4 groups were similar to the above, except that MPP+ was replaced by 200 nM rotenone, and group 3 was not done because no significant difference was found between groups 3 and 4 in the MPP+ experiments.
4.3. Hoechst 53258 staining
At the end of each experiment, cells were harvested and fixed with pre-chilled phosphate-buffered saline (PBS) containing 4% paraformaldehyde for 30 min with gentle agitation. After fixation at room temperature, cells were washed with pre-chilled PBS 3 times and were exposed to 2.5mg/L fluorescent DNA-binding dye Hoechst 53258 (Sigma) in PBS for 10 min at room temperature. After washing, all samples were analyzed under a Nikon fluorescent microscope.
4.4. Measurement of ATP Content with Bioluminescence Assays
Measurement of cellular ATP content in control and experimental groups were as previously described (Wong-Riley et al., 2005) using components of the bioluminescent somatic cell assay kit (Sigma). Briefly, cultured neurons were rinsed with cold PBS, incubated in cold somatic cell ATP releasing reagent for 5 min on ice, and harvested by a cell scraper. They were mixed with the luciferase ATP assay mix and assayed with a luminometer (Berthold Detection systems, Oak Ridge, TN). Values were expressed as nanomolar ATP content per milligram of protein.
References
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neuroscience. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Cappelletti G, Surrey T, Maci R. The parkinsonism producing neurotoxin MPP+ affects microtubule dynamics by acting as a destabilising factor. FEBS Letters. 2005;579:4781–4786. doi: 10.1016/j.febslet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Denton T, Howard BD. A dopaminergic cell line variant resistant to the neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. J Neurochem. 1987;49:622–630. doi: 10.1111/j.1471-4159.1987.tb02909.x. [DOI] [PubMed] [Google Scholar]
- Eells JT, Henry MM, Summerfelt P, Wong-Riley MTT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JT, Wong-Riley MTT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochemistry. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Wood NW. Molecular pathogenesis of Parkinson’s disease. Human Molecular Genetics. 2005;14:2749–2755. doi: 10.1093/hmg/ddi308. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Riederer P, Przuntek H, Youdim MB. MPTP mechanisms of neurotoxicity and their implications for Parkinson’s disease. Eur J Pharmacol. 1991;12:273–286. doi: 10.1016/0922-4106(91)90073-q. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- Karu T, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36:307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- Lane N. Cell biology: power games. Nature. 2006;443:901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 2003;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Liang HL, Whelan HT, Eells JT, Meng H, Buchmann E, Lerch-Gaggl A, Wong-Riley MTT. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Liang HL, Whelan HT, Eells J, Wong-Riley MTT. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008:1–12. doi: 10.1016/j.neuroscience.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Science. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Salach JI, Singer TP. Uptake of the neurotoxin 1-methyl-4-phenylpyridine (MPP+) by mitochondria and its relation to the inhibition of the mitochondrial oxidation of NAD+-linked substrates by MPP+ Biochem Biophys Res Commun. 1986;134:743–748. doi: 10.1016/s0006-291x(86)80483-1. [DOI] [PubMed] [Google Scholar]
- Storch A, Burkhardt K, Ludolph AC, Schwarz J. Protective effects of riluzole on dopamine neurons: involvement of oxidative stress and cellular energy metabolism. J Neurochem. 2000;75:2259–2269. doi: 10.1046/j.1471-4159.2000.0752259.x. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- Veldman BA, Wijn AM, Knoers N, Praamstra P, Horstink MW. Genetic and environmental risk factors in Parkinson’s disease. Clin Neurol Neurosurg. 1998;100:15–26. doi: 10.1016/s0303-8467(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Watababe Y, Himeda T, Araki T. Mechanisms of MPTP toxicity and their implications for therapy of Parkinson’s disease. Med Sci Monit. 2005;11:17–23. [PubMed] [Google Scholar]
- Whelan HT, Smits RL, Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T, Cwiklinski J, Philippi AF, Graf WR, Hodgson B, Gould L, Kane M, Chen G, Caviness J. Effect of NASA lightemitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–314. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- Whelan HT, Buchmann EV, Dhokalia A, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MTT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003;21:67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Bai XT, Buchmann E, Whelan HT. Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport. 2001;12:3033–3037. doi: 10.1097/00001756-200110080-00011. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins. The Journal of Biological Chemistry. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wong-Riley MTT. Expression and regulation of NMDA receptor subunit R1 and neuronal nitric oxide synthase in cortical neuronal cultures: Correlation with cytochrome oxidase. J Neurocytol. 1999;28:525–539. doi: 10.1023/a:1007053204929. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]


