Abstract
Objectives
Many researchers and clinicians continue to believe that (non-modifiable) race/ethnicity is a major contributor to diabetes, prompting a well-intentioned search for genetic and bio-physiological explanations. We seek to reinforce earlier findings showing that socioeconomic status is more strongly associated with diabetes prevalence than race/ethnicity and suggests a very different and potentially modifiable etiologic pathway.
Methods
A community-based epidemiologic survey of 5503 Boston residents aged 30–79 (1767 Black, 1877 Hispanic, 1859 White; 2301 men and 3202 women).
Results
After adjusting for age and gender, Blacks and Hispanics have statistically significantly increased odds of having diabetes: Black (Odds Ratio 2.0 with 95% confidence interval 1.4–2.9) and Hispanic (2.4; 1.6–3.4) compared to Whites. If socioeconomic status (a combination of education and income) is added to the model, these odds are reduced for both Blacks (1.6; 1.1–2.2) and Hispanics (1.6; 1.1–2.3). In a multivariate logistic regression adjusting for age, gender, socioeconomic status, obesity, hypertension, gestational diabetes, physical activity, trouble paying for basics, health insurance status, and family history of diabetes, these odds are reduced further: Black (1.0; 0.7–1.5) and Hispanic (1.3; 0.9–2.1) and are no longer statistically significant.
Conclusions
Consistent with other reports, we find socioeconomic status has a much stronger association with diabetes prevalence than race/ethnicity. Continuing to focus on race/ethnicity as a primary determinant of diabetes prevalence overemphasizes the importance of biomedical factors and diverts effort from socio-medical interventions (e.g. improving social circumstances, access to effective care, and upstream redistributive social policies).
Keywords: Diabetes, race/ethnicity, socioeconomic status
Introduction
Major federal agencies (like the National Institutes of Health and the Centers for Disease Control) and professional organization (like the American Diabetes Association) continue to identify race/ethnicity as a major determinant of the prevalence of diabetes in the United States1–3. This has spawned a well-intentioned search for underlying genetic and bio-physiologic explanations, eventually leading to identification of promising biomedical interventions to reduce race/ethnic disparities in diabetes. In contrast, social epidemiologists continue to find that socioeconomic status may be a more important determinant of diabetes prevalence, even accounting for much of the widely accepted race/ethnic effect4–10. Such findings suggest markedly different explanations (in social circumstances, and environmental and neighborhood influences) and precipitate different types of primary, secondary, and upstream policy interventions. In the United States and many other countries, race/ethnic minorities are more likely to be poorer and less well educated than the majority white population. This has caused researchers to repeatedly ask the question which motivates this paper: is the widely accepted disparity in the prevalence of diabetes really attributable to race/ethnicity (which is considered non-modifiable), or is it due to socioeconomic status (which is potentially modifiable through upstream social policy interventions)? This question has important implications for clinicians, health services researchers, and policy makers. We attempt to answer it using data from a community-based epidemiologic survey of Boston, Massachusetts residents.
Methods
The Boston Area Community Health (BACH) survey is an epidemiologic survey of Boston residents aged 30–79 years. Detailed methods have been described elsewhere11. In brief, a stratified two-stage cluster sample design was used to recruit residents of Boston with the goal of approximately equal number of participants by gender, race/ethnicity (Black, Hispanic, White), and age group (30–39, 40–49, 50–59, 60–79). In total, 5503 adults participated in BACH (1767 Black, 1877 Hispanic, 1859 White respondents; 2301 men and 3202 women). The response rate was 63.3% of screened eligible participants, which is typical of an epidemiologic field survey requiring a lengthy in-home protocol and phlebotomy. Data were collected between 2002 and 2005. After obtaining written informed consent, data were collected during a two hour interview (in English or Spanish), usually in the respondent’s home. All protocols and procedures were approved by the New England Research Institutes’ Institutional Review Board.
Race/ethnicity was determined by self report following Office of Management and Budget requirements12. Socioeconomic status was determined as a combination of standardized levels of education and income in the Northeast13 (with weights of .7 for education and .4 for income), and categorized such that ¼ of the sample was lower, ½ middle, and ¼ upper. Other covariates considered include the risk factors identified by the American Diabetes Association (gender, age (by decade), body mass index (BMI), exercise habits, history of hypertension or gestational diabetes, and family history of diabetes)1. Interviewers directly measured the respondent’s height and weight, from which BMI could be calculated (kg/m2) and was categorized as <25, 25–30, 30+ kg/m2. Information on co-morbidities was obtained by self report: Has a health care provider told you that you have insulin-dependent or juvenile-onset diabetes, non-insulin-dependent or adult-onset diabetes, high blood pressure, or gestational diabetes (if female)? Physical activity was measured by the Physical Activity for the Elderly (PASE) scale14, and categorized into low, moderate, or high. In addition to socioeconomic status, we also considered two additional socioeconomic variables: 1) health insurance status (some private insurance, public insurance only (Medicaid or Medicare), or none; and 2) trouble paying for basics (Are you having trouble paying for transportation, housing, health or medical care, medications, or food? (yes or no)).
Chi-square tests were used to test the assumption of equal distributions by race/ethnicity. A multivariate logistic regression was used to determine the joint effect of covariates on the probability of having diabetes. Multiple imputation was used to impute plausible values for missing observations using SAS 9.1.3 (SAS Institute, Cary, NC). We are missing less than 1 percent of the data on most variables with the exception of income in which we are missing 3 percent for Whites, 4 percent for Blacks and 11 percent for Hispanics. Twenty-five multiple imputations were done by gender and race/ethnicity. Observations were weighted inversely to their probability of selection and weights were post-stratified to the Boston census population in 2000. Analyses were conducted in SUDAAN 9.0.1 (Research Triangle Institute, Research Triangle Park, NC).
Results
The overall prevalence of diabetes was 9.5 percent. As expected, the prevalence of diabetes (and many of its associated risk factors) differed significantly by race/ethnicity (p<.0001) (Table 1). However, the prevalence of diabetes (and many of its associated risk factors) also varied by socioeconomic status (SES) within a race/ethnic categorization (Table 2) with the exception of family history of diabetes for Blacks and Hispanics. There was no significant association of the prevalence of diabetes by race/ethnicity within a socioeconomic level (p=.22 for lower SES, p=.72 for middle SES, p=.24 for upper SES).
Table 1.
Variation in the prevalence of diabetes and its risk factors by race/ethnicity (p value for chi-square test that the distribution is the same by race/ethnicity)
| Race/Ethnicity | ||||
|---|---|---|---|---|
| Black (N=1765) |
Hispanic (N=1877) |
White (N=1859) |
p value | |
| Diabetes (%) | 12.8 | 11.6 | 7.5 | <.0001 |
| High blood pressure (%) | 36.3 | 24.8 | 23.6 | <.0001 |
| Gestational diabetes1 (%) | 4.1 | 5.9 | 3.9 | .32 |
| Body Mass Index (%) | <.0001 | |||
| <25 kg/m2 | 22.4 | 25.3 | 34.8 | |
| 25–30 kg/m2 | 30.5 | 37.0 | 35.6 | |
| 30+ kg/m2 | 47.1 | 37.7 | 29.6 | |
| Physical Activity (%) | .16 | |||
| low | 26.1 | 30.0 | 27.3 | |
| moderate | 49.8 | 51.6 | 50.8 | |
| high | 24.1 | 18.5 | 21.8 | |
| Family history of diabetes (%) | 47.6 | 39.6 | 29.3 | <.0001 |
| Socioeconomic status (%) | <.0001 | |||
| lower | 41.2 | 61.1 | 14.0 | |
| middle | 49.4 | 30.6 | 49.7 | |
| upper | 9.4 | 8.3 | 36.3 | |
| Trouble paying for basics (%) | 37.4 | 30.6 | 18.6 | <.0001 |
| Health insurance status (%) | <.0001 | |||
| private | 51.1 | 35.7 | 64.1 | |
| public only | 36.8 | 39.6 | 24.0 | |
| none | 12.0 | 24.7 | 11.8 |
Table 2.
Variation in the Prevalence of Diabetes and Risk Factors for Diabetes by Race/Ethnicity and Socioeconomic Status (p value is from chi-square test of whether the distribution is the same across socioeconomic status by race/ethnicity).
| Race/Ethnicity | Black | Hispanic | White | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Socioeconomic Status | lower | middle | upper | p value | lower | middle | upper | p value |
lower | middle | upper | p value |
| Sample size (N) | 841 | 797 | 129 | 1312 | 495 | 70 | 413 | 861 | 585 | |||
| Diabetes (%) | 18.2 | 9.4 | 6.9 | .0001 | 14.0 | 7.5 | 8.5 | .0075 | 15.1 | 8.4 | 3.3 | <.0001 |
| High blood pressure (%) | 42.4 | 33.1 | 26.7 | .0040 | 27.2 | 23.0 | 14.3 | .16 | 41.2 | 22.4 | 18.5 | <.0001 |
| Gestational diabetes1 (%) | 5.4 | 3.5 | 0.0 | .0011 | 5.0 | 8.6 | 0.0 | .0140 | 6.2 | 5.3 | 0.1 | .0008 |
| Body Mass Index (%) | .051 | .056 | <.0001 | |||||||||
| <25 kg/m2 | 22.4 | 22.2 | 23.7 | 20.0 | 29.4 | 49.1 | 19.6 | 33.0 | 43.0 | |||
| 25–30 kg/m2 | 25.4 | 33.9 | 34.5 | 39.5 | 36.3 | 20.8 | 31.7 | 35.6 | 37.0 | |||
| 30+ kg/m2 | 52.1 | 43.9 | 41.8 | 40.4 | 34.3 | 30.1 | 48.6 | 31.4 | 20.0 | |||
| Physical Activity (%) | <.0001 | <.0001 | <.0001 | |||||||||
| low | 38.4 | 17.2 | 18.6 | 37.5 | 17.4 | 20.8 | 49.2 | 29.0 | 16.6 | |||
| moderate | 47.8 | 52.8 | 43.5 | 50.5 | 53.9 | 50.6 | 44.5 | 50.4 | 53.8 | |||
| high | 13.8 | 30.0 | 37.9 | 12.0 | 28.6 | 28.6 | 6.3 | 20.5 | 29.6 | |||
| Family history of diabetes (%) | 49.3 | 46.2 | 47.8 | .75 | 39.5 | 40.4 | 37.7 | .95 | 42.6 | 27.7 | 26.4 | .0008 |
| Trouble paying for basics (%) | 49.9 | 31.9 | 12.1 | <.0001 | 36.0 | 26.0 | 7.4 | .0001 | 38.6 | 21.5 | 7.0 | <.0001 |
| Health insurance status (%) | <.0001 | <.0001 | <.0001 | |||||||||
| private | 23.4 | 66.0 | 94.0 | 18.9 | 54.3 | 91.5 | 38.8 | 74.2 | 94.2 | |||
| public only | 61.8 | 22.4 | 3.2 | 51.9 | 24.7 | 3.4 | 48.1 | 13.6 | 3.0 | |||
| none | 14.8 | 11.6 | 2.8 | 29.2 | 21.0 | 5.1 | 13.1 | 12.2 | 2.8 | |||
Among women who have been pregnant
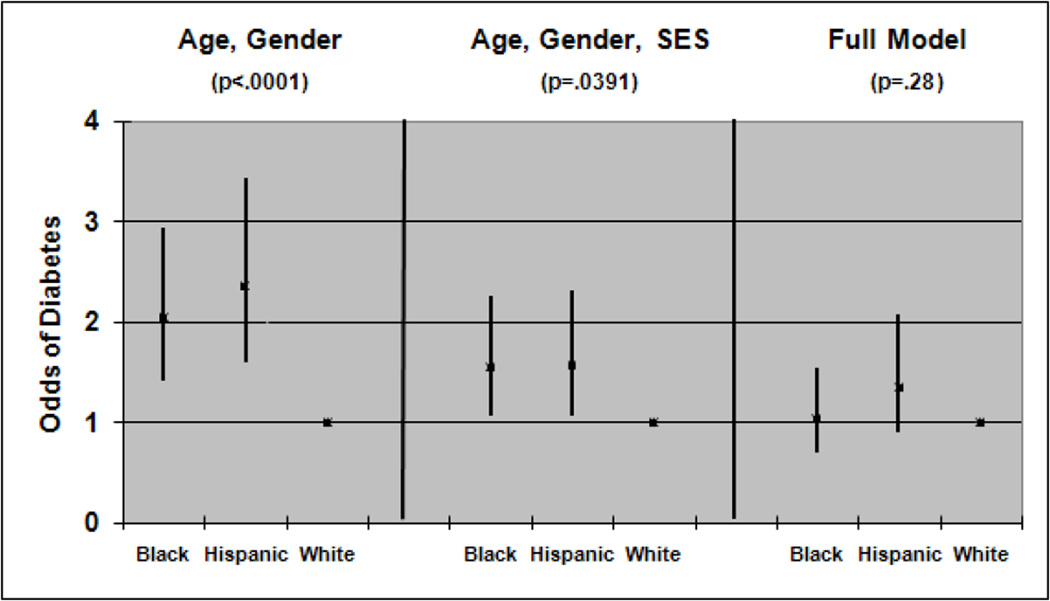
In a logistic regression model (with the dependent variable diagnosed diabetes) after adjusting for gender and age, Blacks (odds ratio=2.04 with 95% confidence interval 1.42–2.94) and Hispanics (odds ratio=2.35 with 95% confidence interval 1.60–3.44) had higher odds of diabetes compared to Whites (Figure 1). When socioeconomic status is added to the model these odds dropped for Blacks (odds ratio=1.55 with 95% confidence interval 1.07, 2.25) and Hispanics (odds ratio=1.57 with 95% confidence interval 1.06, 2.32). In this same model the odds for lower SES compared to upper SES are 3.25 (with 95% confidence interval 1.95, 5.42) and the odds for middle SES compared to upper SES are 2.04 (with 95% confidence interval 1.16, 3.59). After adjusting for all covariates given in Table 1, the odds ratios decreased for both Blacks (odds ratio=1.04 with 95% confidence interval 0.70–1.54) and Hispanics (odds ratio=1.34 with 95% confidence interval 0.89–2.08) and were no longer statistically significant.
Figure 1.
Odds ratios for the prevalence of diabetes (with 95% confidence intervals) for three models ((1) age, gender, race/ethnicity; (2) age, gender, socioeconomic status (SES), race/ethnicity), and (3) age, gender, socioeconomic status, trouble paying for basics, health insurance status, hypertension, gestational diabetes, and family history of diabetes, race/ethnicity). The p value (for race/ethnicity) is from a Wald F test with 2 degrees of freedom in the numerator.
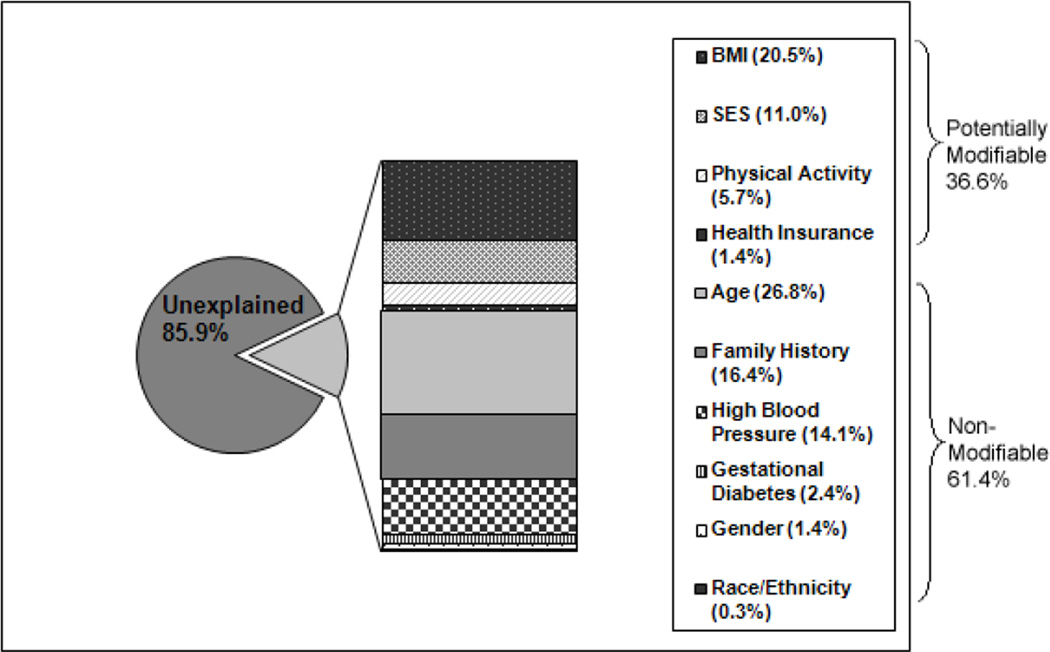
Using a generalized R2 statistic15, entering the potentially modifiable risk factors first and in order of importance by size of the additional variation explained (body mass index, SES (including trouble paying for basics), physical activity, and health insurance status) and then the non-modifiable risk factors (age, family history of diabetes, history of hypertension, history of gestational diabetes, gender, race/ethnicity), we found that they together explain only 14.1 percent of the variation in the prevalence of diabetes (Figure 2). Of that, 38.5 percent is explained by the potentially modifiable risk factors and 61.5 percent is explained by the non-modifiable risk factors. As the least important non-modifiable risk factor entered into the model, race/ethnicity explained only .4 percent of the explainable variation. Health insurance status is associated with the prevalence of diabetes only as it relates to SES (odds of diabetes for public insurance only compared to some type of private insurance 1.36 (p=.06) and odds of diabetes for no health insurance compared to some type of private insurance 0.97 (p=.88).
Figure 2.
Proportion of variation in the prevalence of diabetes by modifiable (BMI, SES, physical activity, health insurance status) and non-modifiable (age, family history of diabetes, history of high blood pressure, history of gestational diabetes, gender, race/ethnicity) risk factors.
Discussion
We have shown that socioeconomic status (a potentially modifiable risk factor) is more important in determining who has diabetes than the non-modifiable risk factor of race/ethnicity. This result is consistent with other reports showing higher prevalence of diabetes in depressed areas, or in people of lower socioeconomic status4–10.
While socioeconomic status is not a biological factor, it is considered a marker for other established risk factors for diabetes such as body mass index, physical activity, hypertension, and gestational diabetes. Thus it allows identification of target groups for primary and secondary interventions.
Our results have important implications for public health policy. They suggest that interventions to prevent the onset of diabetes should be focused more on those of lower socioeconomic status (those who are poorer and who have limited access to effective medical care) than on race/ethnic minorities per se. The large proportion (85.9%) of unexplained variation indicates that other factors associated with the prevalence of diabetes remain to be identified.
Our study has both strengths and limitations. Our major strengths: 1) It employs a random community based population (with sufficient diversity in SES and race/ethnicity) and results appear to be generalizable to the US population11; 2) BACH contains valuable information on a broad range of risk factors associated with diabetes. With respect to limitations: 1) While some variables are directly measured (height and weight), others rely on self-report. However, self report of co-morbidities are well correlated with medical records16–18. 2) It should be noted that individual contributions to an R2 statistic are highly dependent upon the order in which variables are entered into the model. We felt that entering potentially modifiable risk factors first and entering variables in their order of importance was the most appropriate approach. 3) Our study does not include a number of other minority groups (e.g. Asian Americans). Unfortunately, the city of Boston does not have people of other race/ethnic groups in sufficient numbers to include them given our survey sampling design. 4) While a simple combination of education and income may not fully capture what is signified by the concept of SES, it does appear to account for much of the variation in the prevalence of diabetes. 5) This is a cross-sectional study and reported results are associations. However, as BACH is transitioning to a longitudinal study (follow-up is ongoing), we will be able to determine the incidence of newly diagnosed cases by race/ethnicity and SES.
Conclusions
We have shown that socioeconomic status is more important than race/ethnic categorizations as an indicator of who has been told that they have diabetes. There is no suggestion that our findings are entirely novel, or differ from previous work. Our results are consistent with and reinforce findings from other important studies4–10. Given the consistency of these results, it is of concern that research and interventions developed by governments aspiring to reduce worrisome disparities in diabetes continue to focus disproportionately on race/ethnicity categorizations, rather than the apparently more important socioeconomic status. We do not deny that there may be some genetic components in the prevalence of diabetes19, 20 (as family history is the second most important non-modifiable variable), but our concern is that too much attention is being focused on race/ethnicity rather than on socioeconomic circumstances. Race/ethnicity can not be changed, but socioeconomic circumstances are potentially amenable to change.
Acknowledgments
We acknowledge Lynn A. Sleeper, Sc.D., and Thomas G. Travison, Ph.D., for useful conversations concerning this manuscript.
Funding
BACH is supported by NIH, NIDDK U01 DK56842. Additional support for this manuscript was provided by the National Center on Minority Health and Health Disparities (NCMHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center on Minority Health and Disparities, or the National Institutes of Health.
References
- 1.American Diabetes Association. Diabetes risk test. [Google Scholar]
- 2.National Institute of Diabetes, Digestive, and Kidney Diseases, National Institutes of Health. Am I at risk for type 2 diabetes? [Google Scholar]
- 3.Center for Disease Control. National Diabetes Fact Sheet. [Google Scholar]
- 4.Connolly V, Unwin N, Sherriff P, Bilous R, Kelly W. Diabetes prevalence and socioeconomic status: a population based study showing increased prevalence of type 2 diabetes mellitus in deprived areas. J Epidemiol Community Health. 2000;54(3):173–177. doi: 10.1136/jech.54.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabi DM, Edwards AL, Southern DA, Svenson LW, Sargious PM, Norton P, et al. Association of socio-economic status with diabetes prevalence and utilization of diabetes care services. BMC Health Serv Res. 2006;6:124. doi: 10.1186/1472-6963-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamil H, Fakhouri M, Dallo F, Templin T, Khoury R, Fakhouri H. Disparities in Self-Reported Diabetes Mellitus among Arab, Chaldean, and Black Americans in Southeast Michigan. J Immigr Minor Health. 2007 doi: 10.1007/s10903-007-9108-0. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham J, O'Dea K, Dunbar T, Weeramanthri T, Shaw J, Zimmet P. Socioeconomic status and diabetes among urban Indigenous Australians aged 15–64 years in the DRUID study. Ethn Health. 2008;13(1):23–37. doi: 10.1080/13557850701803130. [DOI] [PubMed] [Google Scholar]
- 8.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health. 2007;97(12):2260–2267. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox M, Boyle PJ, Davey PG, Feng Z, Morris AD. Locality deprivation and Type diabetes incidence: a local test of relative inequalities. Soc Sci Med. 2007;65(9):1953–1964. doi: 10.1016/j.socscimed.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Smith JP. Nature and causes of trends in male diabetes prevalence, undiagnosed diabetes, and the socioeconomic status health gradient. Proc Natl Acad Sci U S A. 2007;104(33):13225–13231. doi: 10.1073/pnas.0611234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52(2):389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity: Executive Office of the President of the United States: Office of Management and Budget: Federal Register Notice. 1997 [Google Scholar]
- 13.Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85(9):815–827. [PMC free article] [PubMed] [Google Scholar]
- 14.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR, Snell EJ. The Analysis of Binary Data. Second ed. London: Chapman and Hall; 1989. [Google Scholar]
- 16.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147(10):969–977. doi: 10.1093/oxfordjournals.aje.a009387. [DOI] [PubMed] [Google Scholar]
- 17.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.St Sauver JL, Hagen PT, Cha SS, Bagniewski SM, Mandrekar JN, Curoe AM, et al. Agreement between patient reports of cardiovascular disease and patient medical records. Mayo Clin Proc. 2005;80(2):203–210. doi: 10.4065/80.2.203. [DOI] [PubMed] [Google Scholar]
- 19.Miyake K, Yang W, Hara K, Yasuda K, Horikawa Y, Osawa H, et al. Construction of a prediction model for type 2 diabetes mellitus in the Japanese population based on 11 genes with strong evidence of the association. J Hum Genet. 2009 doi: 10.1038/jhg.2009.17. [DOI] [PubMed] [Google Scholar]
- 20.Franceschini N, Almasy L, MacCluer JW, Goring HH, Cole SA, Diego VP, et al. Diabetes-specific genetic effects on obesity traits in American Indian populations: the Strong Heart Family Study. BMC Med Genet. 2008;9:90. doi: 10.1186/1471-2350-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]