Summary
Activated STAT3 and increased expression of the histone methyltransferase EZH2 are independently associated with the most malignant subset of gliomas. In this issue of Cancer Cell, Kim and colleagues discover that EZH2 enhances STAT3 activation by trimethylatinglysine 180 in STAT3 and does so preferentially in glioma stem-like cells.
Presuming that cancer stem cells are responsible for therapeutic resistance incancer patients, the discovery of the molecular differences that drive cancer stem cell phenotypes holds significant value for improving therapy. More valuable still would be the ability to selectively inhibit these molecular drivers of stemness. In this issue of Cancer Cell, Kim and colleagues (2013) employ cultures of glioma stem-like cells (GSCs) and their isogenic bulk tumor cells along with intracranial xenografts and come one step closer to achieving this ambitious goal.
Mutation or overexpression of enhancer of Zeste homolog 2 (EZH2) can drive the clonal expansion of leukemias and growth of solid cancers, though individual EZH2 alterations follow divergent paths towards malignancy. EZH2 is most known as an enzymatically active component of the polycomb repressive complex 2 (PRC2), which is responsible for depositing methyl groups onto lysine 27(K27) of histone H3 (H3K27). EZH2 plays an important role in maintaining stem cell function. The most common EZH2 mutations in cancer occur within the SET domain at Y641, which result in increased trimethylation activity leading to an increase in global H3K27me3 levels in B-cell lymphomas (Yap et al., 2011). In contrast, inbreast cancer cells, AKT-mediated phosphorylation of S21 reduces EZH2 interaction with histone H3 leading to a decrease in global H3K27me3 levels (Cha et al., 2005). In addition, in pediatric brain tumors, recurrent somatic mutation of K27 on histone H3 variant H3F3A may deplete H3K27me3 on canonical H3 indirectly because of increased interaction between mutant H3F3A and EZH2. However, this occurs concurrently with increased H3K27me3 colocalized with EZH2 at specific genes associated with tumorigenesis (Chan et al., 2013; Lewis et al., 2013). These and other studies suggest altered EZH2 function contributes to tumor development by promoting aberrant expression of cellular oncogenes and tumor suppressor genes, via cancer-associated changes in H3K27 methylation.
Although not appreciated until recently, EZH2 also methylates non-histone proteins. EZH2 monomethylates transcription factors GATA4 and RORα, at lysinesresulting in a reduction in their ability to activate transcription. (He et al., 2012; Lee et al., 2012). EZH2 also functions in a PRC2-independent manner as a transcriptional co-activator with androgen receptor in castration-resistant prostate cancer cells (Xu et al., 2012). This raises the important questions of whether EZH2 methylation of non-histone proteins may contribute to tumorigenesis, and how the balance of histone and non-histone methylation activityis determined.
Kim and colleagues (2013) addressed these questions by first demonstrating that EZH2, along with multiple members of the PRC2 complex, interacts with STAT3. Importantly, this interaction exists preferentially in stem cells – specifically human neural progenitor cells and GSCs but not in their differentiatedisogenic progeny, nor in established glioma cell lines grown in serum. STAT3 has a well characterized role in cancer progression and its activation in glioblastomamultiforme (GBM) is associated with the aggressive mesenchymal subtype and poor overall survival (Carro et al., 2010). The activation of STAT3 is dependent on phosphorylation of Y705 by the Janus kinases (JAKs), but STAT3 can also be dimethylated at K140 by SET9, leading to a decrease in activated STAT3 (Yang et al., 2010). As with EZH2, STAT3 also plays an important role in maintenance of GSCs. Therefore, the newly discovered interaction between EZH2 and STAT3 might underlie a shared functionin maintenance of GSC phenotypes. Inhibiting either of these proteins could potentially help eradicate the GSC pool within a GBM.
As a consequence of their interaction, EZH2 trimethylates STAT3 on K180, which Kim and colleagues found to be essential for STAT3 activation in GSCs and GBM xenografts. This is incontrast to EZH2-mediated methylation of RORα or GATA4 or SET9-mediated STAT3 K140 dimethylation, as these methyl group modifications result in a decrease in transcriptional activity of the modified protein. The exact mechanism by which trimethylation at K180 contributes to STAT3 activation and how this might synergize with Y705 phosphorylationare not yet known. Methylation at K140 on STAT3 can be removed by LSD1 (Yang et al., 2010), but it is unknown at this point which if any protein(s) might be involved with demethylation of K180. Temporal examination of the changing interaction and posttranslational modifications during differentiation of GSCs into non-stem cells could provide insight into the relative ordering of phosphorylation and methylation, the dynamics of EZH2-STAT3 interaction, and whether the loss of interaction is a driving forceor a consequence of differentiation. It is also not yet clear if the EZH2-STAT3 interaction leads to robust STAT3 K180 methylation in normal neural progenitor cells and if this is important for neural progenitor function. It should be noted that GSCs are specifically enriched and maintained by culturing in EGF, which prior studies show is a potent inducer of STAT3 phosphorylation and activation.
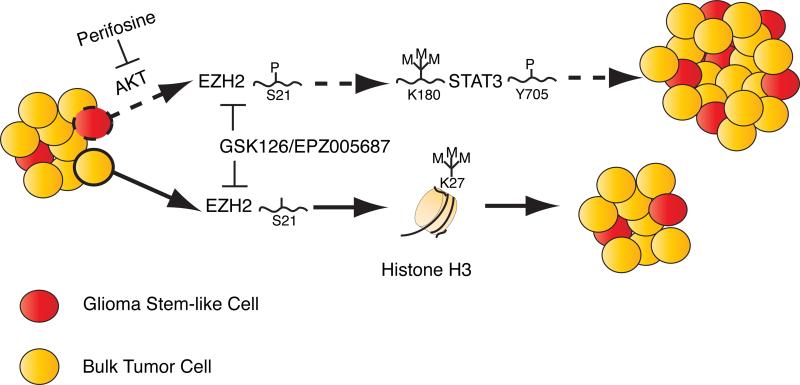
Kim and colleagues (2013) show that AKT phosphorylates EZH2 at S21 in GSCs, and as shown for breast cancer cells, may enable EZH2 to bind substrates other than H3K27. In fact, approximately 90% of primary GBMs have AKT activation. In GSCs, AKT-induced phosphorylation of EZH2 increased activation of STAT3 via K180 trimethylation and decreased survival of mice bearing xenografts of GSCs. These results underscore the importance of AKT activation in GBM and establish a pathway by which EZH2 can contribute to tumor growth independent of, or in addition to, gain or loss of H3K27me3 (Figure 1). However, in primary tumors, AKT is presumably active in both GSC and bulk tumor cell populations. Therefore, it will be important to determine why AKT-mediated S21 phosphorylation of EZH2 and subsequent K180 methylation on STAT3 occurs preferentially in GSCs.
Figure 1. pS21-EZH2 enhances STAT3 activation through K180 trimethylationin glioma stem-like cells.
In GSCs, AKT phosphorylates S21 on EZH2, switching its substrate preference from H3K27 to STAT3 and potentially other non-histone proteins. K180 trimethylation of STAT3 results in increased STAT3 activation, via increased phosphorylation of Y705 by JAKs, promoting tumor growth. In bulk tumor cells, AKT-mediated phosphorylation of EZH2 is diminished; EZH2 with unphosphorylated S21 preferentially binds and trimethylates H3K27. Inhibitors such as GSK126 or EPZ005687 selectively target the methyltransferase domain of EZH2 and, thus, can block both histone- and non-histone-mediatedroutes for GBM progression.
Selective inhibition of STAT3 has been difficult to achieve in patients with cancer. The data from this new study suggested that it might be possible to decrease STAT3 activation by inhibiting EZH2. Emerging selective small molecule inhibitors of EZH2 (GSK126, GSK343, GSK503 and EPZ005687) suppress the growth of lymphoma cell lines and xenografts that have activating EZH2 mutations. Kim et al. (2013) found that GSK126 decreased levels of phosphorylated STAT3 in GSCs. Substantial additional research is required to determine if this early experimental success will translate into notable clinical response.
The findings by Kim and colleagues (2013) are important because they establish an H3K27-independent role for EZH2 in GBM through K180 trimethylation of STAT3, which appears to be involved in the aggressiveness of high-grade glioma (Figure 1). Furthermore, the results provide a new understanding of regulatory pathways that drive GSC phenotypes. If these findings are relevant to cancer stem cells in patients, STAT3 and/or EZH2 will become even more attractive targets for improving therapeutic response and patient survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- Chan KM, Fang D, Gan H, Hashizume R, Yu C, Schroeder M, Gupta N, Mueller S, James CD, Jenkins R, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes & development. 2013;27:985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A, Shen X, Ma Q, Cao J, von Gise A, Zhou P, Wang G, Marquez VE, Orkin SH, Pu WT. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes & development. 2012;26:37–42. doi: 10.1101/gad.173930.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, et al. Cancer Cell. 2013;(this issue) [Google Scholar]
- Lee JM, Lee JS, Kim H, Kim K, Park H, Kim JY, Lee SH, Kim IS, Kim J, Lee M, et al. EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex. Molecular cell. 2012;48:572–586. doi: 10.1016/j.molcel.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Lewis PW, Muller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, Garcia BA, Muir TW, Becher OJ, Allis CD. Inhibition of PRC2 Activity by a Gain-of-Function H3 Mutation Found in Pediatric Glioblastoma. Science. 2013;340:857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, Wu X, Stack EC, Loda M, Liu T, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang J, Dasgupta M, Sears N, Miyagi M, Wang B, Chance MR, Chen X, Du Y, Wang Y, et al. Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc Natl Acad Sci U S A. 2010;107:21499–21504. doi: 10.1073/pnas.1016147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, Morin RD, Mungall AJ, Meissner B, Boyle M, et al. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood. 2011;117:2451–2459. doi: 10.1182/blood-2010-11-321208. [DOI] [PMC free article] [PubMed] [Google Scholar]