Summary
The effects of bacteria on cancer patients have been observed for at least two centuries. Recent studies in animal models of cancer have demonstrated efficacy of both anaerobic bacteria such as Clostridia and Bifidobacteria and facultative anaerobes such as Salmonella. In this issue of Cancer Discovery, Flentie et al have identified five Salmonella promoters that are specifically stimulated by cancer cells as well as by acid pH, a property of most tumors. One of these promoters (STM1787) was linked to a Shiga toxin gene and inserted in a wild-type Salmonella typhimurium strain, which showed in vivo antitumor efficacy. Approaches to further improving the efficacy of S. typhimurium with the use of tumor-targeting mutations are discussed. Since the barriers to efficacy of standard therapy of cancer appear to be opportunities for bacterial cancer therapy, the future of bacterial therapy of cancer appears bright.
“…for over 200 years neoplasms have been observed to regress following acute infections, principally streptococcal. If these cases were not too far advanced and the infections were of sufficient severity or duration, the tumors completely disappeared and the patients remained free from recurrence” (1).
Helen Coley Nauts, author of the quote above, was the daughter of William B. Coley, who was an oncologist at what is now known as Sloan-Kettering Memorial Cancer Center in New York in the late 19th and early 20th centuries, Coley infected cancer patients with Streptococcus pyrogenes and in later years treated the patients with extracts of the bacteria, which became known as Coley's toxins. Coley had remarkable results with his toxins, but this treatment fell out of favor after Coley's death in 1936 (2).
Recently, there has been intense interest to develop bacterial therapy of cancer using modern methods of bacterial genetics, cancer cell and molecular biology, and in vivo imaging (2, 3). The barriers in tumors for standard therapy to be effective such as hypoxia, acidic pH, disorganized vascular architecture, and dissemination can be opportunities for bacteria to target cancer (3).
In the current issue of Cancer Discovery, Flentie et al. (4) designed a promoterless transposon luciferase reporter to analyze a library containing 7,400 independent Salmonella transposon insertion mutants and identified five Salmonella promoter specifically activated by cancer cells, as well as by acidic pH often found in tumors.
Flentie et al. (4) utilized the most pH-sensitive promoter (STM1787) they identified, to demonstrate the utility of tumor-specific Salmonella promoters to drive the Shiga toxin-2 (Stx-2) transgene in a wild-type strain of S. typhimurium. Salmonella expressing Stx-2 from the STM1787 promoter had anti-tumor activity in vivo as well as in vitro. Intratumoral injection of a single high-dose of the wild type S. typhimurium strain SB300A1, transformed with P1787-Stx2, resulted in an 80% inhibition of tumor growth five days after treatment. Mice treated with high-dose SB300A1-P1787-Stx2 died, but mice receiving low-dose P1787-Stx2 were healthy for two weeks, at which point they had a significant reduction in tumor size compared to control.
Other approaches to bacterial therapy of cancer have used the anaerobic bacteria, Bifodobacterium and Clostridium, which replicate in necrotic areas of tumors. These anaerobic bacteria cannot grow in viable tumor tissue which restricts their efficacy. In order for anaerobic bacteria to be effective, they must be used in combination with chemotherapy (3).
S. typhimurium, which is a facultative anaerobe, attenuated with purine and other auxotrophic mutations, has been previously used for cancer therapy (5). S. typhimurium with lipid-A-modified (msbB) and purine auxotrophs (purI) did not have toxicity in mice and swine and also had significantly reduced host TNF-a induction (5). In a Phase I clinical trial on metastatic melanoma patients, the S. typhimurium strain tested (VNP20009) was attenuated by msbB and purI mutations. VNP20009 was safely administered to patients, but poorly colonized the patients' tumors, perhaps because it is over-attenuated (6).
A new strain of S. typhimurium, A1-R, has been developed which has greatly increased antitumor efficacy. S. typhimurium A1-R is auxotrophic for leu-arg which prevents it from mounting a continuous infection in normal tissues. A1-R has no other attenuating mutations as does VNP20009, and therefore, has very high tumor-targeting capability. A1-R was able to eradicate primary and metastatic tumors in monotherapy in nude mouse models of prostate, breast, and pancreatic cancer, as well as sarcoma and glioma (7-13). Tumors with a high degree of vascularity were more sensitive to A1-R and vascular destruction appears to play a role in A1-R antitumor efficacy (14).
A random library of Salmonella enterica typhimurium 14028 genomic DNA was previously cloned upstream of a promoterless gene encoding the green fluorescent protein (GFP). A population of Salmonella containing this library was injected i.v. into tumor-free nude mice and into human PC-3 prostate tumors growing subcutaneously in nude mice. Fluorescence-activated cell sorting was used to enrich for bacterial clones expressing GFP from spleens or tumors. Three candidate tumor-specific promoter clones were individually tested in vivo, using GFP imaging. Two of the three clones (pflE and ansB promoters) were induced in hypoxic conditions found in tumors (15).
The relative fitness of 41,000 Salmonella transposon insertion mutants growing in mouse models of human prostate and breast cancer was also previously tested. Two classes of potentially non-toxic mutants were identified. Class 1 mutants showed reduced fitness in normal tissues and unchanged fitness in tumors. Class 2 mutants showed reduced fitness in tumors and normal tissues. A class 1 mutant (STM3120) effectively targeted tumors after intragastric delivery, suggesting an oral route as an option for bacterial cancer therapy (16). A similar finding of effective oral delivery of S. typhimurium for cancer therapy was recently made by Jia and coworkers (17).
Although Coley's toxins and bacteria themselves may act as immune stimulators, the experiments of Flentie et al (4), and other experiments described above, demonstrate that bacteria such as S. typhimurium directly attack and kill tumors. Tumor-targeting bacteria need to be attenuated to be non-toxic, but not over-attenuated in order not to reduce antitumor efficacy.
Further development of the technology described by Flentie et al. (4) and Arrach et al. (15) are also possible. For example, combinations of two or more promoters that are preferentially induced in tumors by different regulatory mechanisms would allow the delivery of two or more toxins, possibly sequentially. Using highly-selective tumor-targeting bacteria such as S. typhimurium A1-R, inducible Salmonella promters could be combined with tumor-specific Salmonella promoters for controlled expression and greater efficacy.
In addition, use of GFP for imaging the bacteria offers advantages of real-time visualization of single bacteria in vivo (18) which could lead to selection of enhanced cancer cell-targeting variants of S. typhimurium. For example, dual-color labeling of the cancer cells with GFP in the nucleus and red fluorescent protein (RFP) in the cytoplasm, allows simultaneous imaging of intracellularly-infecting GFP-expressing bacteria and apoptotic behavior of the infected cancer cells (Figure 1).
Figure 1.
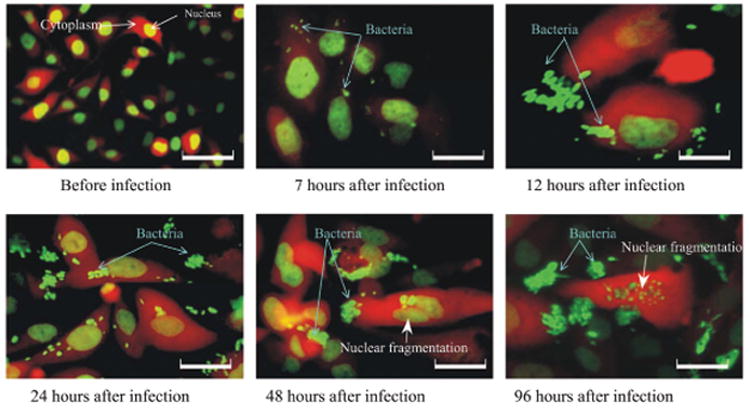
Intracellular growth of S. typhimurium A1. Time course of GFP-labeled S. typhimurium A1 growing in GFP–RFP-labeled PC-3 human prostate cells in vitro. PC-3 human prostate tumor cells were labeled with RFP in the cytoplasm and GFP in the nucleus by means of a fusion with histone H2B. Interaction between bacteria and tumor cells was observed at the indicated time points under fluorescence microscopy magnification. (Bar: 156 μm for Upper Left; otherwise, Bar: 78 μm) (7).
That tumor characteristics which are barriers to standard therapy, are facilitators of bacterial therapy, demonstrate that “bugging tumors” has great promise for treatment of cancer.
Acknowledgments
Experiments in the author's laboratory on bacterial treatment of cancer are supported in part by NCI grant CA126023.
References
- 1.Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley's toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley's mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl. 1953;145:1–102. [PubMed] [Google Scholar]
- 2.Hoffman RM. The preclinical discovery of bacterial therapy for the treatment of metastatic cancer with unique advantages. Expert Opinion on Drug Discovery. 2012;7:73–83. doi: 10.1517/17460441.2012.644534. [DOI] [PubMed] [Google Scholar]
- 3.Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nature Reviews Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flentie K, Kocher B, Gammon ST, Novack DV, McKinney JS, Piwnica-Worms D. A bioluminescent transposon reporter-trap identifies tumor-specific microenvironment-induced promoters in Salmonella for conditional bacterial-based tumor therapy. Cancer Discovery. doi: 10.1158/2159-8290.CD-11-0201. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncology. 2003;4:548–556. doi: 10.1016/s1470-2045(03)01194-x. [DOI] [PubMed] [Google Scholar]
- 6.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, Yang M, Li X-M, Jiang P, Li S, Xu M, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Research. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–1878. [PubMed] [Google Scholar]
- 11.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- 12.Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, et al. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res. 2010;164:248–255. doi: 10.1016/j.jss.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, et al. Targeted therapy of spinal cord glioma with a genetically-modified Salmonella typhimurium. Cell Proliferation. 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle. 2010;9:4518–4524. doi: 10.4161/cc.9.22.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrach N, Zhao M, Porwollik S, Hoffman RM, McClelland M. Salmonella promoters preferentially activated inside tumors. Cancer Res. 2008;68:4827–4832. doi: 10.1158/0008-5472.CAN-08-0552. [DOI] [PubMed] [Google Scholar]
- 16.Arrach N, Cheng P, Zhao M, Santiviago CA, Hoffman RM, McClelland M. High-throughput screening for Salmonella avirulent mutants that retain targeting of solid tumors. Cancer Res. 2010;70:2165–2170. doi: 10.1158/0008-5472.CAN-09-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia LJ, Wei DP, Sun QM, Huang Y, Wu Q, Hua ZC. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci. 2007;98:1107–1112. doi: 10.1111/j.1349-7006.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman RM, Zhao M. Whole-body imaging of bacterial infection and antibiotic response. Nature Protocols. 2006;1:2988–2994. doi: 10.1038/nprot.2006.376. [DOI] [PubMed] [Google Scholar]


