Abstract
Objectives:
Serum 25-hydroxyvitamin D [25(OH)D] concentrations reflect vitamin D status, with deficiency implicated as causative of many diseases. This study assessed vitamin D status and anthropometric indices in a sample of healthy Omanis.
Methods:
Serum 25(OH)D concentrations were measured by high performance liquid chromatography in 206 healthy Omanis, aged 18–55 years (mean age: men 31.1, women 26.8) in Muscat, Oman. Of this number, 95% indicated that they had never taken vitamin D supplements. Findings were compared with published values for populations domiciled in more northerly latitudes. Classical procedures were used to determine global obesity (body mass index [BMI]), and central obesity determined by waist circumference, waist-to-hip ratio (WHR), and waist-to-height ratio.
Results:
Women, as compared to men, had markedly lower concentrations of 25(OH)D. Applying the cut-off point of serum 25(OH)D levels at 50 nmol/L, the prevalence of vitamin D deficiency in the study population was 87.5%; this was higher than the rates reported for the British, and European-, Hispanic-, and African-Americans. At a BMI cut-point of ≥30 kg/m2, the prevalence of obesity was 14.6%; this was lower than the rates reported for European-, Hispanic-, and African-Americans. Levels of 25(OH) D increased relative to age and obesity. WHR was the main predictor of 25(OH)D levels.
Conclusion:
The striking vitamin D deficiency seen in the study population, relative to more northerly populations, may be linked to sun avoidance, inadequate dietary vitamin D, and virtual non-intake of supplemental vitamin D. Age and male-gender determined the status of vitamin D and of obesity.
Keywords: Vitamin D, 25-hydroxyvitamin D, High-performance liquid chromatography, Anthropometric indices, Oman
Advances in knowledge
- The prevalence of vitamin D deficiency in this Omani population was high at 87.5%.
- Vitamin D provided by foods and supplements is inadequate compared to the amount generated by skin exposure to sunlight.
- Vitamin D3 is more effective than vitamin D2 at raising and maintaining the vitamin D blood value.
- Waist circumference, waist-to-hip ratio, and waist-to-height ratio are considered better determiners of the risk of cardiovascular disease than body mass index.
Applications to Patient Care
- The current upper safe limits set by the Institute of Medicine for vitamin D consumption for infants are 1,000 IU/day and 2,000 IU/day for children and adults. The current estimate is that the upper limit could be increased to 10,000 IU/ day. For repletion, intakes above the upper limit may, however, be required and necessary.
- The public should be encouraged to be proactive in protecting their health and have their vitamin D blood levels tested annually.
- Women should be aware of their increased risk of vitamin D deficiency and postmenopausal osteoporosis.
Vitamin D consists of D2 (ergocalciferol) and D3 (cholecalciferol), the former is naturally synthesised from ergosterol by invertebrate fungi in response to ultraviolet B (UVB [280–315 nm]) irradiation, and the latter’s synthesis is exclusively initiated in the skin of vertebrates also in response to the UVB irradiation. During sunlight exposure, UVB photons are absorbed by cutaneous 7-dehydrocholesterol, yielding pre-cholecalciferol, which rapidly rearranges to vitamin D3.1 Vitamin D3 synthesis reaches an equilibrium after several minutes, depending on numerous factors including conditions of sunlight (latitude, season, cloud cover, altitude), age, and skin pigmentation.2 Vitamin D2 and D3 are also commercially synthesised and are used in fortified food and supplements. Endogenously-produced vitamin D enters peripheral circulation where it binds to vitamin D-binding protein for transport to the liver. Dietary vitamin D enters the peripheral circulation through the lymph bound to chylomicron remnants and is transported to the liver. Both forms of vitamin D undergo hydroxylation in the liver on C-25 by the vitamin D-25-hydroxylase to 25-hydroxyvitamin D [25(OH)D]. Further hydroxylation of 25(OH) D occurs in the kidneys by 25-hydroxyvitamin D-1-α-hydroxylase to 1,25-dihydroxyvitamin D [1,25(OH)2D], the biologically-active form of vitamin D.3 This enzyme is also present in a variety of extra-renal sites, including osteoclasts, and the skin, colon, brain and macrophages, which may be the cause of its broad-ranging effects.3 Levels of 25(OH)D largely reflect the amount of vitamin D3 produced in the skin, or the vitamin D2 or D3 ingested.4 Research has shown that 25(OH)D is also the major circulating form of vitamin D that is measured to determine a person’s vitamin D status because it has a half-life in circulation of 3 weeks and correlates with rickets, osteomalacia, and secondary hyperparathyroidism.5 Vitamin D deficiency is implicated as a cause of osteoporosis, cancer, cardiovascular disease (CVD), diabetes mellitus, and multiple sclerosis (MS), among other conditions.6–9
Vitamin D deficiency has been linked to obesity and central adiposity.10 Body mass index (BMI), an indicator of relative weight for height, is frequently used to assess excess body fat, but does not adequately discriminate between body fat and lean body mass.11,12 Visceral adiposity, or intra-abdominal obesity, reflects central body fat distribution and has been suggested as a better marker of obesity risk.11–14 Anthropometric indices used as a substitute for evaluation of central body fat distribution are waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR).11–14
This baseline study aimed to measure serum concentrations of 25(OH)D to assess vitamin D status, and consequently to determine anthropometric indices to assess global obesity and central adiposity in a study population of healthy Omani men and women.
Methods
Oman is situated in the northern hemisphere, 21° north of the equator. The study was carried out from November to March 2010, when the average temperature is 25° C and skies are mostly clear and sunny. The study population, 206 healthy Omani volunteers aged 18–55 years (105 women [mean = 26.8 years] and 101 men [mean = 31.1 years]) was composed of university students, educators, administrators, office secretaries, and their relatives. They completed questionnaires about demographics (age, gender, educational attainment, social status) and their intake of natural and fortified vitamin D-containing foods and vitamin D supplements. The exclusion criteria were pregnancy and medications that raise blood levels, impact absorption, activity, and the activation of vitamin D. Enrolment was voluntary, and participants signed a consent form approved by the Sultan Qaboos University (SQU) Research Review Board’s Ethics Committee (#EC11) for the protection of human subjects in research.
For anthropometric measurements, the participants wore light outfits and the means of triplicate measurements of height, weight, hip circumference (HC) and waist circumference (WC) were recorded. Height was measured with a wall-mounted stadiometer and recorded to the nearest millimetre. Weight was measured with a balance beam scale with two sliding weights and recorded to the nearest 0.1 Kg.
BMI was obtained by dividing the weight by the square of the height. For hip circumference (HC), the participants stood erect with feet together. HC was then measured to the nearest 0.1 cm as the maximal circumference over the buttocks, with the tape position horizontal all around the body. WC was measured at a level midway between the lower rib margin and the iliac crest, with the measuring tape all around the body in a horizontal position and recorded to the nearest millimetre. WHR and WHtR were calculated and recorded as the ratio of the WC to the HC, and the ratio of WC to height, respectively. A BMI ≥30 kg/m2 was used as the cutoff point for determining global obesity in both genders. For central adiposity, the cut-off points were WC ≥94 cm in men and ≥80 cm for women, and a WHR ≥0.90 in men and ≥0.85 in women.14 In both genders, a general cut-off point of ≥0.5 for WHtR was used.12
The samples were taken as follows: venous blood samples were withdrawn into evacuated plain tubes and kept in the dark to clot. They were centrifuged and sera were separated into 1.0 ml capacity Nalgene® cryogenic vials (Nalge Nunc International, Rochester, New York, USA), capped, wrapped in aluminum foil to protect from light, and stored at −80° C, pending assay for 25(OH)D concentrations within a month.
The serum concentrations were measure by high-performance liquid chromatography (HPLC). The method used in the current study was modified from the method of Turpeinen et al.15 Duplicate samples that had been frozen only once were measured. Briefly, 500 μL of serum or Chromsystems 25(OH)-D3/D2 controls levels I and II (Chromsystems Instruments and Chemicals, GmbH, München, Germany) were added to 350 μL of methanol-2-propanol (80:20 by volume) to precipitate protein. The tubes were mixed in a multi-tube vortex mixer for 30 seconds and 25(OH) D was extracted with 2 ml hexane after being mixed 3 times for 60 seconds each time. The phases were separated by centrifugation, and the upper hexane phase was transferred to a glass tube and dried under nitrogen at 40º C. The residue was dissolved in 100 μL of mobile phase (760 ml/L methanol in water), and dispensed in light-protected auto-sampler vials for injection of 25 μL volume. Calibration curves were constructed using 4 concentrations of 25(OH)-D3 and 25(OH)-D2 (15–120 nmol/L) prepared in human serum albumin (50 g/L). Shimadzu HPLC Class VP system with isocratic elution and separation of the vitamin D metabolites was performed on a LiChrospher 60 reversed-phase select B column (4 mm I.D. x 250 mm long; 5 μm bead size) of ∼25º C at a flow rate of 0.7 mL/min. The vitamin D metabolites photodiode array detection was at 265 nm with a sensitivity of 6 nmol/L and linearity 1,250 nmol/L. Chromsystems controls were used for internal quality assessment. Intraand inter-assay coefficients of variation (CV) (%) for 25(OH)-D2 level I were 3.8 and 4.0, respectively, and for 25(OH)-D3, 3.4 and 4.0, respectively. Intraand inter-assay CV (%) for 25(OH)-D2 level II were 7.8 and 9.9, respectively, and for 25(OH)-D3 10.2 and 11.4, respectively. The method was monitored by participation in the Vitamin D External Quality Assessment Scheme (DEQAS, UK).
Serum creatinine concentrations were measured by an enzymatic assay on a Roche Cobas c 111 System (Roche Diagnostics Ltd., Rotkreuz, Switzerland). The intra- and inter-assay CV (%) for Biorad level I were 1.9 and 2.3, respectively; level II were 0.7 and 1.1, respectively, and level III were 1.2 and 1.5, respectively.
Statistical analyses were done as follows: the Gaussian distribution of all data sets was confirmed by the one-sample Kolmogorov-Smirnoff test and by histogram plots, followed by presenting all data as mean ± standard deviation (SD). An independent sample t-test was used to differentiate between two mean groups. Multiple comparison analyses were performed using Scheffe post hoc tests to determine differences between the mean of 3 or more groups. The association between 25(OH)D concentrations and anthropometric measurements were assessed by Pearson correlation. Stepwise linear regression was used to determine which anthropometric index was the main predictor of serum 25(OH)D levels. Probability values were two-tailed and P <0.05 was considered significant. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), Version 17.0 (IBM, Inc., Chicago, Illinois, USA).
Results
Table 1 shows the participants’ responses to the questionnaire items on their intake of dietary and supplemental vitamin D. In decreasing order of intake were fish (51%), cheese (43.2%), laban (a salted yoghurt and water drink) (36.9%), milk (8.3%), and vitamin D supplements (5.3%). Data comparison by gender for age, weight, anthropometric indices, 25(OH)D, and creatinine concentrations are detailed in Table 2. All the values were significantly higher in men than in women.
Table 1:
Participants’ responses to the questionnaire on intake of dietary and supplemental vitamin D
| Diet/Supplement | Positive Response (%) |
|---|---|
| Fish* | 51 |
| Milk | 8.3 |
| Laban** | 36.9 |
| Cheese | 43.2 |
| Yoghurt | 17.5 |
| Vitamin supplement | 5.3 |
Mainly tuna, sardines and other varieties of fish (no salmon, mackerel, or herring) were consumed;
Laban is a drink produced by mixing an equal quantity of yogurt and water and adding salt to taste.
Table 2:
Data comparison (mean ± standard deviation) of various parameters by gender
| Parameters | Total (n = 206) | Men (n = 101) | Women (n = 105) | P value |
|---|---|---|---|---|
| Age (years) | 28.8 ± 8.9 | 31.1 ± 8.6 | 26.8 ± 8.6 | 0.001 |
| Weight (Kg) | 68.1 ± 16.5 | 71.8 ± 16.5 | 64.8 ± 16.0 | 0.003 |
| 25(OH)D (nmol/L) | 32.8 ± 15.4 | 36.8 ± 16.7 | 28.2 ± 12.6 | <0.0001 |
| WC (cm) | 84.8 ± 15.2 | 94.7 ± 12.4 | 75.9 ± 11.1 | <0.0001 |
| WHR | 0.846 ± 0.1 | 0.914 ± 0.069 | 0.782 ± 0.076 | <0.0001 |
| WHtR | 0.514 ± .084 | 0.550 ± 0.074 | 0.481 ± 0.079 | <0.0001 |
| BMI (kg/m2) | 24.9 ± 4.8 | 26.6 ± 4.8 | 23.5 ± 4.7 | <0.0001 |
| Creatinine (μmol/L) | 61.1 ± 13.5 | 72.2 ± 0.9 | 50.6 ± 0.7 | <0.0001 |
Statistical significance was considered at P <0.05; 25(OH)D = serum 25-hydroxyvitamin D; WC = waist circumference; WHR = waist to hip ratio; WHtR = waist to height ratio; BMI = body mass index.
The prevalence of vitamin D deficiency, global obesity, and central adiposity calculated from the respective cut-off point values are represented in Table 3. There was a higher percentage of low concentrations of 25(OH)D in women than in men at the 3 cut-off point values of <25, <50, and <75 nmol/L. Global obesity at the BMI cut-off point value of ≥30 kg/m2 was more prevalent in men than in women. The prevalence of central adiposity at cut-off point values of a WC >102 in males (M) and >88 in females (F); a WHR >0.9 M, >0.88 F, and a WHtR >0.5 M/F were all significantly higher in men than in women.
Table 3:
Prevalence rates at cutoff points for vitamin D deficiency, global obesity, and central adiposity
| Parameter | Cut-off point | Men n=101 % | Women n=105 % | Total N=206 % |
|---|---|---|---|---|
| 25(OH)D (nmol/L) | <25 | 23.8 | 52.4 | 39.0 |
| ≤50 | 81.6 | 93.1 | 87.5 | |
| ≤75 | 97.0 | 100 | 98.5 | |
| BMI (kg/m2) | ≥30 | 19.4 | 9.8 | 14.6 |
| WC (cm) | >102 M >88 F |
24.5 | 13.7 | 19.1 |
| WHR | >0.9 M >0.88 F |
53.1 | 10.8 | 31.6 |
| WHtR | >0.5 M/F | 75.5 | 26.5 | 50.1 |
25(OH)D = serum 25-hydroxyvitamin D; BMI = body mass index; WC = waist circumference; WHR = waist to hip ratio; WHtR = waist to height ratio.
Serum concentrations of 25(OH)D, performed in winter at different latitudes, are presented in Table 4. The Omani study population had lower 25(OH)D values relative to values for studies done in more northerly latitudes.
Table 4:
Reported serum mean serum 25-hydroxyvitamin D (nmol/L) performed at different latitudes
| Location | n | Latitude | Gender | 25(OH)D |
|---|---|---|---|---|
| Oman* | 101 | 21° N | M | 36.8 |
| Oman* | 105 | 21° N | F | 28.2 |
| Oman* | 206 | 21° N | M, F | 32.5 |
| Miami16 | 77 | 26° N | M | 62.3 |
| Miami16 | 135 | 26° N | F | 56.0 |
| Boston17 | 90 | 42° N | F | 60.0 |
| Calgary18 | 188 | 51° N | M, F | 57.3 |
| Britain19 | 7437 | 50–60° N | M, F | 41.1 |
| Denmark20 | 25 | 56° N | M, F | 56.4 |
| Norway21 | 309 | 68° N | F | 49.5 |
* = this study; 25(OH)D = serum 25-hydroxyvitamin D; F = female; M = male.
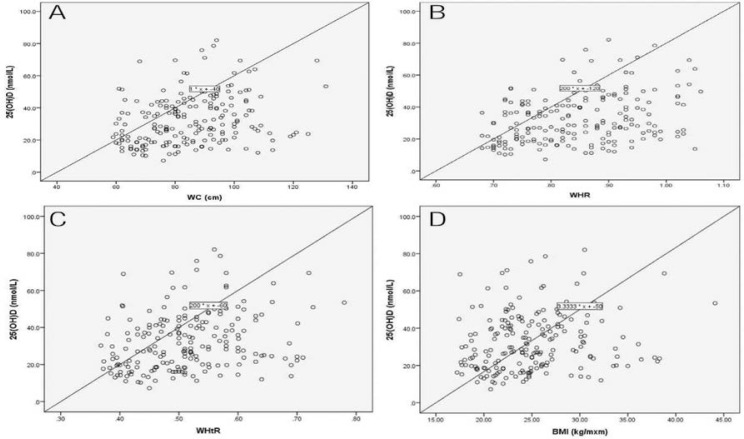
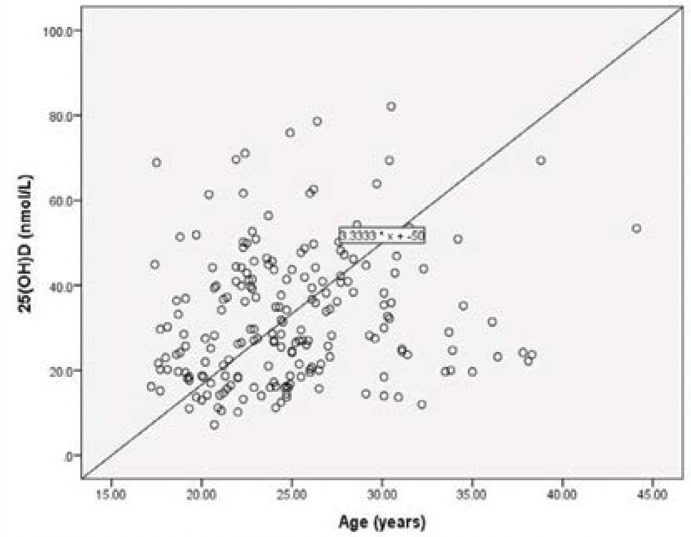
The scatter plot [Figure 1] shows that serum concentrations of 25(OH)D were associated moderately (P = 0.023) with BMI and markedly (P <0.0001) with WC, WHR, and WHtR. Stepwise linear regression analysis revealed WHR as the main predictor (r = 0.336; P <0.0001) of serum 25(OH)D concentrations. A scatter plot of age versus serum concentrations of 25(OH)D [Figure 2] shows the two parameters were strongly correlated.
Figure 1:
Relationship between serum 25-hydroxyvitamin D and anthropometric indices (A, B, C, D).
Figure 2:
Relationship between serum 25-hydroxyvitamin D and age.
Discussion
Research suggests that vitamin D3, because it binds more tightly than D2 to vitamin D receptors, has a longer half-life and is more potent in raising and maintaining vitamin D blood levels.22 According to responses to the questionnaire [Table 1], the Omani study subjects ate a considerable amount of fish, including tuna and sardines, but not the types richer in vitamin D3 such as salmon, herring, and mackerel.23,24 It is likely that because of lactose intolerance the intake of milk was much lower relative to the intake of laban. Like milk, most laban drinks are fortified with vitamin D. A very small percentage of the participants indicated that they took vitamin D supplements. At the time of this study, vitamin D2/D3 capsules of the recommended daily allowance (RDA) of 200 IU were sold over-the-counter in Oman. An RDA of ≥400 IU is more effective in boosting plasma levels of 25(OH)D3.22
The current study utilised HPLC to measure serum concentrations of 25(OH)D because the method isolates and quantifies the D2 and D3 metabolites when present in serum. For the purpose of this study, total 25(OH)D was reported. A comparative study of methods used to measure serum 25(OH)D found HPLC values closest to the reference liquid chromatography/tandem mass spectrometry (LC-MS/MS) defined target value.25
The mean serum 25(OH)D value (36.8 nmol/L) obtained for the Omani study subjects [Table 2] was lower than the mean (54.0 nmol/L) reported by a global meta-analysis of 394 studies.26 The mean serum 25(OH)D concentration (28.2 nmol/L) measured in the Omani women was lower compared to the mean values (56.0, 60.0, and 49.5 nmol/L, respectively) reported for women in Miami, Boston, and Norway [Table 4].16,17,21 The mean serum creatinine values of the study population was normal, indicating unimpaired renal synthesis of 1,25(OH)2D.
The current study determined the prevalence of vitamin D deficiency in the Omani study population by applying 3 recommended cut-off point values of serum 25(OH)D. The first cut-off point was ≥25 nmol/L which is considered sufficient to prevent the severe hypovitaminosis D that leads to a softening of bone tissue, manifesting as rickets in children and osteomalacia in adults.27 The second is that an absolute minimum 25(OH)D of 50 nmol/L is necessary to support and maintain all the actions of vitamin D on bone and mineral health.3 The third is that newer data showing associations of vitamin D status and the prevalence of several diseases such as CVD, hypertension, colon and breast cancers, and MS, as well as involvement of vitamin D in muscle strength and immune functions, indicate that the target levels of 25(OH)D should be 75–100 nmol/L at the minimum.3 The prevalence of vitamin D deficiency at serum 25(OH)D at <25, <50, and <75 nmol/L were 39.0%, 87.5%, and 98.5% in the overall study sample; 23.8%, 81.6%, and 97% in the men, and 52.4%, 93.1%, and 100% in the women, respectively [Table 3]. These values are higher than the prevalence of 15.5%, 46.6%, and 87.1% reported by a British study.19
The observed vitamin D deficiency in the Omani study sample could be the consequence of sun avoidance habits, inadequate intake of dietary vitamin D, and the virtual absence of supplemental vitamin D tablets. The strikingly lower levels of 25(OH)D observed in the Omani women could be attributed to the mentioned consequences and further compounded by the use of sunscreen and dressing in clothing that allows exposure of only the face. There were no veiled females in this study. Clothing impedes cutaneous vitamin D3 photosynthesis; generally, the higher the thread per square inch, the more light attenuation produced.28 The higher the sun protection factor of sunscreen, the more blockage of solar UVB cutaneous synthesis of vitamin D3.23
The current study evaluated anthropometric indices, noting that the men were heavier and had higher values of these indices than the women [Table 2]. BMI is commonly used as an indicator for generalised or global obesity.29 The prevalence of global obesity at a BMI cut-off point of ≥30 kg/m2 was 14.6% in this study population [Table 3]; this was lower than the reported prevalence for whites (23.7%), Hispanics (28.7%), and blacks (35.7%) in America.29 The indices of abdominal obesity (WC, WHR, and WHtR) are considered to be better determiners of the risk of CVD than BMI.12 The cut-off points used to determine the prevalence of central obesity in the Omani study sample were WC >102 cm for men and >88 cm for women, a WHR >0.9 for men and >0.85 for women, and a general cut-off point of >0.5 for WHtR.12,14 Central obesity synonymous with visceral adiposity or abdominal obesity (abnormal WC, WHR, and WHtR) was much more prevalent in men than in women [Table 3]. Visceral fat is located in the peritoneal cavity and is composed of adipose depots including mesenteric, epididymal white adipose tissue, and perirenal fat. Excess visceral fat is a lipid overflow ensuing from a defect of adipose tissue to clear and store the excess triacylglycerols from over-eating and a lack of physical activity.12
The current study further observed that central adiposity indices and not BMI were strongly associated with serum 25(OH)D concentrations [Figure 1], a finding inconsistent with previous reports that serum levels of 25(OH)D were inversely associated with WC and visceral adipose tissue.10,29,30 The discrepancy between the current study and those studies may be linked to the size, age, and health status of the different study populations. The current Omani study population was small, apparently healthy, and relatively young (≤55 years), which may not be the case for the populations of the previous studies.10,29,30 Among the anthropometric indices evaluated in the current study, WHR emerged by stepwise linear regression as the main predictor of serum 25(OH)D levels.
There was an age-related increase of serum levels of 25(OH)D in the current study [Figure 2], which was in accord with the global study’s findings.26 However, this study’s finding did not conform with suggestions that cutaneous vitamin D synthesis diminished with age (≥65 years), possibly because the Omani study population was relatively young (≤55 years).4,27
As this study was done in the winter months of November to March, it was deemed fitting to compare serum 25(OH)D values of the study population domicile in Oman (21° N) with summer values of 25(OH)D for populations dwelling in more northerly latitudes such as Miami (26° N) and Boston (42° N) in the USA, Calgary (51° N) in Canada, Great Britain (50–60° N), Denmark (56° N), and Norway (68° N), which experience summers similar to the Omani winter.16–21 An Omani winter is almost always cloudless and sunny with an average ambient temperature of 25° C. Apart from Miami, the other cities at the more northerly latitudes have more cloud cover, which impedes UVB cutaneous penetration for vitamin D3 synthesis. Most UVB photons from the sun are absorbed by stratospheric ozone. An increase in the sun’s zenith angle results in an increased path length for the UVB photons to travel, and this explains why at higher latitudes (>∼35° N), very little, if any, vitamin D3 is produced in the skin from November through March.2
Ironically, the mean serum 25(OH)D values of this study population of Omanis were lower than values reported for the populations domiciled in upper latitudes [Table 4]. A possible explanation is that those populations were more likely to consume diets richer in vitamin D3 as well as regularly take supplemental vitamin D3. Also, the dress habits of populations in more northerly latitudes allow more skin exposure to sunlight than those of Omanis. Overall, serum concentrations of 25(OH)D reported both for those from more northerly latitudes and Omanis were deficient relative to the recommended cut-off point optimum of ≥75 nmol/L.3
Conclusion
In conclusion, the findings of this study are of great importance in, for example, later development of postmenopausal osteoporosis; particularly, the low level of vitamin D3 in women is worthy of attention. Furthermore, correction of low plasma 25(OH)D concentrations can be attained only if people increase the safe, moderate exposure of skin to ultraviolet light; suitable increases in food fortification with vitamin D3, and the provision of higher prescribed amounts of vitamin D3 in supplements.
References
- 1.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 2.Webb AR. Who, what, where and when - influences on cutaneous vitamin D synthesis. Prog Biophys Molec Biol. 2006;92:17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP, et al. 14th Vitamin D Workshop: Consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2010;121:4–6. doi: 10.1016/j.jsbmb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 4.DeLucia HF. Overview of physiological features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, et al. Prevention of non-vertebral fractures with oral vitamin D dose dependency. A meta-analysis of randomized control trials. Arch Intern Med. 2009;169:551–61. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 7.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 8.Baz-Hecht M, Goldfine AB. The impact of vitamin D deficiency on diabetes and cardiovascular risk. Curr Opin Endocrinol Diabetes Obes. 2010;17:113–9. doi: 10.1097/MED.0b013e3283372859. [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 10.Young KA, Engelman CD, Langfeld CD, Hairston KG, Haffner SM, Bryer-Ash M, et al. Association of plasma vitamin D levels with adiposity in Hispanics and African Americans. Clin Endocrinol Metab. 2009;94:3306–13. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: Elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–60. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 12.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminator of cardiovascular risk factor than BMI: A meta-analysis. J Clin Epidemiol. 2008;61:646–53. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, John U, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Endocrinol Metab. 2010;95:1777–85. doi: 10.1210/jc.2009-1584. [DOI] [PubMed] [Google Scholar]
- 14.Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–7. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 15.Turpeinen U, Hohenthal U, Stenman U-H. Determination of 25-hydroxyvitamin D in serum by HPLC and immunoassay. Clin Chem. 2003;49:1521–34. doi: 10.1373/49.9.1521. [DOI] [PubMed] [Google Scholar]
- 16.Levis S, Gomez A, Jimenez C, Vevas L, Ma F, Lai S, et al. Vitamin D deficiency and seasonal variation in an adult south Florida population. J Clin Endocrinol Metab. 2005;90:1557–62. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 17.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67:1232–6. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 18.Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insufficiency in a population of healthy western Canadians. Can Med Assoc J. 2002;166:1517–24. [PMC free article] [PubMed] [Google Scholar]
- 19.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 years: Nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 20.Thieden E, Philipsen PA, Heydenreich J, Wulf HC. Vitamin D level in summer and winter related to measured UVR exposure and behavior. Photochem Photobiol. 2009;85:1480–4. doi: 10.1111/j.1751-1097.2009.00612.x. [DOI] [PubMed] [Google Scholar]
- 21.Brustad M, Alsaker E, Engelsen O, Aksenes L, Lund E. Vitamin D status of middle-aged women at 65–71° N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2003;7:327–35. doi: 10.1079/PHN2003536. [DOI] [PubMed] [Google Scholar]
- 22.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab. 2011;96:E447–52. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 23.Wolpowitz D, Gilchrest BA. The vitamin D questions: How much do you need and how should you get it? J Am Acad Dermatol. 2006;54:301–17. doi: 10.1016/j.jaad.2005.11.1057. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health (NIH), Office of Dietary Supplements Dietary supplement fact sheet: Vitamin D. From: http://ods.od.nih.gov/factsheets/vitamind.asp. Accessed: Jun 2008.
- 25.Roth HG, Schmidt-Gayk H, Weber H, Nederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–9. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 26.Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, et al. Global vitamin D level in relation to age, gender, skin pigmentation and latitude: an ecological meta-regression analysis. Osteoporosis Int. 2009;20:133–40. doi: 10.1007/s00198-008-0626-y. [DOI] [PubMed] [Google Scholar]
- 27.Souberbielle JC, Friedlander G, Kahan A, Cormier C. Evaluating vitamin D status. Implication for preventing and managing osteoporosis and other chronic diseases. Joint Bone Spine. 2006;73:249–53. doi: 10.1016/j.jbspin.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Salih FM. Effect of clothing varieties on solar photosynthesis of pre-vitamin D3. Photodermatol Photoimmunol Photomed. 2004;20:53–8. doi: 10.1111/j.1600-0781.2004.00068.x. [DOI] [PubMed] [Google Scholar]
- 29.Pan L, Galuska DA, Sherry B, Hunter AS, Rutledge GE, Dietz WH, et al. Differences in prevalence of obesity among Black, White and Hispanic Adults–United States, 2006–2008. MMWR. 2009;58:740–4. [PubMed] [Google Scholar]
- 30.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]