Abstract
Female carriers of balanced translocations involving an X chromosome and an autosome offer genetic counselling challenges. This is in view of the number of possible meiotic outcomes, but also due to the impact of X chromosome-localised genes that are no longer subject to gene silencing through the X chromosome inactivation centre. We present a case where delineation of the extent of X chromosome-localised genes on the derivative autosome using molecular karyotyping offers critical information in the context of genetic counselling.
Keywords: Trisomy; X chromosome, monosomy Xp22 pter; X chromosome inactivation; Receptors, gastrin-releasing peptide; KAL-1 protein; Case report; New Zealand
Female carriers of balanced translocations between an X chromosome and an autosome can exhibit a range of phenotypes from apparently normal to those with a range of abnormalities.1 This outcome is due almost entirely to a skewed X inactivation pattern. In phenotypically normal females, the translocated X is preferentially activated and the intact X is inactivated. From a counselling perspective, this presents problems. There are many possible genotypic outcomes for the offspring of a balanced carrier and, unlike an autosome-autosome translocation, there is the added complication of estimating risk when there is the possibility of functional disomy.
Functional disomy occurs when the translocated portion of the X chromosome is separated from the X chromosome inactivation centre located at Xq13.,2 and is therefore unable to undergo X chromosome-based inactivation. About 30% of genes on the short arm of the X chromosome escape X chromosome inactivation and continue to be expressed from the inactive X chromosomes.2 This is true even in females with trisomy X; the increased dosage is unlikely to have any deleterious effect, as most trisomy X females are phenotypically normal.2 From this we can assume that an abnormal phenotype is the result of the expression of duplicated genes, normally silenced during X chromosome inactivation.
Previously reported cases of functional disomy for chromosome Xp vary substantially both from the extent of the p-arm that is involved and the severity of the phenotype. Not surprisingly, those patients who have all (or almost all) of the Xp arm exhibit more severe phenotypic features.3–5 Those with smaller regions of functional Xp disomy generally exhibit a less severe phenotype.6–9 A comparison of the phenotypic features of these cases is shown in Table 1.
Table 1:
Summary of cases of functional disomy of chromosome Xp
| Reference | Gustashaw et al., 19946 | Matsuo et al., 19993 | Kokalj Vokac et al., 20027 | Kolomietz et al., 20054 | Monnot et al., 20088 | Hunter et al., 20099 | Myszka et al., 20105 | Index case |
|---|---|---|---|---|---|---|---|---|
| Region of functional disomy | Xp21.1→pter | Xp11.21p21.3 | Xp11.2p22.23 | Xp11.2→pter | Xp11.23–p11.4 | Xp11.1p11.22 | Xp11.2→pter | Xp22.33p22.13 |
| IUGR/GR | + | − | − | − | − | − | − | + |
| Facial dysmorphism | + | + | + | + | + | + | + | + |
| Hypertelorism | − | + | − | − | − | + | + | − |
| Ear abnormalities | − | + | + | − | − | + | + | − |
| Myopia | − | − | − | − | − | + | − | − |
| Developmental delay/cognitive impairment | + | + | + | + | + | + | + | + |
| Hypotonia | + | − | − | + | − | + | + | − |
| Myoclonal seizures | + | + | − | + | − | − | + | − |
| Small hands and feet | − | − | + | + | − | − | − | − |
| Clinodactyly | − | − | + | + | − | − | + | − |
| Digital clubbing | + | − | − | − | − | − | − | − |
| Hypermelanosis of Ito | − | + | − | + | − | − | − | − |
| Foramen ovale apertum | − | − | − | + | − | − | + | − |
| Hydronephrosis | − | − | − | + | − | − | + | − |
| Nystagmus | + | − | − | + | − | − | − | − |
| Tachypnea | − | − | − | − | − | − | − | − |
| Supernummery nipple | − | − | − | − | − | − | − | − |
| Sacral Mongolian spot | − | − | − | − | − | − | − | − |
| Broad forehead, posterior scalp line | + | − | − | − | − | − | + | − |
| Respiratory distress | + | + − | − | − | − | − | − | + |
IUGR = intrauterine growth restriction; GR = growth restriction.
Here we describe a case of a maternally inherited 17 Mb duplication of Xp22.33p22.13 detected by molecular karyotype in a 17-month-old female infant. G-banding analysis of the mother and the molecular karyotype data of the proband led to a deduced karyotype of the proband. The molecular karyotype data also allowed for a more informed discussion of the implications of future pregnancies for the mother.
Case Report
The 3 Kg proband was born at term to nonconsanguineous parents. At delivery, her mother and father were 29 and 34 years old, respectively. There was no history of recurrent miscarriages, stillbirths, neonatal deaths, or individuals with intellectual disability and/or unusual physical features.
Poor growth was observed during the last 4 weeks of pregnancy and the mother noted reduced fetal movements compared to her first pregnancy. During the first year of life, the proband’s length followed the 50th centile and her weight the 10th centile. She sat at 5 months of age but did not crawl until 15 months. Repeated significant respiratory infections and minimal weight gain prompted a hospital admission at 17 months of age. At that time, she was pulling to stand but not walking. She was babbling and waving “bye-bye” appropriately. She could point to objects, was developing a pincer grip in both hands, and had a vocabulary of 7 words.
A genetic assessment at that time noted a head circumference at the 10th centile, mild brachycephaly, and a flat bridge of the nose in the absence of telecanthus or hypertelorism. In view of the history of unexplained growth and developmental retardation, a molecular karyotype was requested.
Radiology revealed the presence of right upper and lower lobe pneumonia for which she received appropriate antibiotic treatment and fully recovered. No underlying anatomic or immune abnormalities were identified to explain her repeated respiratory infections and she had no further infections over the ensuing months.
A molecular karyotype analysis was performed on extracted deoxyribonucleic acid (DNA) using the Affymetrix® Cytogenetics Whole-Genome 2.7M Array (Affymetrix, Santa Clara, California, USA). Regions of copy number change were calculated using the Affymetrix® Chromosome Analysis Suite software (ChAS) v.1.0.1 which was interpreted with the aid of the University of California Santa Cruz genome browser (hg18 assembly).10 The proband’s molecular karyotype identified an extra copy of the 17.2 Mb terminal region of the X chromosome; arr Xp22.33p22.13(125,959–17,372,584)x3.
Conventional G-banded chromosome analysis was performed on peripheral blood samples taken from the proband’s mother, which showed an abnormal female karyotype with an apparently balanced translocation between the short arm of one X chromosome and the short arm of chromosome 13; 46,X,t(X;13)(p22.13;p11.2). Conventional G-banded chromosome analysis on blood samples from the mother’s parents showed normal karyotypes, confirming that the balanced translocation in the mother was a de novo event.
Discussion
Due to the uncertain nature of X chromosome inactivation (and prenatal inactivation studies may not be predictive of phenotype), a balanced female chromosome complement identified at prenatal diagnosis is not necessarily associated with a normal phenotype, and some abnormal outcomes may not give rise to a significant abnormality. Male X-autosome translocation carriers have been recorded previously with normal and abnormal phenotypes. Consequently, a detailed fetal ultrasound examination is recommended. The estimated overall risk for liveborn offspring with structural and/or functional imbalance is difficult to estimate but would be in the region of 20–40%.11 The risk of fetal loss is also likely to increase compared to the general population’s risk of 15%.11 Critically, prenatal diagnosis should be available, and unbalanced products of the translocation would be detectable on cultured chorionic villus cells or amniocytes.
The duplicated maternal X chromosome region of 17 Mb reported in the proband contains numerous genes. The genes within the pseudoautosomal region (PAR1), which encompasses approximately 2.7 Mb of the terminal short arm of the X chromosome and comprising 24 genes, obviously escape X inactivation.12 Escape genes are often clustered with as many as 13 genes in large domains ranging in size between 100 Kb and 7 Mb. Interestingly, recent data suggest that those X-linked genes that apparently escape inactivation as determined by somatic cell hybrid data may exhibit monoallelic expression, or expression at levels that may be as low as 25% of the same gene on an active X chromosome.13,14
Of those genes that lie outside the PAR1 region of the maternal 17.2 Mb duplication in the proband, it is largely unclear which ones could have played a role in the proband’s clinical phenotype. Mutations in the KAL1 gene have been detected in patients diagnosed with Kallman syndrome,15–17 but overexpression of this protein in Caenorhabditis elegans induces axon branching and axon misrouting.18,19 In the case of the GRPR gene, Bolton et al. and Ishikawa-Brush et al. reported a female patient with a balanced translocation 46,X,t(X;8) (p22.13;q22.1).20,21 The woman presented with multiple exostoses around the ankles, knees, wrists, and left clavicle. She was short, with small hands, mild brachycephaly, mental retardation, epilepsy, and autism. The translocation breakpoint on the X chromosome occurred in the first intron of the GRPR gene, which suggested that haploinsufficiency of the GRPR gene may have contributed to this patient’s phenotype. Interestingly, and in the context of the proband reported here, recent studies have shown that overexpression of GRPR in the chick brain leads to laminar disorganisation in the telencephalon, tectum, and particularly in the cerebellum, which exhibits severe atrophy.22 It is therefore tempting to suggest that overexpression of KAL1 and GRPR may underlie some aspects of the proband’s phenotype.
This case also highlights genetic counselling issues. Genetic counselling provided an opportunity to explain the proband’s karyotype abnormality to the parents, how it may have accounted for her phenotype, and to discuss recurrence risks. Establishing that the mother carried a de novo balanced translocation removed the need to counsel extended family members, but confirmed the need to explain to the couple the variety of possible outcomes in a future pregnancy.
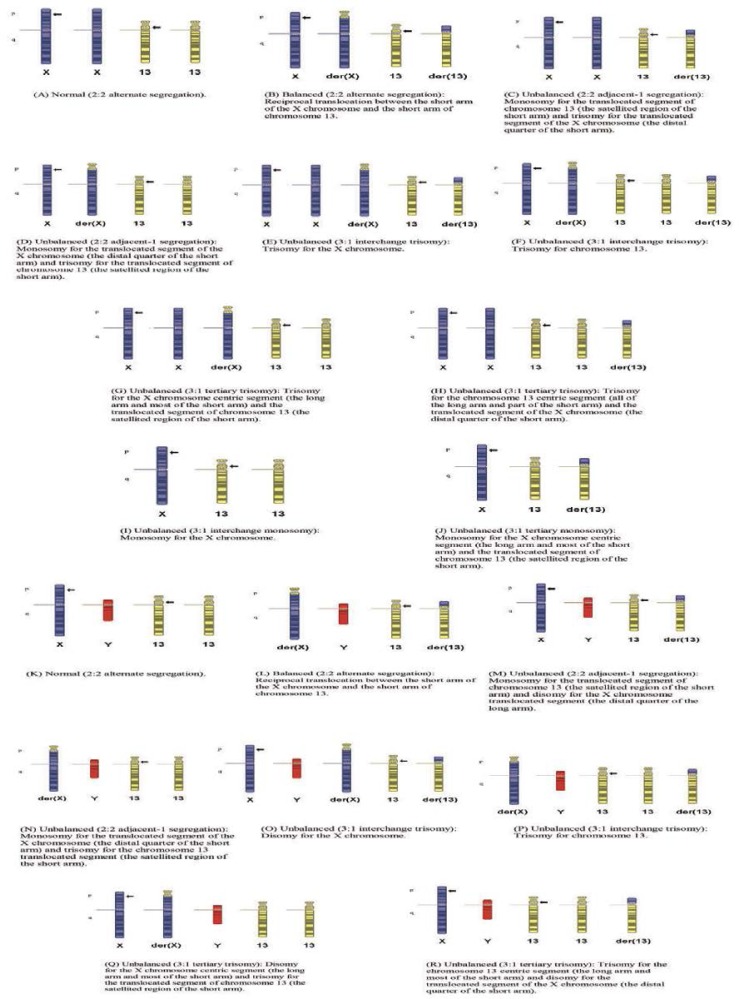
At first glance, and given the overwhelming number of possible segregations [Figure 1; not all possible segregations are shown], a discussion about possible pregnancy outcomes would appear to be daunting. Practically, one way to approach such counselling may be to consider the outcomes by gender.
Figure 1 Panels A–R:
Meiotic outcomes of the proband’s mother’s balanced translocation. Panels A, B, K, and L are the most likely products (normal and balanced) from the proband’s mother’s reciprocal translocation. The Panel L outcome may result in infertility; Panel B may be associated with an abnormal clinical phenotype if the normal X chromosome is not preferentially inactivated; Panel C is known to be viable; Panel M would be expected to have the same degree of viability; Panel D is expected to have a less severe phenotype if the derivative X is preferentially inactivated. The outcomes shown in Panels E, G, I, J, O, and Q are known to be viable, and would be expected to show features of triple X, Turner syndrome or Klinefelter’s syndrome; however, Panels E, G, O, and Q may have a more severe phenotype if the normal X homologues are not inactivated. The outcome shown in Panel N is considered to be less viable and associated with a more severe phenotype. The outcomes shown in Panels F, H, P, and R would result in trisomy 13. Other modes of segregation would have larger imbalances that are considered more unlikely to be viable.
In female fetuses with a Turner, Turner variant, or trisomy 13 chromosome complement (segregation patterns I, J, F, and H, respectively), a high rate of spontaneous miscarriage is to be expected. The Turner variant of pattern D possibly carries a lower risk of abortion. Females with these genotypes who survive to delivery could be expected to have an abnormal phenotype: trisomy 13 would carry the greater degree of morbidity and mortality. Females with greater or lesser degrees of trisomy X (segregation patterns C, E, and G) would be expected to survive to term and have a milder phenotype. These phenotypes, and those resulting from segregation pattern D, may be modified by unpredictable X chromosome inactivation, and may range from normal to the phenotype observed in the proband, or have more severe problems. The carrier mother could also have normal females (patterns A and B) though a female with pattern B may be affected, depending on X chromosome inactivation.
In male fetuses, one would expect those with segregation patterns N, P, and R to have a high risk of spontaneous abortion. Patterns P and R result in trisomy 13 and, for pattern N, some of the genes in the deleted Xp region cause X-linked dominant conditions which are lethal in males in the hemizygous state. Certainly, some males with trisomy 13 may survive to birth. Males with segregation patterns O and Q would be expected to have a Klinefelter’s syndrome phenotype, although those with pattern Q could manifest additional rare X-linked dominant conditions not usually seen in males. Infertility may be part of some phenotypes, but would possibly only be clinically relevant in males with pattern L. It is difficult to predict the clinical outcome of pattern M (partial duplication of Xp22.3p22.13). Given the number of OMIM genes in the region, a range of possibilities (from normal to affected) could be considered. Of relevance in terms of the latter outcome is a report of a male with a mild learning disability and the karyotype 46,Xdup(X)(p22.13–p22.31),Y.23 Tzschach et al. provide a summary of male mental retardation patients with a duplication or disomy of Xp22.24 Critically, the challenge in counselling is the characterisation of patients with smaller duplications than those reported to date that could provide a better insight into those genes that play a significant role in mental retardation.
Genetic counselling was greatly aided in this case by the identification of the exact breakpoints and genes involved in the chromosome rearrangement in the mother of the proband. For almost all the segregation patterns, fairly clear information can be conveyed to the parents about the expected outcome or phenotype. This would aid in reproductive decision-making, either before or during a future pregnancy. The case illustrates how patient care benefits from close collaboration between clinicians and the laboratory.
Conclusion
Carriers of balanced translocations involving the X chromosome offer challenges in terms of genetic counselling; however, delineation of the breakpoints of the rearranged chromosomes can aid the clinician in their conversations with carriers in the context of reproductive decision-making.
References
- 1.Powell CM, Taggart RT, Drumheller TC, Wangsa D, Qian C, Nelson LM, et al. Molecular and cytogenetic studies of an X autosome translocation in a patient with premature ovarian failure and review of the literature. Am J Med Genet. 1994;52:19–26. doi: 10.1002/ajmg.1320520105. [DOI] [PubMed] [Google Scholar]
- 2.Carrel L, Cottle AA, Goglin KC, Willard HF. A first-generation X-inactivation profile of the human X chromosome. Proc Natl Acad Sci USA. 1999;96:14440–4. doi: 10.1073/pnas.96.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo M, Muroya K, Kosaki K, Ishii T, Fukushima Y, Anzo M, et al. Random X-inactivation in a girl with duplication Xp11.21–p21.3: Report of a patient and review of the literature. Am J Med Genet. 1999;86:44–50. [PubMed] [Google Scholar]
- 4.Kolomietz E, Godbole K, Winsor EJ, Stockley T, Seaward G, Chitayat D. Functional disomy of Xp: Prenatal findings and postnatal outcome. Am J Med Genet. 2005;134:393–8. doi: 10.1002/ajmg.a.30652. [DOI] [PubMed] [Google Scholar]
- 5.Myszka A, Karpinski P, Makowska I, Lassota M, Przelozna B, Slezak R, et al. DNA methylation analysis of a de novo balanced X;13 translocation in a girl with abnormal phenotype: Evidence for functional duplication of the whole short arm of the X chromosome. J Appl Genet. 2010;51:331–5. doi: 10.1007/BF03208863. [DOI] [PubMed] [Google Scholar]
- 6.Gustashaw KM, Zurcher V, Dickerman LH, Stallard R, Willard HF. Partial X chromosome trisomy with functional disomy of Xp due to failure of X inactivation. Am J Med Genet. 1994;53:39–45. doi: 10.1002/ajmg.1320530109. [DOI] [PubMed] [Google Scholar]
- 7.Kokalj Vokac N, Seme Ciglenecki P, Erjavec A, Zagradisnik B, Zagorac A. Partial Xp duplication in a girl with dysmorphic features: The change in replication pattern of late-replicating dupX chromosome. Clin Genet. 2002;61:54–61. doi: 10.1034/j.1399-0004.2002.610111.x. [DOI] [PubMed] [Google Scholar]
- 8.Monnot S, Giuliano F, Massol C, Fossoud C, Cossée M, Lambert JC, et al. Partial Xp11.23–p11.4 duplication with random X inactivation: Clinical report and molecular cytogenetic characterization. Am J Med Genet A. 2008;146A:1325–9. doi: 10.1002/ajmg.a.32238. [DOI] [PubMed] [Google Scholar]
- 9.Hunter M, Bruno D, Amor DJ. Functional disomy of proximal Xp causes a distinct phenotype comprising early hypotonia, hypertelorism, small hands and feet, ear abnormalities, myopia and cognitive impairment. Am J Med Genet A. 2009;149A:1763–7. doi: 10.1002/ajmg.a.32954. [DOI] [PubMed] [Google Scholar]
- 10.Genome Browser. University of California Santa Cruz (UCSC). From http://genome.ucsc.edu Accessed: Apr 2012.
- 11.Gardner RJM, Sutherland GR. Chromosome Abnormalities and Genetic Counseling. 3rd ed. New York: Oxford University Press; 2004. [Google Scholar]
- 12.Mangs AH, Morris BJ. The human pseudoautosomal region (PAR): Origin, function and future. Curr Genomics. 2007;8:129–36. doi: 10.2174/138920207780368141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talebizadeh Z, Simon SD, Butler MG. X chromosome gene expression in human tissues: male and female comparisons. Genomics. 2006;88:675–81. doi: 10.1016/j.ygeno.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–4. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 15.Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, et al. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–36. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- 16.Hardelin JP, Levilliers J, del Castillo I, Cohen-Salmon M, Legouis R, Blanchard S, et al. X chromosome-linked Kallmann syndrome: Stop mutations validate the candidate gene. Proc Natl Acad Sci USA. 1992;89:8190–4. doi: 10.1073/pnas.89.17.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardelin JP, Levilliers J, Blanchard S, Carel JC, Leutenegger M, Pinard-Bertelletto JP, et al. Heterogeneity in the mutations responsible for X chromosome-linked Kallmann syndrome. Hum Mol Genet. 1993;2:373–7. doi: 10.1093/hmg/2.4.373. [DOI] [PubMed] [Google Scholar]
- 18.Bülow HE, Berry KL, Topper LH, Peles E, Hobert O. Heparan sulfate proteoglycan-dependent induction of axon branching and axon misrouting by the Kallmann syndrome gene kal-1. Proc Natl Acad Sci USA. 2002;99:6346–51. doi: 10.1073/pnas.092128099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rugarli EI, Di Schiavi E, Hilliard MA, Arbucci S, Ghezzi C, Facciolli A, et al. The Kallmann syndrome gene homolog in C. elegans is involved in epidermal morphogenesis and neurite branching. Development. 2002;129:1283–94. doi: 10.1242/dev.129.5.1283. [DOI] [PubMed] [Google Scholar]
- 20.Bolton P, Powell J, Rutter M, Buckle V, Yates JRW, Ishikawa-Brush Y, et al. Autism, mental retardation, multiple exostoses and short stature in a female with 46,X,t(X;8)(p22.13;q22.1) Psychiat Genet. 1995;5:51–5. doi: 10.1097/00041444-199522000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa-Brush Y, Powell JF, Bolton P, Miller AP, Francis F, Willard HF, et al. Autism and multiple exostoses associated with an X;8 translocation occurring within the GRPR gene and 3-prime to the SDC2 gene. Hum Molec Genet. 1997;6:1241–50. doi: 10.1093/hmg/6.8.1241. [DOI] [PubMed] [Google Scholar]
- 22.Iwabuchi M, Maekawa F, Tanaka K, Ohki-Hamazaki H. Overexpression of gastrin-releasing peptide receptor induced layer disorganization in brain. Neuroscience. 2006;138:109–22. doi: 10.1016/j.neuroscience.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Robertshaw BA, MacPherson J. Scope for more genetic testing in learning disability: Case report of an inherited duplication on the X-chromosome. Br J Psych. 2006;189:99–101. doi: 10.1192/bjp.189.2.99. [DOI] [PubMed] [Google Scholar]
- 24.Tzschach A, Chen W, Erdogan F, Hoeller A, Ropers HH, Castellan C, et al. Characterization of interstitial Xp duplications in two families by tiling path array CGH. Am J Med Genet A. 2008;146A:197–203. doi: 10.1002/ajmg.a.32070. [DOI] [PubMed] [Google Scholar]