Abstract
Matrix metalloproteinase (MMP) inhibition has been shown to reduce adhesive bond degradation when applied as a pre-conditioner, adding to clinical steps in the placement of adhesives, but their incorporation within dental adhesives has not been fully explored. This study examined the effect of including 2 MMP inhibitors (BB94 and GM6001) within the primers of 3 commercially available adhesives. Fluorometric assay and zymography showed that adhesives with MMP inhibitors had high affinity toward both synthetic fluorogenic FRET peptides (95%) and dentin powder substrates, respectively. The immediate microtensile bond strength was enhanced for 2 types of adhesives following the addition of both inhibitors. However, no changes were detected between the control and the inhibitor groups following 3-month storage. The modified two-step etch-and-rinse and single-step systems showed less Rhodamine B penetration to the “hybrid layer” and to the “adhesive”, respectively. The incorporation of BB94 and GM6001 within the primers resulted in the inhibition of dentin MMPs with improved initial bond strength and enhanced sealing ability.
Keywords: matrix metalloproteinases (MMPs), batimastat, galardin, zymography, microtensile bond strength, micropermeability
Introduction
Mechanical, physical, and functional properties of dental adhesives have been improved as a result of numerous investigations into the chemical balance between their hydrophilic and hydrophobic functional components (Pashley et al., 2011). The degradation of the adhesive-dentin interface, including the disorganization of collagen fibrils and loss of resin from interfibrillar spaces, is a limitation of these systems (Hashimoto et al., 2003).
Host-derived matrix metalloproteinases (MMPs), found both in saliva and in etched dentin, have been shown to be involved in the degradation of the unprotected collagen fibrils within the hybrid layer (Chaussain-Miller et al., 2006; Hannas et al., 2007). These proteases are secreted by odontoblasts during dentinogenesis and remain inactive within the dentin extracellular matrix (Tjäderhane et al., 2001). The acidic environment, resulting from adhesive systems or the biological carious process, activates different dentinal MMPs (Visse and Nagase, 2003; Chaussain-Miller et al., 2006).
Studies have investigated the prevention of collagen fibrillar degradation in the dentin extracellular matrix by different matrix metalloproteinases inhibitors (Komori et al., 2009). These inhibitors, including chlorhexidine (Pashley et al., 2004), were applied directly and separately to the acid-etched dentin prior to the placement of the dental adhesive of choice. It has been shown that the activity of MMPs can be inhibited with a reduction in the observed interfacial nanoleakage (Breschi et al., 2010a). Chlorhexidine (0.2-2%) application preserves both the durability and bond strength of dental adhesives (Carrilho et al., 2007; Breschi et al., 2010b).
A recent study showed that quaternary ammonium methacrylate resin monomers have various degrees of inhibitory activity (Tezvergil-Mutluay et al., 2011), suggesting the possibility of incorporating MMP inhibitors into dentin adhesives (Liu et al., 2011). Chlorhexidine was incorporated into the primer of a two-step self-etching adhesive, and its MMP inhibitory effect was partially maintained (Zhou et al., 2011).
The aim of this study was to evaluate the effect of adding MMP inhibitors to 3 commercial dental adhesives on both MMP substrate activity and the adhesives’ physical properties. The null hypotheses were that the addition of MMP inhibitors to each dental adhesive would not significantly affect substrate MMP activity or interfacial physical properties at a significance predetermined at α = 0.05.
Materials & Methods
Caries-free human (aged 18-30 yrs) extracted molars were collected after patients’ informed consent was obtained under a protocol reviewed and approved by the East Central London Research Ethics Committee (Reference 10/H0721/55). All extracted teeth, stored in refrigerated distilled water, were used within 1 mo post-extraction. Commercial recombinant human MMP-1 and MMP-2 were obtained (PeproTech, Rocky Hill, NJ, USA) along with quenched fluorogenic peptide substrates utilizing fluorescence resonance energy transfer (FRET) and intramolecular fluorescence energy transfer (IFET; Biozyme Inc., Apex, NC, USA).
Modified Primer Preparation
Two MMP inhibitors, GM6001 (Millipore Ltd., Watford, UK) and BB94 (British Biotech Ltd., Oxford, UK), were added to the primer component of 3 commercial adhesive systems—Optibond FL “OB” (Kerr, Orange, CA, USA), Prime&Bond NT “PB” (Dentsply, York, PA, USA), and G-Bond “GB” (GC, Tokyo, Japan; see the Appendix for chemical composition)—at a concentration of 5 µM. Nine experimental groups were created, including the positive control groups, which included each adhesive without the inhibitors.
Effect on Synthetic MMP Substrate
Synthetic, internally quenched MMP FRET substrates, as described by Moss and Rasmussen (2007), for MMP-1 [Dabcyl-Pro-Cha-Gly-Cys(Me)His-Ala-Lys(5-FAM)-NH2] and MMP-2 [Dabcyl-Leu-Ala-Gln-Ala-Homophenylalanine-Arg-Ser-Lys(5-FAM)-NH2] were used to evaluate the effects of the modified adhesives on active MMPs. Plates were coated in an N2 anaerobic pouch with 20 µL of adhesive and blocked overnight with 2%(w/v) bovine serum albumin. Recombinant MMP-1 and MMP-2, activated as per manufacturer’s instructions, at a concentration of 500 ng mL–1, were pre-incubated overnight prior to transfer of supernatants to a new plate and addition of the MMP substrate solution. Reactions were carried out in black 96-well microtiter plates (Nunc, Roskilde, Denmark) and were incubated at 37°C for 24 hrs, and fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm by a FluoStar Optima Plate Reader (BMG Labtech GmbH, Ortenberg, Germany). The assays were performed in 3 sets of plates, and corresponding blanks without enzyme were included for evaluation of the background degradation of the fluorogenic substrate.
Effect on Dentin MMPs
The enamel, cementum, and pulp tissue of 5 sound extracted human teeth were removed by means of a water-cooled circular saw diamond blade (Diamond wafering blade XL 12205, Benetec Ltd, London, UK) and a slow-speed diamond bur to produce sound, coronal dentin blocks. Each block was pulverized into a fine powder by 100 MPa compressive load. A 100-mg quantity of powder was placed in 8-µm-pore-diameter Transwell (Corning, NY, USA ) inserts and placed in 24-well adhesive-coated plates. Zymography was performed in SDS-polyacrylamide gels containing gelatin (0.1% w/v) to assess the enzymatic effects of the modified adhesives. Dentin powder was mixed with Laemmli sample buffer without a reducing agent and subjected to electrophoretic analysis without boiling. After incubation with 2.5% Triton X-100 in 50 mM Tris-HCl (pH 7.5), the gelatins were incubated for 24 hrs at 37°C in 50 mM Tris-HCl (pH 7.5) containing 150 mM NaCl, 10 mM CaCl2, and 0.1% Triton X-100. The gels were stained with Coomassie Blue G250, and the disappearance of gelatin staining determined the enzymatic reaction.
Microtensile Bond Strength (µTBS) Measurement
Ninety extracted, sound human teeth were used in this study. A flat, transversely cut dentin surface was created on each tooth by means of a water-cooled circular saw diamond blade. A smear layer was created with 600-grit SiC paper. Each adhesive system was applied to the moist dentin surface following the relevant manufacturers’ instructions. All teeth were restored with Filtek™ Supreme Ultra (3M ESPE, St. Paul, MN, USA) resin composite restorative material according to a standardized protocol. Each sample was stored at 37°C for 24 hrs to ensure complete polymerization and was cut into 12 beams containing 1 mm2 adhesive layers. The beams obtained from 5 teeth in each of the 9 experimental groups were tested immediately. Another 60 beams, obtained from different teeth, were tested after storage in distilled water at 37°C for 3 mos. Samples that failed during sample preparation were noted for each experimental group (pre-test failures).
Each beam was subjected to tensile load in a SMAC LAL300 linear actuator testing machine (SMAC Ltd., West Sussex, UK) with a crosshead speed of 1 mm/min. The force required to break the adhesive bond was recorded in MPa. Two-way ANOVA was used to compare the µTBS of all groups, and the Sidak post hoc test was used to find the differences between any two groups in each adhesive system. Each failed beam, following microtensile testing, was evaluated by stereomicroscopy (Kyowa Optical Co. Ltd., Tokyo, Japan) with a 60 × 0.75 objective, for assessment of the mode and locus of tensile failure. The mode of failure was classified as a percentage of the total surface area of failed dentin exhibiting pure adhesive, cohesive, or mixed failure modes.
Micropermeability Assessment
Twenty-seven teeth were prepared as above, and adhesive/resin composites were applied following the manufacturers’ instructions in a standardized manner. The crown segment of each tooth was obtained by removal of the roots 1 mm beneath the cement-enamel junction (CEJ). Pulpal tissue was extirpated without altering the predentin. The sample was inverted, and an aqueous solution of Rhodamine B was introduced into the pulp chamber and allowed to permeate for 3 hrs under gravity. The tooth was sliced vertically into three 2-mm-thick slabs, each hand-polished with 500-, 800-, and 1,000-grit SiC papers, with 3 min of ultrasonication between polishings. Using a tandem scanning confocal microscope (TSM; Noran Instruments, Middleton, WI, USA), we examined the slabs with a 100 × 1.4 oil-immersion objective, 546 nm excitation, and 640 nm emission filters. The degree of Rhodamine B penetration in 5 constant and pre-selected areas was recorded in each slab. A modified micropermeability index, as reported by Sauro et al. (2008), was used to assess the degree of micropermeability. Order logistic regression analysis was used for between-group comparisons for each adhesive system, by taking into account the clustered analysis of the data.
Results
Effect on MMP Substrates
Both MMP inhibitors resulted in a significant loss of synthetic MMP-1 and MMP-2 activity (Fig. 1a) when they were included within the PB and GB adhesives. The OB treatment group was not analyzable within the constraints of the experimental system used. However, zymographs showed that the enzymatic reaction of the dentin MMPs was inhibited in all experimental groups (Fig. 1b).
Figure 1.
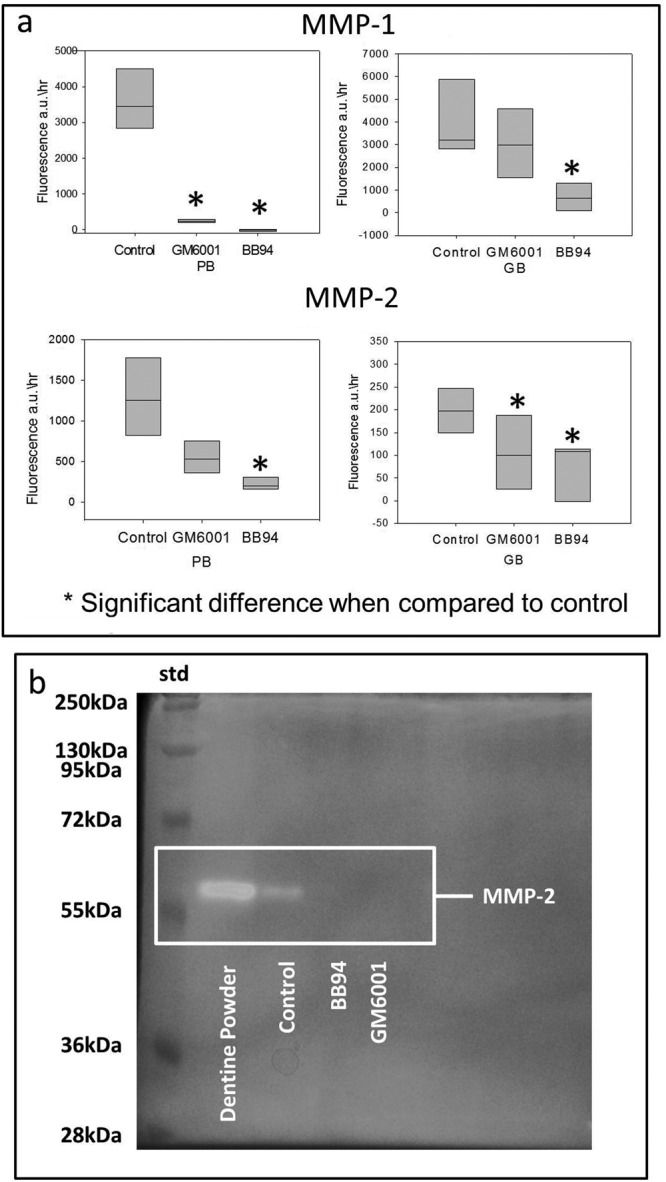
The efficacy of inhibitor-doped commercial adhesives on MMP activity. (a) The effects on recombinant human (rh) MMP-1 and MMP-2 activity. The modified PB and GB showed significant inhibitory effects on MMP-1 and MMP-2. (b) Gelatin zymogram for OB. The highlighted areas represent the protease activity consistent with MMP-2 activity with gelatin. No reaction was observed when both MMP inhibitors adhesives were mixed with dentin powder. Molecular masses, expressed in kDa, are reported in the std lane.
Microtensile Bond Strength (µTBS)
The results of the µTBS tests are summarized in the Table. The immediate µTBS for OB including inhibitors increased significantly (F = 7.13; p = 0.001) when compared with its control. However, both BB94 and GM6001 groups showed significant reduction after 3-month storage.
Table.
The Mean µTBS, the Distribution of the Failure Mode, and the Percentage of Pre-test Failures of the 3 Adhesive Groups Tested Immediately (after 24 hrs) and after 3 Months′ Storage in Distilled Water*
| Dental Adhesive | Group | Storage Time | Mean µTBS MPa (SD) | Failure Mode % (Cohesive/Mixed/Adhesive) | Pre-test Failure % |
|---|---|---|---|---|---|
| Optibond FL “OB” | Control | 24 hrs | 34.7 (18.7)a | (79.2, 16.7,4.1)a | 17.1 |
| 3 mos | 36.8 (17)a | (75, 12.5, 12.5)a | 6.6 | ||
| GM6001 | 24 hrs | 42 (18.7)b | (33.3, 33.3, 33.3)b | 0 | |
| 3 mos | 30.9 (15.7)a | (62.5, 8.3, 29.2)c | 2.5 | ||
| BB94 | 24 hrs | 48 (20.3)b | (83.3, 16.7, 0)a | 1.3 | |
| 3 mos | 34.2 (17)a | (79.2, 16.6, 4.1)a | 7.4 | ||
| Prime&Bond NT “PB” | Control | 24 hrs | 48.2 (20.4)a | (62.5, 29.2, 8.3)a | 19.8 |
| 3 mos | 42.7 (17.5)a | (91.7, 8.3, 0)b | 0 | ||
| GM6001 | 24 hrs | 41.9 (15.8)a | (58.3, 20.8, 20.8)a | 4.2 | |
| 3 mos | 31.4 (16)b | (91.7, 8.3, 0)b | 1.5 | ||
| BB94 | 24 hrs | 43.2 (18.4)a | (45.8, 33.3, 20.8)a | 2.4 | |
| 3 mos | 46.4 (14.5)a | (79.2, 12.5, 8.3)b | 1.4 | ||
| G-Bond “GB” | Control | 24 hrs | 25.3 (15.7)a | (29.2, 20.8, 50)a | 15.3 |
| 3 mos | 12.2 (10)b | (29.1, 16.7, 54.2)a | 25 | ||
| GM6001 | 24 hrs | 41.7 (17.7)c | (29.2, 33.3, 37.5)a | 10.2 | |
| 3 mos | 17 (13.2)b | (29.2, 25, 45.8)a | 10.1 | ||
| BB94 | 24 hrs | 34.8 (19.2)d | (29.2, 37.5, 33.3)a | 3.9 | |
| 3 mos | 18.4 (16.1)b | (37.5, 12.5, 50)a | 25.1 |
The results from each adhesive system were analyzed separately. Cohesive failure mode represents failures occurring mainly within dentin or resin composite. Adhesive type represents failures at the adhesive interface. The mixed failure designates a mixture of adhesive and cohesive failure within the same fractured surface.
Same superscript indicates no significant difference (p > 0.05) within the same adhesive system.
No significant difference was found in the immediate or aged µTBS for all PB groups (p = 0.390), except for the aged GM6001 group, which decreased significantly (p = 0.001).
For GB, the µTBS immediately increased significantly (F = 4.10; p = 0.017) with both inhibitors as compared with their controls. However, no significant differences were found between the control and both MMP inhibitor groups following 3-month storage.
There were fewer pre-test failures when both MMP inhibitors were added to all adhesives in the initial samples (Table).
OB and PB resulted in more cohesive failures than GB (Table). The cohesive failure in OB with GM6001 was increased significantly after 3-month aging. This was the same for all PB groups, with no significant differences between the groups. No significant differences were found in the mode of failure of GB.
Micropermeability Assessment
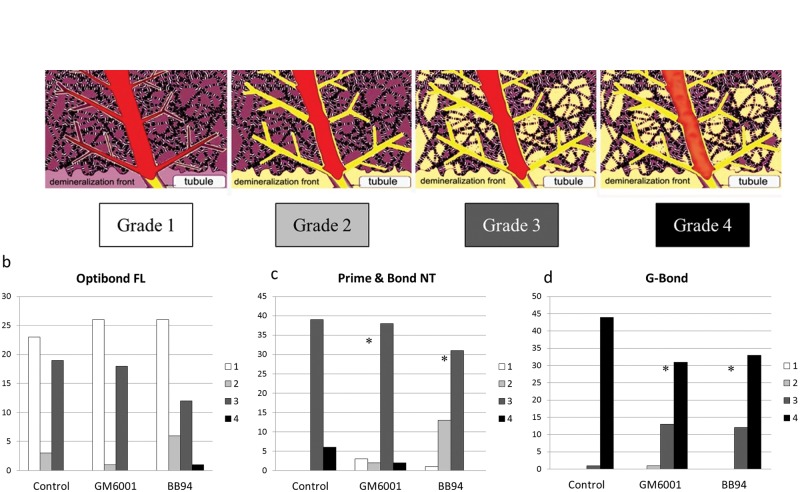
The micropermeability index used in this study is shown in Fig. 2a. The micropermeability of OB (Fig. 2b) did not change significantly (p = 0.791) after the addition of both MMP inhibitors. PB with MMP inhibitors (Fig. 2c) exhibited significantly (p = 0.043) less dye in the hybrid layer than its equivalent control. Additionally, GB exhibited less dye reaching the adhesive layer when both MMP inhibitors were added (Fig. 2d) (p = 0.006). Adding BB94 to PB resulted in a significant better sealing ability than adding GM6001 (p = 0.001). For GB, GM6001 had significantly less dye reaching the adhesive layer when compared with BB94 (p = 0.021). Examples of the micropermeability results for all experimental groups are shown in Fig. 3.
Figure 2.
The evaluation of micropermeability of simulated pulpal fluids into the adhesive interface. (a) The micropermeability index: (1) completely intact hybrid layer with no dye reaching it, (2) the dye reaches the base of the hybrid layer, (3) the dye is completely infiltrated within the hybrid layer, and (4) the dye is reaching the adhesive layer. (b) Micropermeability scores for OB groups. (c) Micropermeability scores for PB groups. (d) Micropermeability scores for GB groups. *Significantly different from the control.
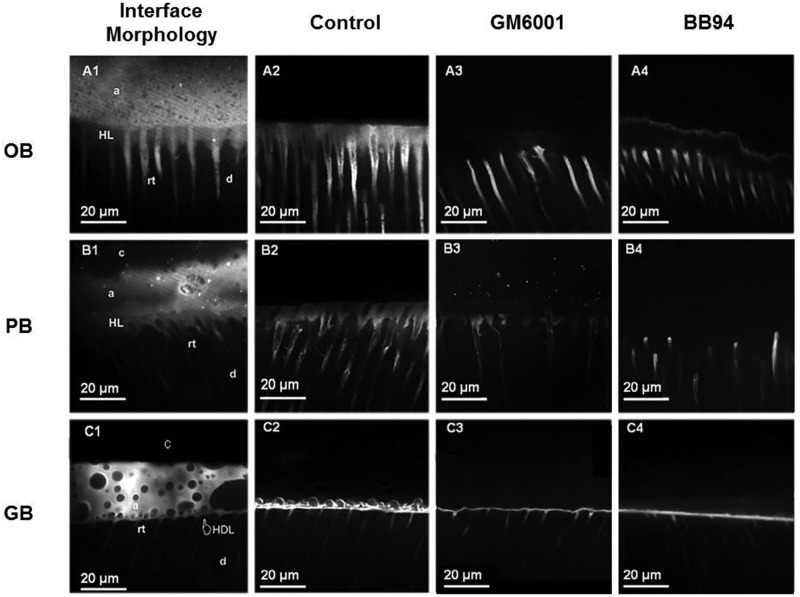
Figure 3.
TSM images of the interface morphology and examples of the 9 experimental groups’ micropermeability. Each row represents the most common features observed in the resin-dentin interfaces created with the 3 adhesive systems used in this study. The TSM images in each column represent the interface morphology (1), control adhesives without MMP inhibitors (2), adhesives with GM6001 (3), and adhesives with BB94 (4). (A1) TSM image captured in fluorescence mode (Rhodamine B excitation/emission), showing the interfacial characterization of the OB-bonded dentin specimens. It is possible to observe long resin tags (rt) and a 5- to 8-µm hybrid layer (HL) localized beneath a thick adhesive layer (a). The OB-bonded dentin showed dye penetration (micropermeability) within the entire thickness of the HL (A2), while the micropermeability of the resin-bonded interface created with the OB doped with GM6001 (A3) or BB94 (A4) was detected only inside the dentinal tubules. (B1) The interfacial characterization of the PB-bonded dentin specimens, also showing, in this case, a clear hybrid layer (HL) localized beneath a thick adhesive layer (a). This type of interface showed micropermeability within the entire thickness of the HL for the control group (B2). PB doped with GM6001 (B3) and BB94 (B4) had less dye reaching the hybrid layer. (C1) A TSM image showing the interfacial characterization of the GB-bonded dentin specimens. It is possible to observe short resin tags (rt) and a 1- to 2-µm hybrid inter-diffusion layer (HDL) localized beneath a thick adhesive layer (a) characterized by the clear presence of phase separation. This type of resin-bonded interface was affected by severe micropermeability within both the hybrid inter-diffusion and part of the adhesive layer, created with GB only (C2). The incorporation of GM6001 (C3) and BB94 (C4) resulted in less dye reaching the adhesive layer.
Discussion
The first null hypothesis was rejected, since the inclusion of MMP inhibitors in the 3 commercial dental adhesives resulted in a significant inhibition of MMP substrate activity. The second null hypothesis was partially rejected, since the microtensile bond strength changed only immediately, and the micropermeability of PB and GB adhesives changed following the addition of MMP inhibitors.
BB-94 (batimastat) is a synthetic low-molecular-weight peptide-like analogue of the collagen substrate that is capable of inhibiting tumor growth and metastatic spread (Wang et al., 1994). It has been used in cancer treatment as an MMP inhibitor. GM6001 (galardin) is a synthetic MMP inhibitor with specific activity against MMP-1, MMP-2, MMP-3, MMP-8, and MMP-9 (Whittaker et al., 1999). It has a collagen-like backbone to facilitate binding to the active site of MMPs and chelating the zinc ion, which is located in the catalytic domain of MMPs (Breschi et al., 2010a).
In this study, the modified adhesives containing both MMP inhibitors showed high affinity for both synthetic and dentin powder substrates. Adding the MMP inhibitors to dental adhesive systems has been previously reported. It was shown that bond degradation was reduced when chlorhexidine and MMP-2/MMP-9 specific inhibitors (SB-3CT) were used within the primer of etch-and-rinse adhesive systems. However, these incorporated MMP inhibitors did not improve the self-etch adhesive systems (De Munck et al., 2009).
The microtensile bond strengths of all adhesive systems including MMP inhibitors were not reduced in the present study when compared with their controls. The reduction in the µTBS for the aged PB with GM6001 could be related to the failure of the resin composite-adhesive interface rather than the failure of the adhesive itself, since 91.67% of the failures were located cohesively within the composite. The aged, modified OB showed a reduction in µTBS when compared with initial data. However, their strength values were no less than those of the controls. Interestingly, the present study showed that adding MMP inhibitors to a single-step adhesive system improved bond strength initially, but not after 3-month aging.
Adding inhibitors to PB and GB improved the micropermeability scores when compared with controls. However, this was not observed with OB, since it has a better sealing ability when compared with other adhesives, and it has been used as a positive control for other adhesives (Griffiths et al., 1999; Sauro et al., 2008).
Different mechanisms could explain the increase in initial bond strength (with minimal pre-test failures) and the reduction in the fluids permeating into the adhesive interface. This could be due to the preservation of the delicate collagen matrix, the enzymatic chemical bond between MMPs and their inhibitors, or a combination of both mechanisms, resulting in improved micromechanical retention of the adhesive within the collagen matrix, suggesting an auxiliary bonding mechanism for these dentin adhesives.
Dentin MMPs can be activated following the demineralization of the dentin surface by acids, resulting in collagenolytic and gelatinolytic activities identified within the hybridized dentin (van Strijp et al., 2003; Chaussain-Miller et al., 2006). The delicate collagen matrix could be degraded at an early stage prior to the complete polymerization of dental adhesives. The inhibition of such activity may result in a rich collagen network, providing improved mechanical interlocking for the dental adhesive.
Effective synthetic MMP inhibitor must contain a functional group (e.g., carboxylic acid, hydroxamic acid, sulfhydryl) capable of chelating the active-site zinc ion in the MMP molecule. This functional group provides a hydrogen bond interaction with the enzyme backbone and one or more side-chains which undergo effective van der Waals interactions with the enzyme subsites (Whittaker et al., 1999). Being competitive toward the zinc-ion-active side, the inhibitor targets the dentin MMPs which are present around the collagen fibrils (Liu et al., 2011). The quaternary ammonium compounds showed an electrostatic attraction within the active site of the MMP and rendered them inactive, while preserving the collagen (Tezvergil-Mutluay et al., 2011). The incorporation of MMP inhibitors within dental adhesive systems may result in the enhancement of micromechanical interlocking through the reaction between the MMP substrate and the inhibitors. As indirect evidence of this, the pre-treatment of the etched dentin with chlorhexidine disinfecting solution was found to reduce the solubility of the collagen through the inhibition of MMPs (Carrilho et al., 2009), observed over time but not initially. The increase in immediate bond strengths shown in our additive systems is unlikely to be due to preservation of collagen from MMP activity alone. Further investigations are required to elucidate mechanisms for inhibiting collagen matrix degradation and its preservation over time. This is important not only for adhesive dentistry but also for finding therapeutic applications for restorative materials in caries prevention.
Footnotes
This study was supported by the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and by the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The authors also acknowledge the College of Dentistry, King Saud University, Saudi Arabia, for grant sponsorship of the first author (grant # 4/52/137605 dated 18/4/2010).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, et al. (2010a). Chlorhexidine stabilizes the adhesive interface: A 2-year in vitro study. Dent Mater 26:320-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. (2010b). Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater 26:571-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Carvalho RM, de Goes MF, di Hipolito V, Geraldeli S, Tay FR, et al. (2007). Chlorhexidine preserves dentin bond in vitro. J Dent Res 86:90-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, et al. (2009). Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater 90:373-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. (2006). The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res 85: 22-32 [DOI] [PubMed] [Google Scholar]
- De Munck J, Van den, Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, et al. (2009). Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res 88:1101-1106 [DOI] [PubMed] [Google Scholar]
- Griffiths BM, Watson TF, Sherriff M. (1999). The influence of dentine bonding systems and their handling characteristics on the morphology and micropermeability of the dentine adhesive interface. J Dent 27:63-71 [DOI] [PubMed] [Google Scholar]
- Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. (2007). The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand 65:1-13 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. (2003). In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials 24:3795-3803 [DOI] [PubMed] [Google Scholar]
- Komori PC, Pashley DH, Tjäderhane L, Breschi L, Mazzoni A, de Goes MF, et al. (2009). Effect of 2% chlorhexidine digluconate on the bond strength to normal versus caries-affected dentin. Oper Dent 34: 157-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. (2011). Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90:953-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss ML, Rasmussen FH. (2007). Fluorescent substrates for the proteinases ADAM17, ADAM10, ADAM8, and ADAM12 useful for high-throughput inhibitor screening. Anal Biochem 366:144-148 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. (2011). State of the art etch-and-rinse adhesives. Dent Mater 27:1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauro S, Pashley DH, Mannocci F, Tay FR, Pilecki P, Sherriff M, et al. (2008). Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: a comparison study using a double-staining/confocal microscopy technique. Eur J Oral Sci 116:184-193 [DOI] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, et al. (2011). The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res 90:535-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjäderhane L, Palosaari H, Wahlgren J, Larmas M, Sorsa T, Salo T. (2001). Human odontoblast culture method: the expression of collagen and matrix metalloproteinases (MMPs). Adv Dent Res 15:55-58 [DOI] [PubMed] [Google Scholar]
- van Strijp AJ, Jansen DC, DeGroot J, ten Cate JM, Everts V. (2003). Host-derived proteinases and degradation of dentine collagen in situ. Caries Res 37:58-65 [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827-839 [DOI] [PubMed] [Google Scholar]
- Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM. (1994). Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumor growth and spread in a patient-like orthotopic model in nude mice. Cancer Res 54:4726-4728 [PubMed] [Google Scholar]
- Whittaker M, Floyd CD, Brown P, Gearing AJ. (1999). Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev 99:2735-2776 [DOI] [PubMed] [Google Scholar]
- Zhou J, Tan J, Yang X, Xu X, Li D, Chen L. (2011). MMP-inhibitory effect of chlorhexidine applied in a self-etching adhesive. J Adhes Dent 13:111-115 [DOI] [PubMed] [Google Scholar]