Abstract
Teeth develop from interactions between embryonic oral epithelium and neural-crest-derived mesenchyme. These cells can be separated into single-cell populations and recombined to form normal teeth, providing a basis for bioengineering new teeth if suitable, non-embryonic cell sources can be identified. We show here that cells can be isolated from adult human gingival tissue that can be expanded in vitro and, when combined with mouse embryonic tooth mesenchyme cells, form teeth. Teeth with developing roots can be produced from this cell combination following transplantation into renal capsules. These bioengineered teeth contain dentin and enamel with ameloblast-like cells and rests of Malassez of human origin.
Keywords: biotooth, tissue engineering, root formation, oral epithelium, ameloblast
Introduction
Current implant-based methods of whole-tooth replacement fail to reproduce a natural root structure, and, as a consequence of the non-buffered forces of mastication, jaw bone resorption is commonly observed around the implant (Maiorana et al., 2005; Shin et al., 2006; Moeintaghavi et al., 2007; Fugazzotto, 2008; Heinemann et al., 2010; Kim et al., 2010; Shapoff et al., 2010; Hasan et al., 2011). A biological implant that forms a tooth root that is fully integrated into bone via a natural periodontal ligament would be a considerable advance in tooth replacement. Research toward achieving the aim of producing bioengineered teeth (bioteeth) has largely focused on the generation of tooth primordia that mimic those in the embryo that can be transplanted as small cell “pellets” into the adult jaw to develop into functional teeth (Yamamoto et al., 2003; Duailibi et al., 2004; Ohazama et al., 2004; Nakao et al., 2007; Ikeda et al., 2009; Oshima et al., 2011). Remarkably, despite the very different environments, embryonic tooth primordia can develop normally in the adult mouth, and thus, if suitable cells can be identified that can be combined in such a way to produce a tooth primordium, there is a realistic prospect that bioteeth can become a clinical reality. The first demonstration of the success of adult non-dental cells being used to form a biotooth came from recombinations between embryonic tooth epithelium and adult bone marrow stromal cells (Ohazama et al., 2004). Subsequent studies have largely focused on the use of embryonic cells, and although it is clear that embryonic tooth primordia cells can readily form teeth following dissociation into single-cell populations and subsequent recombination, such cell sources have little relevance for the development of a clinical therapy. What is required is the identification of adult sources of human epithelial and mesenchymal cells that can be obtained in sufficient numbers to make biotooth formation a viable alternative to dental implants.
Teeth develop from reciprocal interactions between embryonic oral epithelial cells and neural-crest-derived mesenchyme (Jernvall and Thesleff, 2000; Tucker and Sharpe, 2004; Zhang et al., 2005). At the initiation of tooth development, the epithelium provides the first instructional signals to the mesenchyme (Thesleff et al., 2001; Hu et al., 2005; Lesot and Brook, 2009). The mesenchyme then reciprocates signals back to the epithelum. To bioengineer a tooth primordium from non-embryonic tooth cells, one of the cell populations, epithelial or mesenchymal, must be capable of providing these inductive signals to the other. Thus, in heterotypic tissue recombinations, early dental epithelium is able to induce tooth formation in a non-dental mesenchyme, as long as such mesenchyme has stem-cell-like properties. Similarly, tooth bud mesenchyme is able to induce tooth formation in a non-dental epithelium as long as the epithelial cells are undifferentiated. The first experiments with adult bone marrow stroma containing mesenchymal stem cells as a source showed that the embryonic inductive tooth epithelium could induce tooth formation in an adult non-dental mesenchymal cell population (Ohazama et al., 2004). We show here that the reciprocal inductive interaction between adult epithelial cells and embryonic tooth-inducing mesenchyme will also produce teeth.
Materials & Methods
Isolation of Human Gingival Epithelial Cells
From patients who provided informed consent, gingival tissue was collected while they underwent routine oral surgery procedures at the Department of Oral Surgery, King’s College Hospital, London. The study was approved and followed the guidelines set by the Ethical Committee for human studies at King’s College Hospital, King’s College University of London. The gingival tissues were washed with Ca2+(−), Mg2+(−), and Dulbecco’s phosphate-buffered saline [DPBS(−); Gibco Life Technologies Ltd, Paisley, UK] containing 100 U/mL penicillin/streptomycin, and incubated in 1.2 U/mL Dispase II (Roche, Lewes, UK) in DPBS(−) at 37°C for 60 min. After enzymatic separation, the epithelium was readily peeled off the underlying fibrous connective tissue by means of fine tungsten needles. For creation of the epithelial explants, the isolated gingival epithelia were cut into pieces in DPBS(−) and plated in 6-well dishes (BD Primaria™, Franklin Lakes, NJ, USA). The cells were grown in feeder-free and serum-free conditions with progenitor cell targeted media CNT24 (CELLnTECTM media, Bern, Switzerland) at 37°C. After 2 days in culture, outgrowth of typical cobblestone epithelial cells around the explant was evident, and there were no mesenchymal cells present. At 90% confluence, the cells were trypsinized with 0.04% Trypsin and 0.03% EDTA solution (PromoCell™, Heidelberg, Germany) and centrifuged at 400 g for 5 min. The cell pellets were then re-suspended in CNT24 (CELLnTEC™ media) and plated 6 x 103 per sm2 on Hydrocell low binding plates (Nunc, Thermo Fisher Scientific Inc., Waltham, MA, USA).
After 3 days in culture, cell clusters were collected by means of pipettes, and gentle pipetting was used to break the clusters into single cells, which were centrifuged at 400 g for 5 min; the resulting pellets were used in the re-association experiment.
Re-association and Tissue Culture
Tooth germs were dissected from lower molars of CD-1 mouse embryos at E14.5 and trimmed from any surrounding tissue. The tooth germs were incubated in DPBS (−) containing 1.2 U/mL Dispase II (Roche) for 15 min at RT. Epithelium and mesenchyme were separated with fine needles. The molar mesenchyme tissues were used for re-association with human gingival epithelial cells.
Using fine Eppendorf Geloader tips (200), we injected the human epithelial cells into the top of the mesenchyme tissue until the surface of the mouse mesenchyme was completed covered with cells inside a 20-μL gel drop of Cellmatrix type I-A (Nitta gelatin, Osaka, Japan), placed on a cell culture insert (4.0-μm pore size; BD, Franklin Lakes, NJ, USA). The re-association was cultured for 5 days on the cell culture insert containing 1.5 mL/well DMEM containing 20% FCS, 100 U/mL penicillin/streptomycin, and 0.18 mg/mL L-ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA).
Kidney Transplantation and microCT Scans
After 7 days of culture, the re-associations were transplanted into kidney capsules of adult immunocompromised (SCID) mice according to procedures approved by a Home Office Project license to P. Sharpe. The host mice were sacrificed after 6 wks, and the kidneys were fixed in 4% paraformaldehyde (PFA) in PBS overnight at 4°C. After being washed with PBS, specimens for microCT were scanned by means of a GE Locus SP microCT scanner (GE Healthcare, Little Chalfont, UK). The specimens were scanned to produce 6.5-μm-voxel-size volumes, with an x-ray tube voltage of 80 kVp and a tube current of 80 μA. An aluminum filter (0.05 mm) was used to adjust the energy distribution of the x-ray source. The specimens were characterized further by three-dimensional slice volumes, generated and measured with Microview software (GE).
Histology and Immunohistochemistry
Specimens were decalcified with 10% ethylenediaminetetraacetic acid (EDTA) solution for 2 wks at 4°C, dehydrated in graded ethanol, and then embedded in paraffin. Serial sections (8 μm thick) were mounted on slides and stained with hematoxylin-eosin (H&E).
For immunohistochemical analysis, the transplanted tissues were fluorescently stained with: anti-human MHC class 1 antibody (Abcam ab52922, Cambridge, UK); anti-dentin sialoprotein DSPP (MABT 37) (Millipore, Temecula, CA, USA); and osteopontin OPN (LFMb-14) sc-73631 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Further, we used a Tyramide Signal Amplification kit (PerkinElmerTM RenaissanceR TSA fluorescence systemTM, Waltham, MA, USA), following the instructions of the manufacturer.
Results
Isolation and Growth of Human Gingival Epithelial Cells
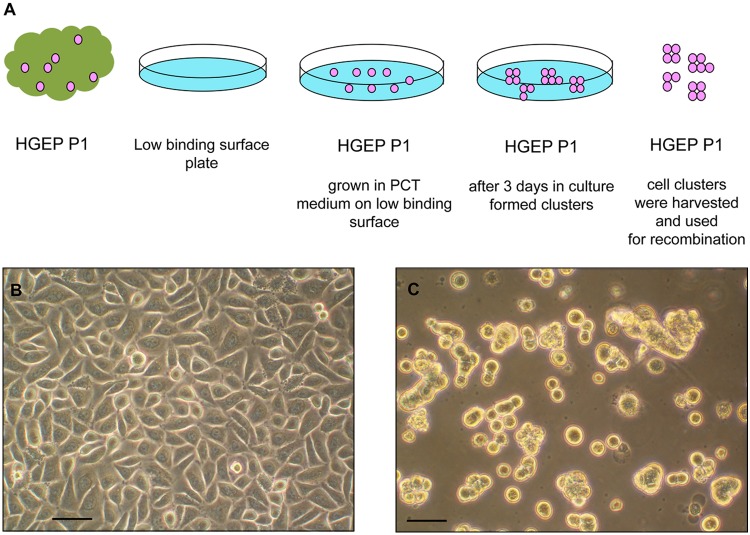
We used a combined method of enzymatic treatment and explant culture to establish epithelial cell cultures from gingivae. Dispase was used to separate the epithelium from the underlying connective tissue of the gingivae. The resulting epithelial explants were cultured to allow cells to grow from the explants. The epithelial cells exhibited typical cuboidal morphology and cobblestone growth (Fig. 1A). Upon reaching 90% confluence, the cells were harvested and centrifuged at 400 g for 5 min. The cell pellets were then re-suspended in CNT24, harvested, and centrifuged at 400 g for 5 min. The pellets were then re- suspended in CNT24 (CELLnTEC™ media) and plated on low-binding plates.
Figure 1.
Growth of gingival epithelial cells. (A) Diagrammatic presentation of the growing conditions of primary human gingival epithelial cells. Human gingival epithelial cells were used on passage 1, grown in feeder- and serum-free conditions. On passage 1, they were plated on low-binding plates and grown in progenitor-cell-targeted (PCT) medium. The formed cell clusters were collected after 3 days and used in the experiment. (B) Primary human gingival epithelial cells were isolated to establish the epithelial cell cultures. The epithelial cells exhibited typical cuboidal morphology and cobblestone growth. (C) Epithelial cells were plated on low-binding plates, where the cells formed cell clusters of different sizes. Scale bar = 10 µm.
This procedure of growing epithelial cell clusters on low-binding plates allowed us to harvest these cells without enzymes (Fig. 1B).
Tooth Formation in Renal Capsules
Human gingival epithelial cells were combined with embryonic tooth mesencyme cells isolated from 24 mouse mandibular molar tooth primordia by means of a collagen microinjection system (see Materials & Methods). Cultures were observed between 3 and 7 days. After 3 days in culture, cell aggregations were formed, and by day 5, round, tooth-like structures were visible with an appearance of the late cap stage (Appendix Fig. 1). On the seventh day of culture, the explants were surgically transferred into renal capsules of SCID mice and left for 6 wks. MicroCT analysis of the transferred tissues revealed hard-tissue formation, and 3D reconstruction showed obvious tooth-like structures (Fig. 2). From each individual tooth primordium transferred to renal capsules, 1 in 5 formed teeth (n = 30) (Fig. 2).
Figure 2.
MicroCT analysis of tissues after development. (A) Epithelial-mesenchymal recombinations after 6 wks in vivo development in renal capsules. Hard-tissue formation is evident, and 3D reconstructions show obvious multiple tooth-like structures. (B) The teeth show typical tooth appearance, with well-developed crowns and roots. (C) Higher density mineralization can be observed in the coronal part of the tooth-like structure (marked in pink), corresponding to tooth crown enamel. (D) A “slice” CT scan image of a higher mineralized region visible with higher density, while a lower density mineralization corresponding to dentin area is elongated beyond the coronal part (as root area), forming a chamber (dental pulp chamber).
Higher density mineralization was observed in the coronal part of the tooth-like structure, corresponding to enamel of the tooth crown, while a lower density mineralization corresponding to dentin area was elongated beyond the coronal part (as root area) and formed a chamber (dental pulp chamber) (Fig. 2C, Appendix Fig. 2).
Histology was performed on the tooth structures from the renal capsules following decalcification to remove the enamel. Sections confirmed the presence of obvious teeth, with dentin, enamel spaces, and well-vascularized pulp with odontoblast-like cells expressing DSPP lining the dentin surface (Fig. 3A). Cells with an epithelial appearance and cuboidal shape were visible on the outer surface of the enamel space, indicative of an ameloblast-like cell population at a post-secretory stage (reduced enamel epithelium) (Figs. 3A, 3B).
Figure 3.

Tooth-like structures from cell recombinations. (A) Hematoxylin-eosin staining of a tooth-like structure with visible dental pulp tissue (DP), well-developed blood vessels (BV), tubular dentin (D), and cementum (C). Reduced enamel epithelium cells (black arrows) border the space where the enamel was present before decalcification (ES). Boxed areas show high-magnification images of odontoblasts and reduced enamel epithelium (REE). (B) MHC class I positive cells of human origin (white arrows) are localized around the empty space, marked as enamel space (ES) in the crown region of the bioengineered tooth structure. Scale bar = 100 µm. C = cementum, BV = blood vessel, D = dentin, ES = enamel space.
Human gingival epithelial cells, grown in the same conditions as described, and mouse embryonic tooth mesenchyme (E14.5) were used separately as controls. Six wks following renal transfer, the epithelial cells formed cyst-like structures (Appendix Fig. 3A), and the mesenchymal cells formed mineralized (bone-like) tissue (Appendix Fig. 3B), but no tooth-like structures were ever formed in either of these controls.
Contributions of Human Epithelial Cells to Tooth Tissues
To determine the origin of the ameloblast-like epithelial cells, we performed immunohistochemistry for human MHC class 1 protein. The MHC class I positive cells of human origin were localized around the empty space in the crown region of the bioengineered tooth structure, where we presume that enamel was present before the specimens were decalcified (visible in the microCT scans) (Fig. 3B).
The microCT scans revealed evidence of early stages of root formation (Fig. 2). Since the only other location for epithelial-derived cells is the rests of Malassez, we were interested to determine if these cells could be detected, and, if so, if their origin could confirm the human origin of dental epithelial cells. Immunohistochemistry for human MHC class 1 protein showed localized patches of positive-staining cells close to root dentin (Fig. 4). Histology and expression of osteocalcin confirmed these human-derived epithelial cells as being embedded within the root cementum, consistent with their being rests of Malassez (Fig. 4) (Huang et al., 2009).
Figure 4.

Tooth root formation. (A) Hematoxylin-eosin staining of sections of tooth-like structures with dentin (D) and dental pulp (DP) as well as cementum (C). Cells were visible embedded in the cellular cementum region (black arrows). Boxed area shows a high-power view of a cell arrowed. (B) Human-derived epithelial cells (white arrows) positive for the anti-human MHC class I antibody were found embedded within the root cellular cementum, consistent with their being rests of Malassez. Scale bar = 100 µm. D = dentin, DP = dental pulp, C = cementum.
Discussion
Despite some significant advances, the prospect of a clinically viable, cell-based approach for whole-tooth replacement remains a distant goal. The concept of using the basic understanding of cell interactions that occur during the early stages of natural tooth development to reproduce such interactions in vitro was first established by showing the formation of teeth from combinations of adult mesenchymal stem cells and dental epithelial cells (Ohazama et al., 2004). In this “proof-of-concept” study, the natural tooth-inducing capacity of early-stage dental epithelium was used to induce non-dental cells from adult bone marrow to participate in tooth development. Several subsequent studies have used cell combinations consisting entirely of re-associated embryonic tooth cells (Hu et al., 2005; Nakao et al., 2007; Ikeda et al., 2009; Oshima et al., 2011). Although human embryonic-like tooth cells could, in theory, be obtained from developing third molars, the potential for this as a general tooth-replacement therapy is limited. For tooth development to occur, only one of the two cell sources used, epithelial or mesenchymal, needs to be inductive, as long as the other is responsive. Classic experiments involving heterotypic recombinations established that the dental epithelium provides the first inductive signals to the underlying neural-crest-derived mesenchyme (Mina and Kollar, 1987; Lumsden, 1988). Similarly, recombination of tooth mesenchyme that had received the first inductive signals from the epithelium with non-dental epithelium forms teeth. Significantly, these experiments also suggested that responding mesenchyme needed to have stem-cell-like properties in common with those of cranial neural crest cells, whereas responding epithelial cells did not. This is consistent with the relative contributions of epithelial and mesenchymal cells to tooth structure, where mesenchymal cells need to be capable of forming multiple differentiated cell types (Tucker and Sharpe, 2004). These developmental results establish that, for bioengineered tooth primordial to be formed, a source of inducing cells needs to be combined with a source of responding cells, and the source of mesenchymal cells, either inducing or responding, needs to have stem-cell-like properties. More recent recombinations with dissociated tooth primordia epithelial and mesenchymal cells have confirmed the original tissue recombination results and, in addition, showed that such cell-based recombinations require significantly larger numbers of cells than are present in a single tooth primordium (Hu et al., 2005; Nakao et al., 2007; Ikeda et al., 2009). The logical conclusion, therefore, is that clinically feasible (i.e., non-embryonic) sources of either epithelial or mesenchymal cells with tooth-inducing or responding capacity need to be identified that can be expanded in culture to provide sufficient cell numbers for successful tooth formation following recombination. To date, the only non-dental source of adult cells that have successfully been used in bioengineered tooth formation is mesenchymal cells derived from bone marrow stroma (Ohazama et al., 2004). This cell population contains mesenchymal stem cells and, when combined with inducing embryonic dental epithelium, is capable of forming teeth.
We describe here the isolation and culture of a population of human adult epithelial cells from oral mucosa that, when combined with mouse embryonic tooth-inducing mesenchyme cells, form teeth. The epithelial cell contribution to these teeth includes ameloblast-like cells and rests of Malassez (Kat et al., 2003; Becktor et al., 2007; Sonoyama et al., 2007; Shinmura et al., 2008; Huang et al., 2009). Thus, in addition to being able to respond in an embryonic-like manner to the mesenchymal-inducing signals, the adult epithelial cells are also able to differentiate into appropriate specialized epithelial cell derivatives and fully contribute to tooth structure. Whereas tooth primordia formed in culture with a high frequency from the recombination of the adult human epithelial cells and mouse embryonic mesenchyme, only 20% of these transplanted into renal capsules formed complete teeth. We attribute this to the difficulties in handling the primordia that, during transfer, often showed physical separation of the epithelium and mesenchyme.
The epithelial-derived ameloblast-like cells are located in the position consistent with ameloblasts adjacent to the enamel space. They do not have the classic elongated shape of ameloblasts but more closely resemble reduced enamel epithelium that is formed when maturation is complete.
The method we describe for the growth of epithelial cells from gingival tissue is simple, does not require feeder cells, and uses serum-free medium, important considerations for clinical applications. Previous studies have suggested that palatal mucosal epithelium from post-natal mice can be grown as cell sheets in serum-supplemented medium in the presence of the ROCK inhibitor, Y-27632, and form tooth-like structures when combined with mouse-inducing mesenchyme (Nakagawa et al., 2009). In another study, with the oral epithelium of p53- deficient fetal mice at embryonic day 18 (E18), clonal cell lines were established that formed calcified structures as seen in natural teeth, when recombined with E16.5 dental mesenchyme (Takahashi et al., 2010). Another study provided evidence that the addition of exogenous FGF proteins was required for the formation of ameloblast-like cells in recombinations between human newborn skin keratinocyte progenitors and mouse-inducing mesenchymal cells (Wang et al., 2010). In our study of human epithelial cells grown in serum-free, chemically defined conditions, we show root structures with human epithelial cells present in the cellular cementum. This is in accordance with the previously reported role of the Hertwig’s epithelial root sheath, an ectoderm-derived outer and inner enamel epithelium, which, during root development, breaks up into epithelial rests and cords (known as rests of Malassez). These cells were reported to be embedded in the cellular cementum in the same way as observed in our study. Thus, epithelial cells derived from adult human gingival tissue are capable of responding to tooth-inducing signals from embryonic tooth mesenchyme in an appropriate way to contribute to tooth crown and root formation and give rise to relevant differentiated cell types, following in vitro culture. These epithelial cells are thus a realistic source for consideration for use in human biotooth formation.
Supplementary Material
Acknowledgments
We thank Tara Renton and Zehra Yilmaz for recruitment of patients. We thank our collaborators, Christopher Healy for the MicroCT and Alex Huhn for the mouse surgery.
Footnotes
This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Becktor KB, Nolting D, Becktor JP, Kjaer I. (2007). Immunohistochemical localization of epithelial rests of Malassez in human periodontal membrane. Eur J Orthod 29:350-353. [DOI] [PubMed] [Google Scholar]
- Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. (2004). Bioengineered teeth from cultured rat tooth bud cells. J Dent Res 83:523-528. [DOI] [PubMed] [Google Scholar]
- Fugazzotto PA. (2008). Shorter implants in clinical practice: rationale and treatment results. Int J Oral Maxillofac Implants 23:487-496. [PubMed] [Google Scholar]
- Hasan I, Heinemann F, Bourauel C. (2011). The relationship of bone resorption around dental implants to abutment design: a preliminary 1-year clinical study. Int J Prosthodont 24:457-459. [PubMed] [Google Scholar]
- Heinemann F, Hasan I, Schwahn C, Biffar R, Mundt T. (2010). Crestal bone resorption around platform-switched dental implants with fine threaded neck after immediate and delayed loading. Biomed Tech (Berl) 55:317-321. [DOI] [PubMed] [Google Scholar]
- Hu B, Nadiri A, Bopp-Kuchler S, Perrin-Schmitt F, Wang S, Lesot H. (2005). Dental epithelial histo-morphogenesis in the mouse: positional information versus cell history. Arch Oral Biol 50:131-136. [DOI] [PubMed] [Google Scholar]
- Huang X, Bringas P, Slavkin HC, Chai Y. (2009). Fate of HERS during tooth root development Dev Biol 334:22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, et al. (2009). Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci USA 106:13475-13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. (2000). Reiterative signalling and patterning during mammalian tooth morphogenesis. Mech Dev 92:9-29 [DOI] [PubMed] [Google Scholar]
- Kat PS, Sampson WJ, Wilson DF, Wiebkin OW. (2003). Distribution of the epithelial rests of Malassez and their relationship to blood vessels of the periodontal ligament during rat tooth development. Aust Orthod J 19:77-86. [PubMed] [Google Scholar]
- Kim JJ, Lee DW, Kim CK, Park KH, Moon IS. (2010). Effect of conical configuration of fixture on the maintenance of marginal bone level: preliminary results at 1 year of function. Clin Oral Implants Res 21:439-444. [DOI] [PubMed] [Google Scholar]
- Lesot H, Brook AH. (2009). Epithelial histogenesis during tooth development Arch Oral Biol 54(Suppl 1):25-33. [DOI] [PubMed] [Google Scholar]
- Lumsden AG. (1988). Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103(Suppl):155-169. [DOI] [PubMed] [Google Scholar]
- Maiorana C, Sigurt ÃD, Mirandola A, Garlini G, Santoro F. (2005). Bone resorption around dental implants placed in grafted sinuses: clinical and radiologic follow-up after up to 4 years. Int J Oral Maxillofac Implants 20:261-266. [PubMed] [Google Scholar]
- Mina M, Kollar EJ. (1987). The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol 32:123-127. [DOI] [PubMed] [Google Scholar]
- Moeintaghavi A, Radvar M, Kadkhodazadeh M, Arab HR. (2007). Clinical and radiographical evaluation of maximus implants, with immediate nonfunctional loading. Int J Oral Maxillofac Surg 36:1092. [Google Scholar]
- Nakagawa E, Itoh T, Yoshie H, Satokata I. (2009). Odontogenic potential of post-natal oral mucosal epithelium. J Dent Res 88:219-223. [DOI] [PubMed] [Google Scholar]
- Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. (2007). The development of a bioengineered organ germ method. Nat Methods 4:227-230. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Modino SA, Miletich I, Sharpe PT. (2004). Stem-cell-based tissue engineering of murine teeth. J Dent Res 83:518-522. [DOI] [PubMed] [Google Scholar]
- Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, et al. (2011). Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 6:e21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapoff CA, Lahey B, Wasserlauf PA, Kim DM. (2010). Radiographic analysis of crestal bone levels around Laser-Lok collar dental implants. Int J Periodontics Restorative Dent 30:129-137. [PubMed] [Google Scholar]
- Shin YK, Han CH, Heo SJ, Kim S, Chun HJ. (2006). Radiographic evaluation of marginal bone level around implants with different neck designs after 1 year. Int J Oral Maxillofac Implants 21:789-794. [PubMed] [Google Scholar]
- Shinmura Y, Tsuchiya S, Hata K, Honda MJ. (2008). Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. J Cell Physiol 217:728-738. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Seo BM, Yamaza T, Shi S. (2007). Human Hertwig’s epithelial root sheath cells play crucial roles in cementum formation. J Dent Res 86:594-599. [DOI] [PubMed] [Google Scholar]
- Takahashi C, Yoshida H, Komine A, Nakao K, Tsuji T, Tomooka Y. (2010). Newly established cell lines from mouse oral epithelium regenerate teeth when combined with dental mesenchyme. In Vitro Cell Dev Biol Anim 46:457-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I, Keränen S, Jernvall J. (2001). Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv Dent Res 15:14-18. [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe P. (2004). The cutting-edge of mammalian development: how the embryo makes teeth. Nat Rev 5:499-508. [DOI] [PubMed] [Google Scholar]
- Wang B, Li L, Du S, Liu C, Lin X, Chen Y, et al. (2010). Induction of human keratinocytes into enamel-secreting ameloblasts. Dev Biol 344:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kim EJ, Cho SW, Jung HS. (2003). Analysis of tooth formation by reaggregated dental mesenchyme from mouse embryo. J Electron Microsc (Tokyo) 52:559-566. [DOI] [PubMed] [Google Scholar]
- Zhang YD, Chen Z, Song YQ, Liu C, Chen YP. (2005). Making a tooth: growth factors, transcription factors and stem cells. Cell Res 15:301-316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.