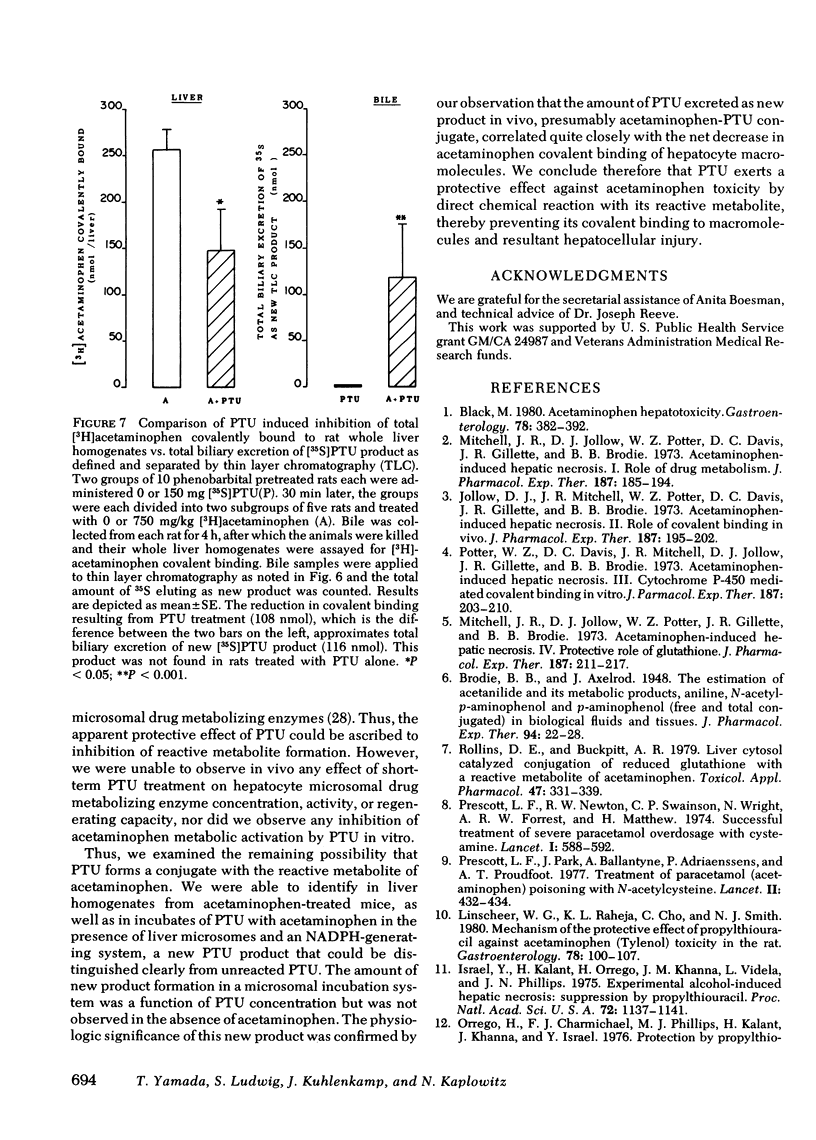
Abstract
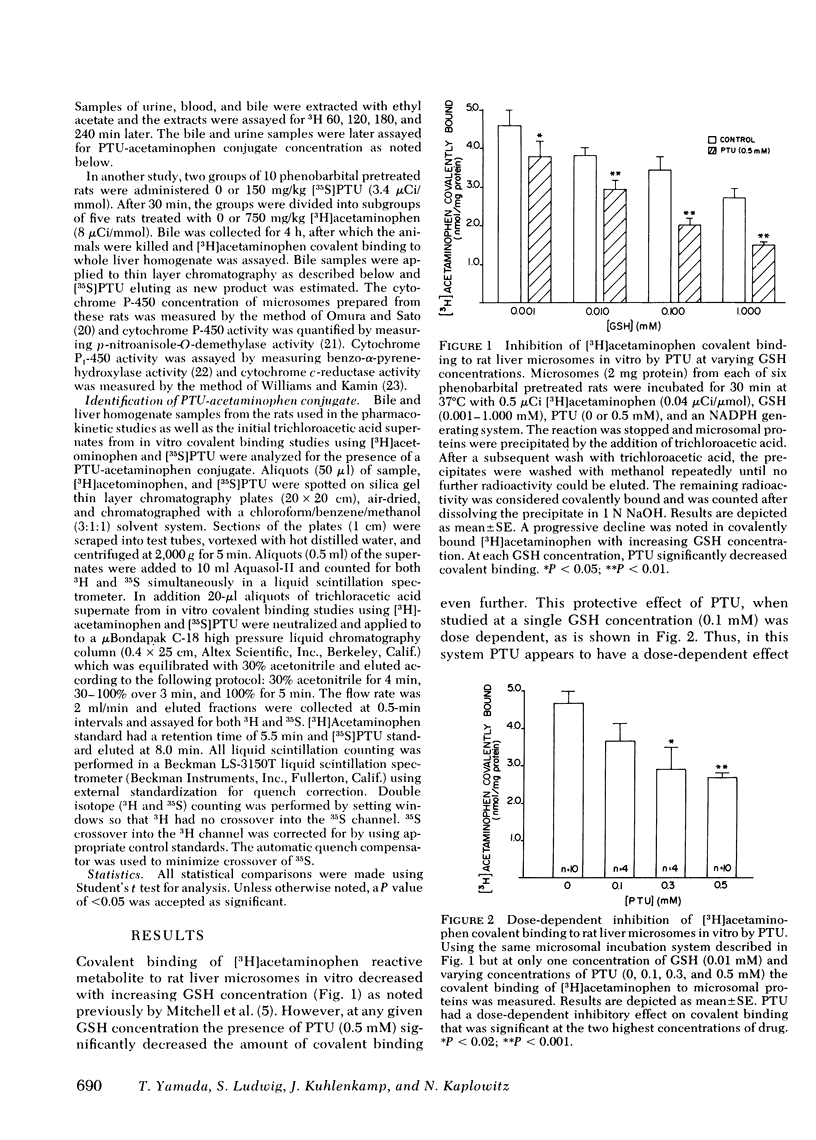
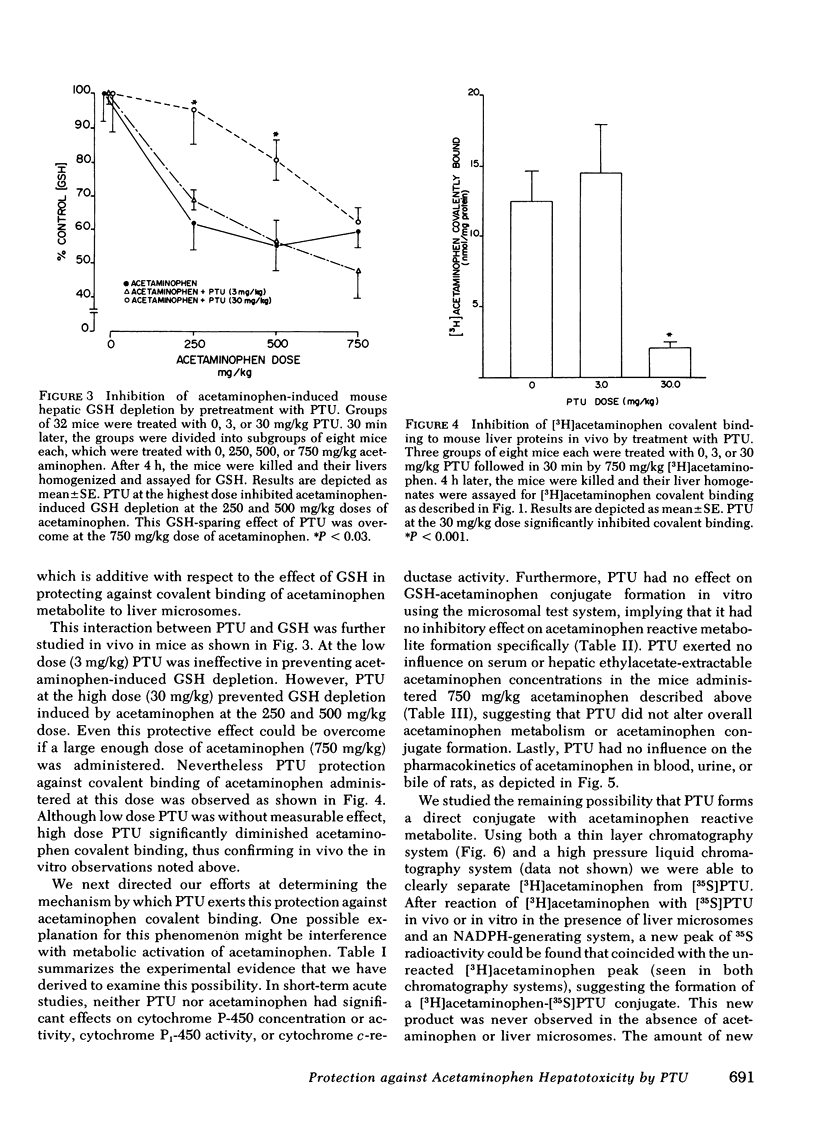
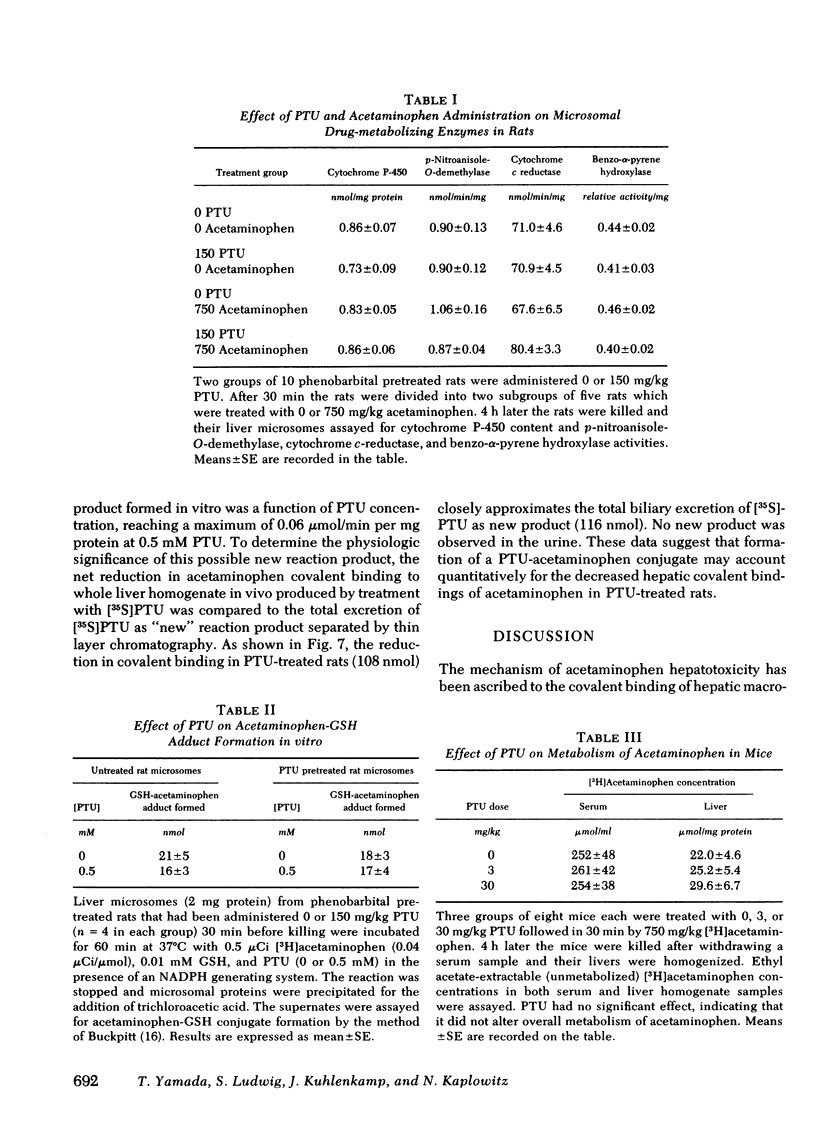
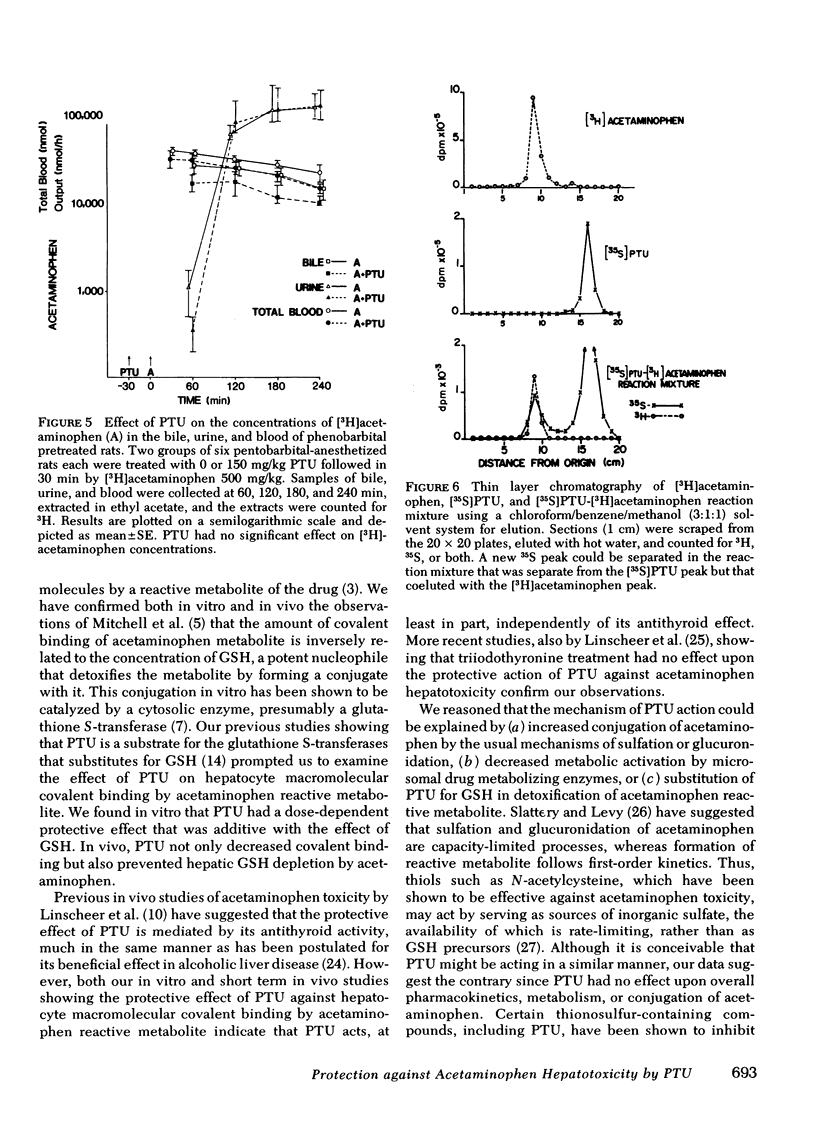
Hepatotoxicity caused by acetaminophen can be prevented by enzyme-catalyzed conjugation of its reactive metabolite with glutathione (GSH). Since we have shown in previous studies that 6-N-propyl-2-thiouracil (PTU) can substitute for GSH as a substrate for the GSH S-transferases, we examined the possibility that PTU might also protect against acetaminophen hepatotoxicity by direct chemical interaction with the reactive metabolite of acetaminophen. In an in vitro system consisting of [3H]acetaminophen, liver microsomes from phenobarbital-pretreated rats, and an NADPH-generating system, we found that PTU had a dose-dependent additive effect with GSH on inhibition of acetaminophen covalent binding. PTU administration also resulted in a dose-dependent decrease in both GSH depletion and covalent binding in vivo in acetaminophen-treated mice. To examine the possible mechanisms by which PTU exerts its protective effect, we studied the action of PTU on both acetaminophen conjugation and metabolic activation. PTU had no effect upon acetaminophen pharmacokinetics in phenobarbital-pretreated rats, as examined by measuring acetaminophen concentration in bile, urine, and blood after an intraperitoneal dose, nor did it alter the total amount of polar conjugates formed. Microsomes from PTU-treated rats were unaltered in cytochrome P-450 concentrations and p-nitroanisole-O-demethylase, benzo-α-pyrene hydroxylase, and cytochrome c-reductase activities. Furthermore PTU did not decrease acetaminophen-GSH adduct formation in vitro, suggesting that there was no reduction in drug activation. However, in bile from [35S]PTU and [3H]acetaminophen treated rats, as well as in incubates of the two drugs with liver microsomes, a new 35S- and 3H-containing product could be identified. By both thin layer chromatography and high pressure liquid chromatography this new product, which co-eluted with [3H]acetaminophen, was separated from unreacted [35S]PTU. The formation of this product in vitro was a function of PTU concentration and reached a maximum of 0.06 μmol/min per mg protein at 0.5 mM PTU. In vivo, the total biliary excretion of this product over 4 h (116 nmol) equaled the net reduction in acetaminophen metabolite covalent binding in the liver of phenobarbital-pretreated rats (108 nmol). We conclude that PTU, independent of its antithyroid effect, diminishes hepatic macromolecular covalent binding of acetaminophen reactive metabolite both in vivo and in vitro, and it does so by detoxifying the reactive metabolite through direct chemical interaction in a manner similar to GSH. These observations may define the mechanism by which PTU is protective against liver injury caused by acetaminophen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black M. Acetaminophen hepatotoxicity. Gastroenterology. 1980 Feb;78(2):382–392. [PubMed] [Google Scholar]
- Buckpitt A. R., Rollins D. E., Nelson S. D., Franklin R. B., Mitchell J. R. Quantitative determination of the glutathione, cysteine, and N-acetyl cysteine conjugates of acetaminophen by high-pressure liquid chromatography. Anal Biochem. 1977 Nov;83(1):168–177. doi: 10.1016/0003-2697(77)90522-x. [DOI] [PubMed] [Google Scholar]
- Dehnen W., Tomingas R., Roos J. A modified method for the assay of benzo(a)pyrene hydroxylase. Anal Biochem. 1973 Jun;53(2):373–383. doi: 10.1016/0003-2697(73)90083-3. [DOI] [PubMed] [Google Scholar]
- Galinsky R. E., Levy G. Effect of N-acetylcysteine on the pharmacokinetics of acetaminophen in rats. Life Sci. 1979 Aug 20;25(8):693–699. doi: 10.1016/0024-3205(79)90511-3. [DOI] [PubMed] [Google Scholar]
- Hunter A. L., Neal R. A. Inhibition of hepatic mixed-function oxidase activity in vitro and in vivo by various thiono-sulfur-containing compounds. Biochem Pharmacol. 1975 Dec 1;24(23):2199–2205. doi: 10.1016/0006-2952(75)90052-0. [DOI] [PubMed] [Google Scholar]
- Israel Y., Kalant H., Orrego H., Khanna J. M., Videla L., Phillips J. M. Experimental alcohol-induced hepatic necrosis: suppression by propylthiouracil. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1137–1141. doi: 10.1073/pnas.72.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y., Walfish P. G., Orrego H., Blake J., Kalant H. Thyroid hormones in alcoholic liver disease. Effect of treatment with 6-n-propylthiouracil. Gastroenterology. 1979 Jan;76(1):116–122. [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973 Oct;187(1):195–202. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linscheer W. G., Raheja K. L., Cho C., Smith N. J. Mechanism of the protective effect of propylthiouracil against acetaminophen (Tylenol) toxicity in the rat. Gastroenterology. 1980 Jan;78(1):100–107. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- NETTER K. J., SEIDEL G. AN ADAPTIVELY STIMULATED O-DEMETHYLATING SYSTEM IN RAT LIVER MICROSOMES AND ITS KINETIC PROPERTIES. J Pharmacol Exp Ther. 1964 Oct;146:61–65. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OWENS C. W., BELCHER R. V. A COLORIMETRIC MICRO-METHOD FOR THE DETERMINATION OF GLUTATHIONE. Biochem J. 1965 Mar;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego H., Kalant H., Israel Y., Blake J., Medline A., Rankin J. G., Armstrong A., Kapur B. Effect of short-term therapy with propylthiouracil in patients with alcoholic liver disease. Gastroenterology. 1979 Jan;76(1):105–115. [PubMed] [Google Scholar]
- Potter W. Z., Davis D. C., Mitchell J. R., Jollow D. J., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther. 1973 Oct;187(1):203–210. [PubMed] [Google Scholar]
- Prescott L. F., Newton R. W., Swainson C. P., Wright N., Forrest A. R., Matthew H. Successful treatment of severe paracetamol overdosage with cysteamine. Lancet. 1974 Apr 6;1(7858):588–592. doi: 10.1016/s0140-6736(74)92649-x. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A. T. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977 Aug 27;2(8035):432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Rollins D. E., Buckpitt A. R. Liver cytosol catalyzed conjugation of reduced glutathione with a reactive metabolite of acetaminophen. Toxicol Appl Pharmacol. 1979 Feb;47(2):331–339. doi: 10.1016/0041-008x(79)90328-4. [DOI] [PubMed] [Google Scholar]
- Slattery J. T., Levy G. Acetaminophen kinetics in acutely poisoned patients. Clin Pharmacol Ther. 1979 Feb;25(2):184–195. doi: 10.1002/cpt1979252184. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]
- Yamada T., Kaplowitz N. Propylthiouracil. A substrate for the glutathione S-transferases that competes with glutathione. J Biol Chem. 1980 Apr 25;255(8):3508–3513. [PubMed] [Google Scholar]



