Abstract
Background
Low dietary intakes of vitamin D and calcium hasten bone loss and osteoporosis. Because vitamin D metabolites may also alter the inflammatory response and have anti-microbial effects, we studied whether use of vitamin D and calcium supplements affects periodontal disease status.
Methods
A cohort of 51 subjects receiving periodontal maintenance therapy was recruited from 2 dental clinics. Of these, 23 were taking vitamin D (≥400 international units/day) and calcium (≥1000mg/day) supplementation, and 28 were not taking such supplementation. All subjects had ≥2 interproximal sites with ≥3 mm clinical attachment loss. Daily calcium and vitamin D intakes (from food and supplements) were estimated by nutritional analysis. The following clinical parameters of periodontal disease were recorded for the mandibular posterior teeth: gingival index, probing depth, cementoenamel junction-gingival margin distance (attachment loss), bleeding upon probing, and furcation involvement. Posterior photostimulable-phosphor bitewing radiographs were taken to determine cementoenamel-junction-alveolar-crest distances (alveolar crest height loss). Data were analyzed with a repeated-measures, multivariate analysis of variance.
Results
Relative to subjects who did not take vitamin D and calcium supplementation, supplement takers had shallower probing depths, fewer bleeding sites, lower gingival index values, fewer furcation involvements, less attachment loss and less alveolar crest height loss. The repeated-measures analysis indicated that collectively these differences for clinical parameters were borderline significant (P=0.08).
Conclusion
In these subjects receiving periodontal maintenance therapy, there was a trend for better periodontal health with intake of vitamin D and calcium supplementation. More expanded longitudinal studies are required to determine the potential of this relationship.
Keywords: vitamin D, calcium, chronic periodontitis, alveolar bone
Introduction
Most epidemiological data suggest that alveolar bone loss is greater in individuals with low bone mass or osteoporosis,1-12 although such an association is not universally accepted.13, 14 It is likely that chronically low intakes of vitamin D and calcium may lead to a negative calcium balance, thus causing a secondary increase in calcium removal from bone, including the alveolar bone. Such bone loss may contribute to weakening the tooth attachment apparatus. In addition to its action on skeletal homeostasis, vitamin D and in particular its hormonally active form, 1α,25-dihydroxyvitamin D has anti-inflammatory and anti-microbial effects, via modulation of inflammatory cytokine production by immune cells, and stimulated secretion of peptides with anti-bacterial action by cells of the monocyte-macrophage lineage.15-20 These multiple actions of vitamin D are potentially appealing for the management of patients with periodontal disease, whose pathogenesis is based on chronic bacterial-driven inflammation. The inflammatory response leads to tissue destruction either by direct action of bacterial products, or by activation of host defense cells and secretion of inflammatory mediators. These locally produced factors eventually result in connective-tissue breakdown and bone loss via activation of osteoclast mediated bone resorption.21, 22
Average vitamin D and calcium intakes in the general population are below current recommendations of 400-600 IU and 1000-1200 mg daily, respectively. 23 Although there is a growing consensus that such daily targets are inadequate, and higher vitamin D intakes (800-1000 IU daily) are now recommended by professional organizations,24 it has been estimated that one billion people worldwide have vitamin D deficiency or insufficiency. 25, 26 While the beneficial effects of calcium and vitamin D supplementation on bone health have been well recognized; 27-29 their potential role in periodontal disease has not been fully determined. A number of studies have suggested that vitamin D and/or calcium intake results in reduced alveolar bone loss, gingival inflammation, and/or attachment loss.30-37 Most relevant are data on subjects (about 12,000) enrolled in the Third National Health and Nutrition Examination Survey (NHANES III), suggesting that lower dietary intake of calcium increased attachment loss in a dose-dependent fashion.38 In another large cohort (about 6700 subjects), an association between serum concentrations of 25-hydroxyvitamin D (25OHD) and gingival inflammation was found, possibly linked to vitamin D′s anti-inflammatory effect.39 A potential role of vitamin D in periodontal health is also supported by findings that polymorphisms of the vitamin D receptor gene are associated with periodontitis, alveolar bone loss, clinical attachment loss, and/or tooth loss. 40, 41 Thus, there is evidence pointing to a potential role of vitamin D and calcium intake on dental health; however, the possible effects of such dietary supplementation on periodontal disease parameters and outcomes has not been addressed.
The objective of our study was to determine if the use of calcium and vitamin D oral supplementation by subjects attending periodontal disease maintenance programs would have an impact on clinical parameters of periodontal health.
Materials and Methods
Study Design
This cross-sectional, observational study was conducted with subjects who were recruited between June 2007 and February 2008 from the Graduate Periodontics clinics at Saint Louis University Center for Advanced Dental Education and Southern Illinois University School of Dental Medicine. Our Health-Insurance-Portability-and-Accountability-Act, compliant study was approved by the Institutional Review Boards at Washington University School of Medicine, Saint Louis University Center for Advanced Dental Education, and Southern Illinois University School of Dental Medicine. All subjects signed informed consent documents for participation in the study. They were patients in periodontal maintenance programs and received regular maintenance therapy.
Inclusion and exclusion criteria
Our goal was to enroll 23 subjects who were taking vitamin D (≥400 international units/day) and calcium (≥1000 mg/day) supplementation for more than 18 months (“takers”) and 23 subjects who were taking neither vitamin D nor calcium supplementation and had dietary intakes of calcium <1000 mg/day and of vitamin D < 400 international units/day (“non-takers”). Subjects were asked to bring their bottles of oral supplementation to their baseline appointments and were asked how long they had been taking oral supplements. Subjects had previously filled out institutional-review-board-approved questionnaires to determine their levels of oral supplementation.42 Each subject's food intake was analyzed for calcium and vitamin D using a semi–quantitative, food-frequency questionnaire1 which was originally based on the second National Health and Nutrition Examination Survey (NHANES) II data and revised using NHANES-III, food-intake data. 43, 44 The results were reviewed by a registered dietitian (CAS) at Washington University's Center for Applied Research Sciences.
Additionally, subjects had to have moderate to severe chronic periodontal disease (≥ 2 interproximal sites with 3 mm or greater clinical attachment loss)45; and a minimum of one maxillary and two mandibular posterior teeth. We enrolled postmenopausal women (≥ 5 years since last menstrual period) and men aged 50 to 80.
The following exclusion criteria were used: 1) periodontal surgery within the last year; 2) scaling and root planing as part of initial periodontal therapy, within the past six months; 3) history of diabetes; 4) history of diseases, conditions, or use of medications that might affect bone and mineral metabolism and/or periodontal health ; 5) treatment with fluorides for more than 2 weeks within 24 months of enrollment; 6) treatment with estrogen within the last 6 months; 7) treatment with bisphosphonates in the past 12 months or lifetime exposure to bisphosphonates for more than 3 years; 8) body mass indexes (wt/ht2) < 18.5 or ≥ 33; 9) behavioral eating disorders; and 10) current treatment with antibiotics.
Clinical assessment
The following periodontal measurements were recorded: gingival index,46 probing depth, cementoenamel junction-gingival margin distance (CEJ-GM) (attachment loss), bleeding upon probing, and furcation involvement.47 A University of North Carolina (UNC) #15 probe2 was used for the measurements. Clinical measurements were recorded at six sites (buccal, lingual, mesiolingual, mesiobuccal, distolingual, distobuccal) for each mandibular posterior tooth. All clinical data were entered into computerized forms at chair side.
All probing depth and CEJ-GM measurements were made twice for each patient and the average recorded for each site. If the two measurements at a site varied by more than 1 mm, the measurement for that site was repeated until two consecutive identical measurements were obtained.48. One masked examiner (NMG) performed all clinical procedures at both institutions. She was calibrated for intra and inter-examiner error. The calibration protocol involved measuring five representative subjects twice by the operator and the reference standard examiner. The operator was considered calibrated for the gingival and furcation indices once she achieved at least 80% intra- and inter-examiner exact reproducibility plus 95% intra- and inter-examiner reproducibility within ± 1 index unit. For clinical attachment loss and probing depth, calibration was based on: 1) 85% and 90% intra-examiner reproducibility within ± 1 mm respectively; 2) at least, 95% intra-examiner reproducibility within ± 2 mm for both parameters; and 3) at least, 75% inter-examiner agreement for probing depth within ± 1 mm and at least 60% inter-examiner agreement for clinical attachment loss within ± 1 mm. For bleeding on probing, the examiner and the reference standard examiner observed each other and discussed and evaluated the criteria for this variable.
Radiographic assessment
Photostimulable-phosphor bitewing radiographs were taken of the mandibular posterior teeth. Methods are described in detail elsewhere.49, 50 In brief, subjects were rigidly attached to the x-ray tube by means of a vacuum coupling device and custom cross-arch bite plates with occlusal registration. Custom software and NIH ImageJ 51 were used for image registration and measurements. Cementoenamel-junction-alveolar-crest-height (CEJ-AC) measurements were made at the mesial and distal of posterior teeth. The root-mean-square standard deviation for these measurements is 0.18 mm (0.14 - 0.24 mm, 95% confidence interval). 52
Statistical analyses
Means and 95% confidence intervals were calculated for all measurements, including vitamin D and calcium intakes. The Student's t test was used to test for differences between takers and non-takers for each clinical measurement. Repeated measures multivariate analysis of variance was used to determine the extent to which clinical measurements when considered collectively were significantly different between the 23 subjects who took oral supplementation (takers) and the 28 subjects who did not (non-takers) take oral supplementation. In this analysis, clinical measures were the dependent variables (repeated measures, within subject factors) and whether or not subjects did or did not take oral supplementation was the between-group factor. The covariates of race, smoking, gender, and sunshine exposure53 were included in the analysis. Our study was powered (80%) to detect a between-group mean CEJ-AC difference of 0.2 mm over a period of 1 year. Here we report cross-sectional, baseline differences for the two groups.
Results
Although all of the subjects enrolled in the non-taker group took no calcium nor vitamin D oral supplementation, full dietary analysis revealed that 5 had dietary intakes of calcium above 1000 mg/day and one had a vitamin D intake above 400 IU/day. These subjects were retained in the non-taker group, but 5 additional subjects taking no supplements were enrolled in the non-taker group, and these met all the enrollment criteria. In addition, one subject on oral calcium supplementations was found to have a total intake of calcium of only 897 mg/day. This subject was retained in the taker group. The final study population included 23 subjects in the taker group and 28 subjects in the non-taker group. Of the 51 subjects enrolled, 24 were enrolled at Saint Louis University and 27 were enrolled at Southern Illinois University. Subjects using supplements had been taking them for an average of 10.6 (6.3-23 years, 95% confidence interval). The average age of the taker group was 63.9 (61.1-66.7 years). The average age of the non-taker group was 62.0 (58.4-65.7 years). Table 1 contains demographic data on gender, race, and smoking history for both groups.
Table 1.
Demographics.
| Taker n (%) | Non-taker n (%) | Totals n (%) | |
|---|---|---|---|
| Gender | |||
| Male | 6 (13%) | 16 (31%) | 32 (44%) |
|
|
|||
| Female | 17 (33%) | 12 (23%) | 29 (56%) |
|
|
|||
| Totals | 23 (46%) | 28 (54%) | 51 (100%) |
|
|
|
|
|
| Race | |||
| African American | 2 (4%) | 6 (12%) | 8 (16%) |
|
|
|||
| Asian | 0 (0%) | 1 (2%) | 1 (2%) |
|
|
|||
| Caucasian | 20 (40%) | 19 (36%) | 39 (76%) |
|
|
|||
| Hispanic | 0 (0%) | 1 (2%) | 1 (2%) |
|
|
|||
| Hispanic/Latino | 1 (2%) | 1 (2%) | 2 (4%) |
|
|
|||
| Totals | 23 (46%) | 28 (54%) | 51 (100%) |
|
|
|
|
|
| Smoking | |||
| Smoker | 1 (2%) | 1 (2%) | 2 (4%) |
|
|
|||
| Non-smoker | 16 (33%) | 20 (38%) | 36 (71%) |
|
|
|||
| Past smoker | 6 (12%) | 7 (13%) | 13 (25%) |
|
|
|||
| Totals | 23 (46%) | 28 (54%) | 51 (100%) |
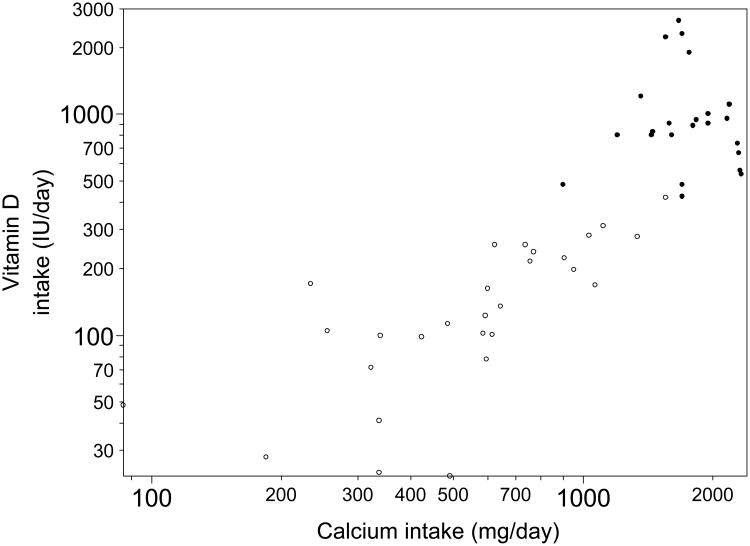
As shown in Figure 1, there was a good separation between the two groups in terms of total daily intake of calcium and vitamin D. For subjects who did not take oral supplementation, the mean daily calcium intake was 642 mg (505-779, 95% CI) and the mean daily vitamin D intake was 156 IU (117-195). For subjects who did take oral supplementation, the mean daily calcium intake was 1769 mg (1606-1933) and the mean daily vitamin D intake was 1049 IU (781-1317). The differences between groups in calcium and vitamin D supplementation were significant (Table 2).
Figure 1.
Total calcium and vitamin D daily intake (from oral supplementation and diet). Subjects who took vitamin D and calcium supplementation are labeled with ●. Subjects who took no oral supplementation are labeled with ○. Log scales are used for axes.
Table 2.
Mean values and 95% confidence intervals for clinical and radiographic measurements plus total calcium and vitamin D intakes (oral supplementation plus diet).
| Measurement | Taker (n=23) | Non-taker (n=28) |
|---|---|---|
| Probing depth | 2.18 (2.00-2.36) mm | 2.33 (2.09-2.57) mm‡ |
| Attachment level | 1.80 (1.39-2.20) mm | 2.01 (1.59-2.42) mm‡ |
| Bleeding* | 60 (52-69) % | 66 (58-74) %‡ |
| Gingival Index | 0.73 (0.52-0.94) | 1.00 (0.77-1.23) ‡ |
| Furcation Involvement† | 47 (26-68) % | 72 (42-100) %‡ |
| CEJ-AC | 1.71 (1.34-2.09) mm | 2.04 (1.63-2.45) mm‡ |
| Calcium intake | 1769 (1606-1933) mg/day | 642 (505-779) mg/day§ |
| Vitamin D intake | 1049 (781-1317) IU/day | 156 (117-195) IU/day§ |
percentage of bleeding sites.
percentage of molar sites with furcation involvement.
P > 0.05.
P < 0.01.
Attachment level = cementoenamel junction-gingival margin distance, CEJ-AC = cementoenamel junction-alveolar crest distance.
The mean scores for all clinical measurements of periodontal health were greater in the subjects who did not take oral supplementation compared with those who did (Table 2). Probing depths, attachment loss, and CEJ-AC distances were, respectively 7% (0.15 mm/2.18 mm), 12% (0.21 mm/1.80 mm), and 19% (0.32 mm/1.71 mm) greater in subjects who took no oral supplementation. There were similar differences for the additional clinical measurements. Because of the relatively large standard deviations, none of the univatiate test results for the clinical measurements was significant (Table 2); however, when these measurements were considered collectively, a repeated measures multivariate analysis of variance indicated that these differences between the 23 subjects who took supplementation and the 28 subjects who took no supplementation were borderline statistically significant (p = 0.08). Controlling for race, smoking, gender, and sunlight exposure did not substantively change this result (p ≥ 0.08).
Discussion
Through dietary analysis and a positive or negative history of calcium and vitamin D supplementation, we were able to enroll two groups of subjects who were fairly distinct from one another. We had difficulty finding subjects taking adequate amounts of oral supplementation to meet our enrollment criteria, most likely because only a small proportion of the general population meets the dietary standards for these nutrients. In a previous survey of patients in our periodontal recall programs, only 15 (7%) met the U.S. Food and Nutrition Board's recommended intake levels for calcium and vitamin D through oral supplementation.42 These recommendations are supposedly adequate for preventing ostomalacia in adults but were not set to assure the beneficial actions of vitamin D discussed in this paper54. In fact, it has been suggested that doses as large as 3,800 to 5,000 IU/day of vitamin D3 are required to ensure that vitamin D deficiency or insufficiency is resolved in more than 80% of supplemented people.55-57
We made a concerted effort to recruit takers who had the highest levels of vitamin D oral supplementation in our patient pool. Our efforts yielded a mean total vitamin D intake (diet plus supplements) of 1049 IU/day, and only 3 subjects had vitamin D intake greater than 2000 IU/day. On the other hand, the average daily total calcium intake in the takers group was 1769 mg, above the current recommendation; whereas in the non-takers group average daily calcium intake was only 642 mg, well below the current recommendations, but quite consistent with the U.S. median intake values of 708 mg/day for men, and 571 mg/day for women 50-70 years of age, and of 702 and 517 mg/day, over 70.58 It is also possible that sunlight exposure (which results in the synthesis of vitamin D) varied between our two groups; however, the groups were well balanced throughout the enrollment period, thus making it unlikely that seasonal variation to sunlight exposure might have affected them differently.
All the periodontal parameters assessed in this study were higher (worse) in subjects who did not take oral supplementation compared with those who did. It could be argued that subjects habitually taking dietary supplements may be more health conscious than those not taking supplements, and thus the differences in periodontal health parameters may reflect a better general health. This possibility cannot be ruled out; however, all subjects in our study were medically healthy, and all had been enrolled in periodontal-maintenance programs for at least six months and received regular treatments, which appear to have been successful, as on average they had about 2 mm or less of attachment loss. Therefore, they were all well motivated for maintaining good dental health.
If vitamin D and calcium oral supplementation were effective in improving periodontal health above the level provided by periodontal-maintenance programs, this would lessen the damage (clinical attachment and alveolar crest height loss) caused by periodontal disease and would be a useful adjunctive treatment. Our results seem to support this idea because the differences we detected between groups (0.21 mm in attachment loss and 0.32 mm in alveolar crest height) are similar to the 0.26 mm difference in attachment level that was reported between subjects with high and low serum levels of 25OHD, a good index of vitamin D status, in an analysis of NHANES III data.30 Although 25OHD was not measured in our study, it is highly likely that vitamin D status might have been better in takers than in non-takers.59 Based on the difference in vitamin D intake between supplement takers and non-takers of 905 IU/day, and assuming that serum 25OHD levels increase ∼0.70 nmol/l for each μg (40 IU) of vitamin D supplementation59, the two groups would have differed by approximately 15 nmol/l (6 ng/ml). This can be considered a substantial difference, though presumably not sufficient to normalize serum 25OHD in most individuals.
Of relevance to our findings, an analysis of the NHANES-III database (men and women older than 50) revealed that serum levels of 25OHD were significantly and inversely associated with periodontal attachment loss, but independently of bone mineral density 30. In a related study, a linear association between 25OHD and bleeding with probing was found39. The investigators suggested that vitamin D may reduce the susceptibility to gingival inflammation through anti-inflammatory effects. It was also demonstrated in a 3 year study that intake levels of calcium and vitamin D had a beneficial effect on tooth retention32. Additional studies, with findings similar to ours are reviewed elsewhere36.
In our study, the attachment loss and alveolar crest height loss measurements were similar, while one would expect the attachment loss measurements to be less than the alveolar crest height loss measurements. We found that a number of our subjects had relatively large buccal attachment loss measurements, but this buccal loss of the attachment apparatus was not reflected in our alveolar-crest-height measurements.
Despite our having recruited subjects who had at least two interproximal sites with 3 mm or greater clinical attachment loss, this parameter averaged 1.80 mm (taker group) and 2.01 mm (non-taker group) in the mandibular posterior regions, a sign of a modest degree of periodontal disease. Nonetheless, all of the clinical and radiographic measurements indicated better periodontal health for subjects who took oral vitamin D and calcium supplementation. Furthermore, since all of our subjects were enrolled in periodontal maintenance programs, our data are consistent with the notion that taking vitamin D and calcium supplements may have beneficial effects above and beyond those of standard periodontal care.
Thus, within the limitations of a cross-sectional design in a relatively small population, our results would suggest that vitamin D and calcium supplementation could be advocated as a component of periodontal disease management. More expanded longitudinal studies are required to determine the potential of this relationship.
Acknowledgments
This publication was made possible by Grant Number R21 DE016918-01A2 from the National Institute of Dental and Craniofacial Research and Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIDCR, NCRR, or NIH.
Footnotes
Brief Block 2000, Block Dietary Data Systems, Berkeley, CA, USA
Hu-Friedy, Chicago, IL, USA
Conflicts of Interest: The authors report no financial relationships related to any products involved in this study.
References
- 1.Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol. 1997;26:3–15. doi: 10.1038/sj.dmfr.4600226. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoat M. The association between osteoporosis and oral bone loss. J Periodontol. 2005;76:2125–2132. doi: 10.1902/jop.2005.76.11-S.2125. [DOI] [PubMed] [Google Scholar]
- 3.Jeffcoat MK, Reddy MS. Alveolar bone loss and osteoporosis: Evidence for a common mode of thearpy using the bisphoshonate alendronate. In: Davidovitch Z, Norton L, editors. The Biologic Mechanism of Tooth Resorption and Replacement by Implants. Boston: Harvard Society for the Advancement of Orthodontics; 1996. pp. 365–373. [Google Scholar]
- 4.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 5.Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol. 1997;68:24–31. doi: 10.1902/jop.1997.68.1.24. [DOI] [PubMed] [Google Scholar]
- 6.Southard KA, Southard TE, Schlechte JA, Meis PA. The relationship between the density of the alveolar processes and that of post-cranial bone. J Dent Res. 2000;79:964–969. doi: 10.1177/00220345000790041201. [DOI] [PubMed] [Google Scholar]
- 7.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–1498. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- 8.von Wowern N. General and oral aspects of osteoporosis: a review. Clin Oral Investig. 2001;5:71–82. doi: 10.1007/s007840100105. [DOI] [PubMed] [Google Scholar]
- 9.Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67:1076–1084. doi: 10.1902/jop.1996.67.10s.1076. [DOI] [PubMed] [Google Scholar]
- 10.Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76:2116–2124. doi: 10.1902/jop.2005.76.11-S.2116. see comment. [DOI] [PubMed] [Google Scholar]
- 11.White SC. Oral radiographic predictors of osteoporosis. Dentomaxillofac Radiol. 2002;31:84–92. doi: 10.1038/sj.dmfr.4600674. [DOI] [PubMed] [Google Scholar]
- 12.Brennan RM, Genco RJ, Hovey KM, Trevisan M, Wactawski-Wende J. Clinical attachment loss, systemic bone density, and subgingival calculus in postmenopausal women. J Periodontol. 2007;78:2104–2111. doi: 10.1902/jop.2007.070155. [DOI] [PubMed] [Google Scholar]
- 13.Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol. 2005;76:11–15. doi: 10.1902/jop.2005.76.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipps KR, Chan BK, Madden TE, et al. Longitudinal study of bone density and periodontal disease in men. J Dent Res. 2007;86:1110–1114. doi: 10.1177/154405910708601117. [DOI] [PubMed] [Google Scholar]
- 15.Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13:719–764. doi: 10.1210/edrv-13-4-719. [DOI] [PubMed] [Google Scholar]
- 16.D'Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannell JJ, Vieth R, Willett W, et al. Cod liver oil, vitamin A toxiicty, frequent respiratory infections, and the vitamin D defiency epidemic. Ann Otol Rhinol Laryngol. 2008c;117:864–870. doi: 10.1177/000348940811701112. [DOI] [PubMed] [Google Scholar]
- 19.Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E. On the epidemiology of influenza. Virol. 2008b;5:29. doi: 10.1186/1743-422X-5-29. serial online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. see comment. [DOI] [PubMed] [Google Scholar]
- 21.Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 22.Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 23.Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. J Am Diet Assoc. 2004;104:980–983. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 24.NOP. [Accessed March 26, 2009];National Osteoporosis Foundations's Updated Recommendations for Calcium and Vitamin D Intake. 2007 http://www.nof.org/prevention/calcium_and_VitaminD.htm.
- 25.Glerup H, Mikkelsen K, Poulsen L, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heaney RP. The importance of calcium intake for lifelong skeletal health. Calcif Tissue Int. 2002a;70:70–73. doi: 10.1007/s00223-001-0032-3. [DOI] [PubMed] [Google Scholar]
- 28.Heaney RP. Ethnicity, bone status, and the calcium requirement. Nutr Res. 2002b;22:153–178. [Google Scholar]
- 29.Heaney RP. Long-latency deficiency disease: insights from calcium and vitamin D. Am J Clin Nutr. 2003;78:912–919. doi: 10.1093/ajcn/78.5.912. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 31.Groen JJ, Duyvensz F, Halsted JA. Diffuse alveolar atrophy of the jaw (non-inflammatory form of paradental disease) and pre-senile osteoporosis. Gerontol Clin. 1960;2:68–86. doi: 10.1159/000244610. [DOI] [PubMed] [Google Scholar]
- 32.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001a;111:452–456. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 33.Krook L, Lutwak L, Whalen JP, Henrikson PA, Lesser GV, Uris R. Human periodontal disease. Morphology and response to calcium therapy. Cornell Vet. 1972;62:32–53. [PubMed] [Google Scholar]
- 34.Krook L, Whalen JP, Lesser GV, Lutwak L. Human periodontal disease and osteoporosis. Cornell Vet. 1972;62:371–391. [PubMed] [Google Scholar]
- 35.Lutwak L, Krook L, Henrikson PA, et al. Calcium deficiency and human periodontal disease. Isr J Med Sci. 1971;7:504–505. [PubMed] [Google Scholar]
- 36.Hildebolt CF. Effect of vitamin D and calcium on periodontitis. J Periodontol. 2005;76:1576–1587. doi: 10.1902/jop.2005.76.9.1576. [DOI] [PubMed] [Google Scholar]
- 37.Hildebolt CF, Pilgram TK, Dotson M, et al. Estrogen and/or calcium plus vitamin D increase mandibular bone mass. J Periodontol. 2004;75:811–816. doi: 10.1902/jop.2004.75.6.811. [DOI] [PubMed] [Google Scholar]
- 38.Nishida M, Grossi SG, Dunford RG, Ho AW, Trevisan M, Genco RJ. Calcium and the risk for periodontal disease. J Periodontol. 2000;71:1057–1066. doi: 10.1902/jop.2000.71.7.1057. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–580. doi: 10.1093/ajcn.82.3.575. [DOI] [PubMed] [Google Scholar]
- 40.Inagaki K, Krall EA, Fleet JC, Garcia RI. Vitamin D receptor alleles, periodontal disease progression, and tooth loss in the VA dental longitudinal study. J Periodontol. 2003;74:161–167. doi: 10.1902/jop.2003.74.2.161. [DOI] [PubMed] [Google Scholar]
- 41.Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontol 2000. 2007;43:102–132. doi: 10.1111/j.1600-0757.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 42.Dixon D, Hildebolt CF, Miley DD, et al. Calcium and vitamin D use among adults in periodontal-disease maintenance programs. Br Dent J. 2009 doi: 10.1038/sj.bdj.2009.519. Accepted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139:1190–1196. doi: 10.1093/oxfordjournals.aje.a116965. [DOI] [PubMed] [Google Scholar]
- 45.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38:610–616. doi: 10.1902/jop.1967.38.6.610. Suppl. [DOI] [PubMed] [Google Scholar]
- 47.Ammons WF, Jr, Harrington GW. Furcation: Involvement and Treatment. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Clinical Periodontology. Vol. 10. St. Louis: Saunders; 2006. pp. 991–993. [Google Scholar]
- 48.Osborn J, Stoltenberg J, Huso B, Aeppli D, Pihlstrom B. Comparison of measurement variability using a standard and constant force periodontal probe. J Periodontol. 1990;61:497–503. doi: 10.1902/jop.1990.61.8.497. [DOI] [PubMed] [Google Scholar]
- 49.Couture RA, Dixon D, Hildebolt C. A precise receptor-positioning device for subtraction radiography, based on cross-arch stabilization. Dentomaxillofac Radiol. 2005;34:231–236. doi: 10.1259/dmfr/22285074. [DOI] [PubMed] [Google Scholar]
- 50.Couture RA, Hildebolt CF. Precise image-receptor calibration and monitoring of beam quality with a step wedge. Dentomaxillofac Radiol. 2002;31:56–62. doi: 10.1038/sj/dmfr/4600659. [DOI] [PubMed] [Google Scholar]
- 51.Rasband W. ImageJ. National Institues of Health; USA: 2002. [Google Scholar]
- 52.Hildebolt C, Couture R, Garcia N, Dixon D, Miley DD, Mueller C, Langenwalter E, Logan L, Anderson C, Rhodes L, Walker SY, Civitelli R. Bone Active Periodontitis Treatments: Radiographic Measurement Reliability. J Dent Res. 2008;87(Spec Iss B):0145. [Google Scholar]
- 53.Villareal DT, Civitelli R, Chines A, Avioli LV. Subclinical vitamin D deficiency in postmenopausal women with low vertebral bone mass. J Clin Endocrinol Metab. 1991;72:628–634. doi: 10.1210/jcem-72-3-628. [DOI] [PubMed] [Google Scholar]
- 54.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3:1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aloia JF, Patel M, Dimaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–1958. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 56.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008a;9:107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 57.Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86:1657–1662. doi: 10.1093/ajcn/86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes (DRI) for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997. pp. 388–389. [PubMed] [Google Scholar]
- 59.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]