Abstract
Systemic iron homeostasis is regulated by the interaction of the peptide hormone, hepcidin and the iron exporter, ferroportin. Mutations in FPN1, the gene that encodes ferroportin, result in iron-overload disease that shows dominant inheritance and variation in phenotype. The inheritance of ferroportin-linked disorders can be explained by the finding that ferroportin is a multimer and the product of the mutant allele participates in multimer formation. The nature of the ferroportin mutant can explain the variation in phenotype, which is due to either decreased iron export activity or decreased ability to be downregulated by hepcidin. Iron export through ferroportin is determined by the concentration of ferroportin in plasma membrane, which is the result of both synthetic and degradation events. Ferroportin degradation can occur by hepcidin-dependent and hepcidin-independent internalization. Ferroportin expression is regulated transcriptionally and posttranslationally.
Keywords: Ferroportin, iron, hepcidin, genetics
Iron is an essential element due to its ability to easily gain and lose electrons and to bind oxygen. Iron is required for a wide range of oxidation/reduction reactions and as a substrate for heme. Heme in the form of hemoglobin is the essential oxygen-carrying molecule in vertebrates. Heme bound to proteins such as P450 is required for the metabolism of both endogenous and exogenous substrates. Both iron deficiency and iron excess may result in disease. Insufficient iron results in developmental defects, failure to thrive, and a deficit in hemoglobinization leading to anemia. Iron excess may lead to tissue pathology resulting in hepatic fibrosis, diabetes, and adrenal insufficiency. All eukaryotes tightly regulate iron acquisition and iron storage. Regulation of iron acquisition is the predominant method for maintaining iron homeostasis, as eukaryotes do not have regulated mechanisms for iron excretion. Single-cell eukaryotes such as yeast and multicellular organisms such as humans face the same issues of coordinating iron acquisition with iron utilization. The confounding issue for multicellular organisms is that iron acquisition and iron utilization occur in different cell types and tissues. Dietary iron enters the body through absorptive intestinal mucosal cells, which mediate net iron accumulation. Most of the iron that enters plasma, however, is exported from macrophages that recycle iron from senescent or damaged erythrocytes. Iron that enters plasma from macrophages or the gut is bound to plasma transferrin and delivered to cells through the interaction of diferric transferrin and cell-surface transferrin receptors. Under conditions in which iron entry into plasma exceeds the iron-binding capacity of transferrin, iron is rapidly removed from plasma and deposited in parenchymal tissues such as hepatocytes and islet cells of the pancreas.
The coordination between iron entry into plasma, iron utilization, and iron storage is accomplished by the interaction of the peptide hormone hepcidin and the iron transporter, ferroportin (FPN) encoded by FPN1 (SLC40A1). Hepcidin is a member of the defensin family of antimicrobial peptides. Hepcidin is an amphipathic peptide of 25 amino acids, which contains six cysteine residues resulting in a highly disulfide-bonded structure. It is synthesized as a prepropeptide, which is cleaved to the mature 25 amino acid molecule. Hepcidin is highly conserved in all vertebrates, although there are differences in the amino terminal of hepcidin from cold-blooded versus warm-blooded vertebrates. The iron-related function of hepcidin was identified through a deletion of the gene HAMP1, which encodes hepcidin. A targeted gene deletion in mice resulted in massive iron overload of parenchymal tissues.1 In contrast, mice that overexpress hepcidin as a transgene exhibited the opposite phenotype, severe iron limitation.2 These results showed that hepcidin is a negative regulator of iron acquisition; high hepcidin levels give rise to decreased iron uptake and conversely low hepcidin levels result in increased iron uptake. The recessive genetic disorders resulting in hereditary hemochromatosis are due to decreased expression of hepcidin. Conversely, the acquired disorder anemia of chronic inflammation3,4 and the genetic disorder iron-refractory iron resistant anemia are due to increased levels of hepcidin.5 Studies elsewhere in this issue describe how hepcidin expression is regulated.
The mechanism of how hepcidin regulates iron acquisition was clarified by showing that hepcidin binds to and induces the degradation of the iron exporter, FPN.6 FPN is the only known vertebrate iron exporter and is a membrane protein with a predicted 10 to 12 transmembrane domains.7 FPN was identified by three groups, each using a different approach. One group identified FPN through analysis of a mutant gene that resulted in decreased iron-dependent hemoglobinization in zebrafish;8 the second group identified FPN1 as a transcript highly expressed in duodenal mucosa in iron deficiency9 and the third group identified FPN1 as an iron-responsive element (IRE)-containing mRNA.10 Mutation of FPN1 in zebrafish or targeted deletion of FPN1 in mice resulted in an inability to absorb iron from the intestine and to recycle iron from macrophages.8,11 These biochemical and genetic studies were confirmed by studies in humans, which demonstrated that FPN mutations gave rise to iron-linked disorders.12,13 Recently, it was shown that deletion or mutations in FPN1 in mice led to severe developmental abnormalities including decreased neural tube closure,14 demonstrating the importance of FPN and iron in development.
THE ROLE OF FPN IN MAMMALIAN IRON METABOLISM
The importance of FPN in human iron homeostasis was demonstrated by the finding that mutations in FPN led to human iron-overload diseases. An important defining feature of FPN-linked iron disease is that it shows dominant inheritance12. The dominant transmission of FPN-linked hemochromatosis is in marked contrast to the genetically recessive transmission of iron-overload disorders due to mutations in HFE, TFR2, hemojuvelin (HJV), or HAMP1. Those disorders all result from decreased levels of hepcidin, whereas hepcidin levels in FPN-linked disorders are either normal or above normal. Patients with FPN-linked iron disorders show two distinct sets of symptoms. One class of patients presents with high serum ferritin, low transferrin saturation, and iron accumulation in Kupffer cells. These patients may have a normal hematocrit, but when phlebotomized show a decreased hematocrit and reduced transferrin saturation.15 The second presentation is indistinguishable from classic hereditary hemochromatosis. Patients have an elevated hematocrit, high serum ferritin, high transferrin saturation, and accumulate iron in hepatocytes. The symptoms associated with this class of FPN-linked iron disorder improve upon phlebotomy.
Studies in cultured cells, Xenopus oocytes or zebrafish have provided an explanation for the different phenotypes associated with FPN-linked iron disorders. The macrophage form of FPN-linked iron disease or “classic” FPN disease is due to FPN mutations that result in an inability to transport iron.7,16,17 Some of the FPN mutants (e.g., deletion of valine 162) do not traffic to the cell surface appropriately. Other mutants show normal targeting to the cell surface, but are unable to transport iron (e.g., asparagine 174 to isoleucine). There are discrepancies in the behavior of specific FPN mutants, as some studies report that FPN mutants showed defective trafficking,7,16,17 whereas other reports showed normal trafficking, but defective iron export.18–20 The difference in results may be due to expression levels of transfected FPN or to the specific cell type employed. Regardless of whether the mutant FPN does not traffic well or is transport incompetent, the result is the same, defective iron export from cells. Decreased iron export explains reduced transferrin saturation and high serum ferritin, as decreased iron export results in increased iron retention in the specialized iron exporting cells. The cells most affected are macrophages, which recycle iron from phagocytosed red blood cells. In contrast, the amount of FPN in the intestine of a human or mouse fed a standard diet, which is fairly iron rich, is only a fraction of the total FPN levels. Thus, in intestinal mucosa the effect of a mutation that compromises iron export might be compensated for by increased expression of FPN. The overall result would be increased or relatively normal iron absorption from the intestine yet decreased iron export from macrophages.
The hepatocyte form of FPN-linked hemochromatosis is due to the constitutive expression of FPN even in the face of high levels of plasma and liver iron. The high levels of FPN result from decreased FPN degradation in response to the hormone hepcidin.7,16,21 Hepcidin resistance leads to continued iron export through FPN independent of hepcidin levels.
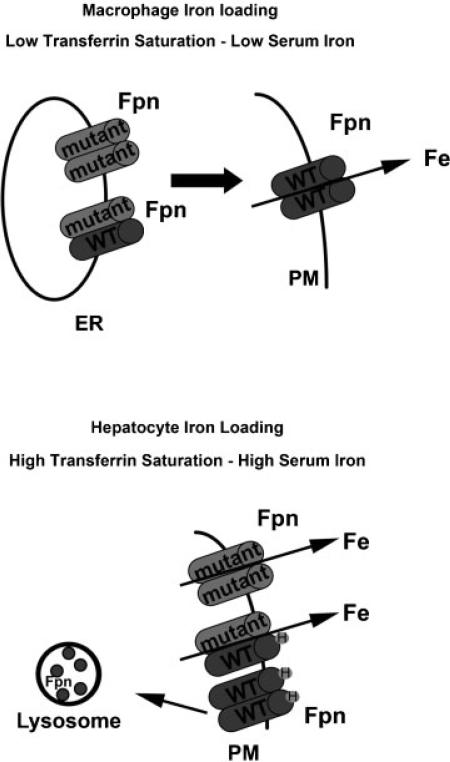
There are two possible mechanisms that would explain dominant transmission of FPN-linked iron disorders: haploinsufficiency or gain-of-function. Al-most all human FPN1 mutations are missense mutations. There is a report of a case of FPN-disease due to a mutation in the promoter region of FPN1, but this mutation leads to a gene product.22 No nonsense mutations in FPN1 have been identified. Additionally, mice that are heterozygous for a targeted deletion in the FPN1 gene do not show FPN disease.11 These data argue against haploinsufficiency. In contrast, there is support for a dominant negative model for the genetic basis of FPN disease. Most critically, there is evidence that FPN is a dimer and that the monomers, which are the products of mutant alleles can interact with the wild-type monomer and affect the behavior of the dimer. Evidence in support of an FPN dimer comes from biochemical studies including the coprecipitation of different epitope-tagged FPN, crosslinking studies and the observations that FPN mutants that do not traffic appropriately can affect the trafficking of wild-type FPN.16,23,24 The conclusion that FPN is a dimer has been the subject of some controversy as there are studies that indicate that FPN is a monomer.18,25–27 Strong support for a dimer structure for FPN came from studies in which an N-ethyl-N-nitrosourea-generated mouse mutant exhibited “classical” FPN disease with low transferrin saturation, high serum ferritin and iron accumulation in Kupffer cells and not hepatocytes.28 The mutation was identified as a missense mutation in FPN (H32R). The flatiron (ffe/+) mouse was heterozygous for the H32R mutation resulted in defective trafficking of the mutant FPN, which in turn affected the trafficking of wild-type FPN to the cell surface. This flatiron mouse showed mild anemia and iron accumulation in Kupffer cells. An equally compelling result came from studies in which fertilized zebrafish eggs were injected with plasmids containing GFP-tagged wild-type or mutant FPN.29,30 The FPN-GFP was expressed throughout the developing embryo. Expression of either a known human FPN mutant construct that results in FPN disease or the H32R FPN cloned from the flatiron mouse led to a defect in hemoglobinization of developing red blood cells in the developing embryos. In contrast, expression of wild-type human or mouse FPN or human FPN mutations that are hepcidin resistant did not lead to iron-limited erythropoiesis. It is important to note that under these experimental protocols the zebrafish express their endogenous FPN, therefore, the only explanation for these results is that FPN is a multimer and FPN mutants interact with wild-type FPN affecting the behavior of the dimer (Fig. 1). Thus, FPN missense mutants are dominant negative or gain-of function alleles explaining the dominant transmission of the disorder.
Figure 1.
Effects of FPN mutation on iron homeostasis. FPN disease is a dominantly inherited disorder caused by mutations in the SLC40A1/FPN1 gene. Patients present with either of two phenotypes. Some subjects have low transferrin saturation, high serum iron, and iron loading in Kupffer cells. These phenotypes are due to mutations in FPN that impair its localization at the plasma membrane or its ability to transport iron. Other patients have a presentation indistinguishable from classic hereditary hemochromatosis: high transferrin saturation, serum iron, and hepatocyte iron loading. These phenotypes are due to FPN mutants that do not bind hepcidin or FPN mutants that bind hepcidin but do not get internalized. FPN mutant monomers can heterodimerize with wild-type FPN and affect their localization and/or function.
HEPCIDIN-MEDIATED FPN INTERNALIZATION
The effect of hepcidin on FPN internalization was first demonstrated in cultured cells expressing an FPN-green fluorescent protein (GFP) chimeric protein.6 The presence of FPN-GFP on the cell surface led to increased iron export as shown by measurement of the level of the cytosolic iron protein ferritin and by direct export of radioactive iron. Addition of hepcidin resulted in the clearance of FPN-GFP from the cell surface and its accumulation in lysosomes where it was degraded. Subsequent studies in mice showed that hepcidin induced the loss of FPN from intestinal mucosal cells,31 and splenic31 and bone marrow macrophages32 and hepatocytes.33
Transfection of FPN-expressing plasmids in different cell types has shown that the degradation of FPN by hepcidin is independent of cell type. The intrinsic nature of hepcidin-mediated FPN degradation is because hepcidin binds to FPN and that interaction is critical for degradation.6 The binding of hepcidin to FPN was first shown with crosslinking agents in which hepcidin crosslinked to FPN could be identified on SDS-polyacrylamide gel electrophoresis. Subsequently, iodinated or fluorescently labeled hepcidin was shown to bind to cells in a FPN-dependent manner, but no hepcidin binding was found on cells expressing a class of FPN mutants that led to hepcidin-resistant FPN disease.34 The hepcidin-binding site has been localized to an extracellular domain between loops five and six.35 Further analysis using peptides encompassing this loop have identified amino acids required for binding. An essential amino acid is cysteine 326, as mutations in this amino acid gives rise to hepcidin-resistant hemochromatosis.36 This amino acid could provide a free sulfhydryl residue and alkylation of this residue would prevent hepcidin binding. It has been speculated that this sulfhydryl residue may react with sulfhydryls on FPN leading to a mixed disulfide that would stabilize hepcidin FPN interactions.36 The generation of derivative hepcidins has identified that the first five amino acids are critical for FPN binding.34 Serial deletion of the first five amino acids led to a progressive loss of activity such that hepcidin derivatives lacking all five amino acids had only a fraction of binding activity. The predominant forms of urinary hepcidin are lacking two to five of the amino terminal amino acids,35 suggesting that there may be an inactivation/processing of hepcidin. Additionally, poikilotherms such as fish may have multiple hepcidin genes. Only one of the multiple genes encodes a 25 amino acid form of hepcidin. The other gene products encode forms of hepcidin lacking some of the amino terminal residues. Truncated and full-length hepcidins have antimicrobial activity, but only the 25-amino acid form can regulate iron metabolism. As mentioned above, cold-blooded vertebrates have a different amino terminus for hepcidin compared with homeotherms. Mammalian hepcidin cannot stably bind to mammalian FPN at temperatures below 15°C. In contrast, zebrafish hepcidin can bind to mammalian FPN regardless of temperature. The difference in binding is due to mammalian hepcidin changing its structure at low temperature while fish hepcidin does not. Hepcidin is a heavily disulfide crosslinked molecule, but removal of many of the cysteines does not affect its ability to bind FPN.34 This suggests that the disulfide crosslinks may be important for its activity in vivo, perhaps increasing hepcidin's circulating half-life or protecting it from inactivation by plasma proteins.
The mechanism of hepcidin-mediated FPN internalization has been analyzed in some detail. Binding of hepcidin to FPN results in the binding of the cytosolic Janus kinase 2 (Jak2) to FPN.37 FPN is a dimer and each monomer must bind hepcidin for Jak2 to bind. Dimers comprised of a wild-type FPN and a C326S FPN mutant will not bind Jak2 upon addition of hepcidin. This result supports the in vivo phenotype that hepcidin resistance of FPN is transmitted dominantly. Once Jak2 is bound to FPN, the two molecules of Jak2 are autophosphorylated and phosphorylated Jak2 then phosphorylates FPN on either of two adjacent tyrosine residues (Y302–303). Phosphorylation of these residues results in the internalization of the FPN-hepcidin complex via dynamin and epsin-dependent endocytosis.38 FPN mutants that bind hepcidin, but are not internalized will still export iron. These results show that hepcidin does not affect the iron transport activity of FPN, but affects the concentration of cell surface FPN.
Phosphorylation of FPN is a transient event as upon internalization the phosphates are rapidly removed.38 Internalized FPN is then monoubiquitinated on lysine 253. Ubiquitination is critical for FPN degradation. A series of cytosolic proteins termed endosomal sorting complex required for transport (ESCRT) complexes recognize ubiquitinated FPN permitting FPN to be captured by vesicles that bud into the lumen of endosomes forming the multivesicular body. The multivesicular body fuses with lysosomes resulting in the degradation of internalized FPN. Mutation of K253 or reduction in ESCRT proteins by RNAi result in decreased degradation of internalized FPN.
FPN can also be internalized by hepcidin-independent mechanisms. Studies have shown that iron entry into plasma requires the activity of a multicopper oxidase to convert Fe(II) to Fe(III).39–41 Ceruloplasmin (Cp) is a copper-containing enzyme that can be found as a plasma protein or bound to select cell types through a glycosylphosphatidylinositol linkage.42 Cp mediates the conversion of Fe(II) to Fe(III) by oxidizing iron atoms and storing the electrons.43 When four atoms are oxidized the stored electrons reduce oxygen to water. The oxidation of Fe(II) to Fe(III) can be accomplished without Cp; however, the rate of oxidation is slow at low oxygen concentrations and the spontaneous oxidation of iron may give rise to toxic oxygen radicals.44 Cp-mediated iron oxidation is coupled to the reduction of molecular oxygen, which prevents oxygen radical formation. In the absence of ferroxidase activity, there is a decrease in the entry of iron into plasma. Studies published over 50 years ago demonstrated that copper-deficient pigs were anemic.45 Copper deficiency leads to the absence of active ceruloplasmin. This work was confirmed by a targeted gene deletion of Cp in mice.46 Cp–/– mice showed an inability to export iron from stores upon stress. Mammals express another membrane bound homologue of ceruloplasmin termed hephaestin. Hephaestin is found at the basal lateral membrane of the gut and assists in net iron absorption in the duodenum. The sex-linked anemia mouse, which has a mutation in hephaestin, exhibits a microcytic anemia due to defective iron transport by the intestine.47 This genetic data confirm the biochemical data that multicopper oxidase activity is required for FPN-mediated iron export.
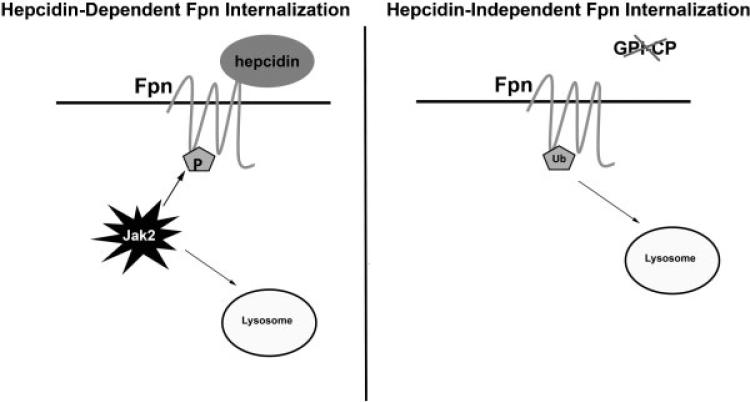
It is thought that the role of multicopper oxidases is to convert Fe(II) to Fe(III), as Fe(III) is the form of iron that binds to apotransferrin to be distributed to tissues. A curious issue, however, is that the phenotype of humans with aceruloplasminemia is different than humans with atransferrinemia. Mutations in Cp result in mild systemic iron accumulation but a predominant symptom is progressive neurodegeneration of the retina and basal ganglia due to iron accumulation.48 In contrast, atransferrinemia is characterized by anemia and hemosiderosis in the heart and liver.49,50 There is no evidence of neurologic symptoms associated with atransferrinemia. The difference in phenotype between atransferrinemia and aceruloplasminemia was reconciled by the finding that multicopper oxidase activity is required to remove iron from FPN and prevent FPN degradation. In the absence of multicopper oxidase activity, FPN on the cell surface of macrophages and cultured glial cells is rapidly internalized and degraded.51 This loss of FPN is independent of hepcidin and is not Jak2-mediated (Fig. 2). It is dependent, however, on the presence of lysine residue 253 in FPN and ubiquitination. In the absence of multicopper oxidase activity, iron is bound to FPN but cannot be released into the extracellular media. The presence of bound iron appears to trap FPN in a conformation that is recognized by an E3-ubiquitin ligase, which ubiquitinates FPN leading to its internalization and degradation. Mutation of K253 or mutations that prevent FPN-mediated iron transport will maintain FPN on the cell surface.
Figure 2.
Hepcidin-dependent and independent FPN degradation. Binding of hepcidin to FPN leads to the binding of the cytosolic protein kinase Jak2 to FPN. Once Jak2 is bound, FPN is phosphorylated and internalized. Internalized FPN is degraded in lysosomes. In the absence of hepcidin, the loss of Cp activity will lead to the internalization of FPN. Cell surface FPN can be ubiquitinated resulting in its internalization and degradation in lysosomes.
The finding of a second mechanism for FPN degradation implies that FPN can be regulated independent of hepcidin. Loss of hepcidin expression results in severe iron-overload disease in organs such as the liver, heart, and pancreas. There is, however, no evidence of iron overload in neural tissues or retina as seen in aceruloplasminemia. This result suggests that iron homeostasis in tissues separated from the general circulation is regulated differently than in tissues that are exposed to the circulation.
REGULATION OF FPN SYNTHESIS
The steady state concentration of FPN reflects a balance between FPN synthesis and degradation. An indication of the importance of FPN in iron homeostasis is that expression of FPN is regulated at several levels. FPN is most highly expressed on cell types that have a “professional” role in iron export, however, FPN1 transcription can occur in a wide variety of cell types in response to different stimuli. FPN1 transcription increases in macrophages in response to heme or iron.52,53 Heme-induced FPN1 transcription is the result of heme acting on the transcription factor Bach1.53 Iron can also induce FPN1 transcription, although the relevant transcription factor has not been identified. It is important to note that there may be cell type differences in FPN1 expression. FPN1 transcription is increased in J774 cells in response to copper, but not zinc or manganese.54 In Caco-2 cells and in cultured mouse bone marrow macrophages, FPN1 mRNA levels increase in response to a wide variety of transition metals including zinc, copper, manganese, cobalt, and cadmium.55,56 In the cultured macrophage cell line RAW264.7, heme as well as protoporphyrin IX were shown to be potent inducers of FPN1 transcription.53 Heme-induced FPN1 transcription in the J774 macrophage cell line, however, was blocked by iron chelation suggesting that it was the iron in heme that induced FPN1 transcription.57 In bone marrow macrophages, iron salts alone have been shown to induce FPN1 transcription.58
In addition to iron, other metals can induce FPN1 transcription. Depending on cell type, manganese, zinc, and copper induce FPN1 transcription. The effect of zinc on FPN1 transcription requires the activity of the zinc sensitive transcription factor MTF1.58 There are zinc-responsive elements in the promoter of FPN1 and mutation of those elements in reporter constructs abrogates zinc-sensitive transcription of FPN1, but not iron-sensitive transcription. An explanation for zinc-dependent transcription of FPN1 arises from the fact that FPN can also transport zinc. Zinc-induced expression of FPN may be a mechanism preventing zinc toxicity. Troadec et al suggested that copper-induced FPN1 transcription was not mediated by MTF1.58 The effect of copper might well be mediated through the Nrf2 transcription factor.53 This transcription factor, which is activated by oxidants and nucleophilic agents, binds to sites in the FPN promoter. Two independent studies showed that in cultured macrophages and in macrophage-like cell lines activation of Nrf2 led to FPN1 transcription.53,59 Increased oxidants are released by macrophages during the phagocytosis of red blood cells. This may be a signal to increase FPN levels during macrophage iron recycling.
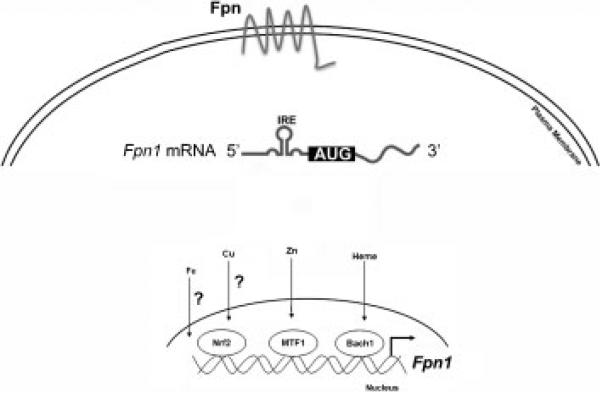
FPN levels are also regulated posttranscriptionally. One of the first identifications of FPN was based on the presence of an IRE in the 5′-untranslated region of FPN mRNA.60 IREs are stem loop structures in RNA that are capable of binding iron-regulated proteins (IRP). Under conditions of low cytosolic iron, IRPs occupies the IREs preventing 5′-IRE containing mRNA from being translated.61 If cytosolic iron rises, the IRP is removed from the IRE permitting translation (Fig. 3). In the case of FPN, increased cytosolic iron would result in increased FPN1 translation, which by exporting iron would decrease cytosolic iron. The involvement of the IRE/IRP system in regulating FPN protein levels was confirmed in cultured cells through the use of FPN1 reporter constructs containing or lacking the 5′-IRE.60 The importance of the FPN1 5′-IRE in mammalian iron homeostasis was shown in the Pcm mouse, which has a radiation-induced 58-base pair microdeletion in the promoter region of the FPN1 locus.62 This deletion, located four nucleotides upstream of the TATA box resulted in the absence of the IRE in the 5′ untranslated region of the vast majority of hepatic FPN1 transcripts in Pcm homozygotes. Pcm mutant mice showed a significant elevation in FPN protein levels in liver and duodenum, as well as organismal iron overload during early postnatal development. These results are consistent with increased expression of FPN due to the absence of the IRE/IRP translation repression control system. Recently, Rouault and colleagues identified a naturally occurring splice variant of FPN that lacks the 5′-IRE.63 The variant mRNA is expressed primarily in intestinal mucosal cells and surprisingly erythroblasts. The authors suggest that the presence of this transcript may permit FPN-mediated iron transport that is not regulated by cytosolic iron.
Figure 3.
Transcription and posttranscriptional regulation of FPN. Transcription of FPN can be regulated by different stimuli acting through a variety of transcription factors. Transcription of FPN1 can be regulated by transcription factors Nrf2, MTF1, and Bach1. The FPN1 promoter has Nrf2 binding sites and it is hypothesized that Nfr2 can be activated by copper (Cu). MTF1 and Bach1 are activated by zinc (Zn) and heme, respectively. Iron has also been shown to affect FPN1 transcription although the transcription factor(s) responsible has not been identified. Once transcribed, FPN mRNA can be regulated translationally. FPN1 mRNA contains an iron-responsive element in its 5′ untranslated region. Under low iron conditions, iron-responsive protein (IRP) binds to the 5′ IRE in FPN1 mRNA and blocks translation. When cytosolic iron levels are high, IRPs are degraded or removed allowing FPN1 translation.
Studies in the last decade using genetics, model organisms, and biochemical analyses have advanced our understanding of systemic iron homeostasis and the roles of hepcidin and FPN in iron metabolism. We can now describe the genetics and pathophysiology of iron-overload diseases and or iron-linked anemias in molecular terms. It is expected that this information in the next decade will lead to improved therapies that will alleviate the morbidity and mortality associated with these disorders.
ACKNOWLEDGMENTS
This work is supported by NIH grant DK070947 to J.K. and DK090257 to I.D.D.
ABBREVIATIONS
- Cp
ceruloplasmin
- ESCRT
endosomal sorting complex required for transport
- FPN
ferroportin
- GFP
green fluorescent protein
- IRE
iron-responsive element
- IRP
iron-responsive protein
REFERENCES
- 1.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98(15):8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99(7):4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy CNMH, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109(9):4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100(10):3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 5.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 7.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35(1):33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 9.McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the baso-lateral transfer of iron to the circulation. Mol Cell. 2000;5(2):299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 10.Troadec MB, Warner D, Wallace J, et al. Targeted deletion of the mouse Mitoferrin1 gene: from anemia to protoporphyria. Blood. 2011;117(20):5494–5502. doi: 10.1182/blood-2010-11-319483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Montosi G, Donovan A, Totaro A, et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108(4):619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietrangelo A, Montosi G, Totaro A, et al. Hereditary hemochromatosis in adults without pathogenic mutations in the hemochromatosis gene. N Engl J Med. 1999;341(10):725–732. doi: 10.1056/NEJM199909023411003. [DOI] [PubMed] [Google Scholar]
- 14.Mao J, McKean DM, Warrier S, Corbin JG, Niswander L, Zohn IE. The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure. Development. 2010;137(18):3079–3088. doi: 10.1242/dev.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32(1):131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.De Domenico I, Ward DM, Nemeth E, et al. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci U S A. 2005;102(25):8955–8960. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schimanski LM, Drakesmith H, Merryweather-Clarke AT, et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105(10):4096–4102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- 18.Rice AE, Mendez MJ, Hokanson CA, Rees DC, Björkman PJ. Investigation of the biophysical and cell biological properties of ferroportin, a multipass integral membrane protein iron exporter. J Mol Biol. 2009;386(3):717–732. doi: 10.1016/j.jmb.2008.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace DF, Harris JM, Subramaniam VN. Functional analysis and theoretical modeling of ferroportin reveals clustering of mutations according to phenotype. Am J Physiol Cell Physiol. 2010;298(1):C75–C84. doi: 10.1152/ajpcell.00621.2008. [DOI] [PubMed] [Google Scholar]
- 20.McDonald CJ, Wallace DF, Ostini L, Bell SJ, Demediuk B, Subramaniam VN. G80S-linked ferroportin disease: classical ferroportin disease in an Asian family and reclassification of the mutant as iron transport defective. J Hepatol. 2011;54(3):538–544. doi: 10.1016/j.jhep.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Drakesmith H, Schimanski LM, Ormerod E, et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106(3):1092–1097. doi: 10.1182/blood-2005-02-0561. [DOI] [PubMed] [Google Scholar]
- 22.Cunat S, Giansily-Blaizot M, Bismuth M, et al. CHU Montpellier AOI 2004 Working Group Global sequencing approach for characterizing the molecular background of hereditary iron disorders. Clin Chem. 2007;53(12):2060–2069. doi: 10.1373/clinchem.2007.090605. [DOI] [PubMed] [Google Scholar]
- 23.De Domenico I, Ward DM, Musci G, Kaplan J. Evidence for the multimeric structure of ferroportin. Blood. 2007;109(5):2205–2209. doi: 10.1182/blood-2006-06-032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGregor JA, Shayeghi M, Vulpe CD, et al. Impaired iron transport activity of ferroportin 1 in hereditary iron overload. J Membr Biol. 2005;206(1):3–7. doi: 10.1007/s00232-005-0768-1. [DOI] [PubMed] [Google Scholar]
- 25.Pignatti E, Mascheroni L, Sabelli M, Barelli S, Biffo S, Pietrangelo A. Ferroportin is a monomer in vivo in mice. Blood Cells Mol Dis. 2006;36(1):26–32. doi: 10.1016/j.bcmd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Schimanski LM, Drakesmith H, Talbott C, et al. Ferroportin: lack of evidence for multimers. Blood Cells Mol Dis. 2008;40(3):360–369. doi: 10.1016/j.bcmd.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Yeh KY, Yeh M, Mims L, Glass J. Iron feeding induces ferroportin 1 and hephaestin migration and interaction in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296(1):G55–G65. doi: 10.1152/ajpgi.90298.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zohn IE, De Domenico I, Pollock A, et al. The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood. 2007;109(10):4174–4180. doi: 10.1182/blood-2007-01-066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Domenico I, Vaughn MB, Yoon D, Kushner JP, Ward DM, Kaplan J. Zebrafish as a model for defining the functional impact of mammalian ferroportin mutations. Blood. 2007;110(10):3780–3783. doi: 10.1182/blood-2007-07-100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Domenico I, Lo E, Ward DM, Kaplan J. Human mutation D157G in ferroportin leads to hepcidin-independent binding of Jak2 and ferroportin down-regulation. Blood. 2010;115(14):2956–2959. doi: 10.1182/blood-2009-10-251306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaston T, Chung B, Mascarenhas M, et al. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut. 2008;57(3):374–382. doi: 10.1136/gut.2007.131722. [DOI] [PubMed] [Google Scholar]
- 32.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112(3):866–874. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2010;95(3):501–504. doi: 10.3324/haematol.2009.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107(1):328–333. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Domenico I, Nemeth E, Nelson JM, et al. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8(2):146–156. doi: 10.1016/j.cmet.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Fernandes A, Preza GC, Phung Y, et al. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood. 2009;114(2):437–443. doi: 10.1182/blood-2008-03-146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci U S A. 2009;106(10):3800–3805. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Domenico I, Ward DM, Langelier C, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18(7):2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241(12):2746–2751. [PubMed] [Google Scholar]
- 40.Dowdy RP, Matrone G. A copper-molybdenum complex: its effects and movement in the piglet and sheep. J Nutr. 1968;95(2):197–201. doi: 10.1093/jn/95.2.197. [DOI] [PubMed] [Google Scholar]
- 41.Lee GR, Nacht S, Lukens JN, Cartwright GE. Iron metabolism in copper-deficient swine. J Clin Invest. 1968;47(9):2058–2069. doi: 10.1172/JCI105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem. 1997;272(32):20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- 43.Osaki S, Johnson DA, Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971;246(9):3018–3023. [PubMed] [Google Scholar]
- 44.Sarkar JSV, Seshadri V, Tripoulas NA, Ketterer ME, Fox PL. Role of ceruloplasmin in macrophage iron efflux during hypoxia. J Biol Chem. 2003;278(45):44018–44024. doi: 10.1074/jbc.M304926200. [DOI] [PubMed] [Google Scholar]
- 45.Lahey ME, Gubler CJ, Chase MS, Cartwright GE, Wintrobe MM. Studies on copper metabolism. II. Hematologic manifestations of copper deficiency in swine. Blood. 1952;7(11):1053–1074. [PubMed] [Google Scholar]
- 46.Harris ED. The iron-copper connection: the link to ceruloplasmin grows stronger. Nutr Rev. 1995;53(6):170–173. doi: 10.1111/j.1753-4887.1995.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 47.Vulpe CD, Kuo YM, Murphy TL, et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21(2):195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- 48.Harris ZL, Klomp LW, Gitlin JD. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am J Clin Nutr. 1998;67(5, Suppl):972S–977S. doi: 10.1093/ajcn/67.5.972S. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein SE. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J Lab Clin Med. 1987;110(6):690–705. [PubMed] [Google Scholar]
- 50.Hamill RL, Woods JC, Cook BA. Congenital atransferrinemia. A case report and review of the literature. Am J Clin Pathol. 1991;96(2):215–218. doi: 10.1093/ajcp/96.2.215. [DOI] [PubMed] [Google Scholar]
- 51.De Domenico I, Ward DM, di Patti MC, et al. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26(12):2823–2831. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411(1):123–131. doi: 10.1042/BJ20071474. [DOI] [PubMed] [Google Scholar]
- 53.Marro S, Chiabrando D, Messana E, et al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95(8):1261–1268. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park BY, Chung J. Effects of various metal ions on the gene expression of iron exporter ferroportin-1 in J774 macrophages. Nurs Res Pract. 2008;2(4):317–321. doi: 10.4162/nrp.2008.2.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zoller H, Theurl I, Koch R, Kaser A, Weiss G. Mechanisms of iron mediated regulation of the duodenal iron transporters divalent metal transporter 1 and ferroportin 1. Blood Cells Mol Dis. 2002;29(3):488–497. doi: 10.1006/bcmd.2002.0587. [DOI] [PubMed] [Google Scholar]
- 56.Jacolot S, Férec C, Mura C. Iron responses in hepatic, intestinal and macrophage/monocyte cell lines under different culture conditions. Blood Cells Mol Dis. 2008;41(1):100–108. doi: 10.1016/j.bcmd.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003;102(12):4191–4197. doi: 10.1182/blood-2003-04-1250. [DOI] [PubMed] [Google Scholar]
- 58.Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood. 2010;116(22):4657–4664. doi: 10.1182/blood-2010-04-278614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harada N, Kanayama M, Maruyama A, et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopoly-saccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch Biochem Biophys. 2011;508(1):101–109. doi: 10.1016/j.abb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275(26):19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 61.Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 62.Mok H, Mendoza M, Prchal JT, Balogh P, Schumacher A. Dysregulation of ferroportin 1 interferes with spleen organo-genesis in polycythaemia mice. Development. 2004;131(19):4871–4881. doi: 10.1242/dev.01342. [DOI] [PubMed] [Google Scholar]
- 63.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9(5):461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]