Abstract
Purpose
To compare rates of severe late toxicities following concomitant chemoradiotherapy and radiotherapy alone for cervical cancer.
Methods and Materials
Patients with cervical cancer were treated at a single institution with radiotherapy alone or concomitant chemoradiotherapy for curative intent. Severe late toxicity was defined as grade ≥3 vaginal, urologic, or gastrointestinal toxicity or any pelvic fracture, using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE), occurring ≥6 months from treatment completion and predating any salvage therapy. Severe late toxicity rates were compared after adjusting for pertinent covariates.
Results
At 3 years, probability of vaginal severe late toxicity was 20.2% for radiotherapy alone and 35.1% for concomitant chemoradiotherapy (P=.026). At 3 years, probability of skeletal severe late toxicity was 1.6% for radiotherapy alone and 7.5% for concomitant chemoradiotherapy (P=.010). After adjustment for case mix, concomitant chemoradiotherapy was associated with higher vaginal (hazard ratio [HR] 3.0, 95% confidence interval [CI], 1.7–5.2, P<001), and skeletal (HR 7.0, 95% CI 1.4–34.1, P=.016) severe late toxicity. Compared to high dilator compliance, moderate (HR 3.6, 95% CI 2.0–6.5, P<.001) and poor (HR 8.5, 95% CI 4.3–16.9, P<.001) dilator compliance was associated with higher vaginal severe late toxicity. Age >50 was associated with higher vaginal (HR 1.8, 95% CI 1.1–3.0, P=.013) and skeletal (HR 5.7, 95% CI 1.2–27.0, P=.028) severe late toxicity. Concomitant chemoradiotherapy was not associated with higher gastrointestinal (P=.886) or urologic (unadjusted, P=.053; adjusted, P=.063) severe late toxicity.
Conclusion
Compared to radiotherapy alone, concomitant chemoradiotherapy is associated with higher rates of severe vaginal and skeletal late toxicities. Other predictive factors include dilator compliance for severe vaginal late toxicity and age for severe vaginal and skeletal late toxicities.
Keywords: Cervical cancer, Chemotherapy, Late toxicity, Radiotherapy, Vaginal stricture
Introduction
Prospective analyses of severe late toxicities (SLT) following concomitant chemoradiotherapy (CRT) compared to radiotherapy alone (RT) have been limited (1, 2). This may, in part, be due to insufficient late toxicity observation of patients in prospective randomized clinical trials. In a recent meta-analysis of 13 prospective randomized trials comparing CRT to RT, data were available for late rectal toxicity in 7 trials, late bladder toxicity in 5 trials, and late intestinal and late vaginal toxicity in 4 trials. In addition, each of these trials contained substantial missing data (3). Given the established survival advantage of CRT, an opportunity to prospectively compare SLT with and without concomitant chemotherapy may never again arise. As a result, we sought to address this question through a 20-year historical cohort analysis spanning 2 eras of cervical cancer management. Inclusion of multiple clinical and treatment-related covariates permitted evaluation of and adjustment for case mix differences between cohorts.
Methods and Materials
Patient population
The study population consisted of 480 consecutive patients with cervical cancer treated at a single academic medical center from 1989 to 2009 with RT or CRT for curative intent. This study was approved by the academic medical center's Institutional Review Board.
Radiotherapy
RT consisted of pelvic external beam RT (EBRT) and cervical brachytherapy. According to institutional practice, pelvic EBRT was delivered using a standard 4-field technique, with the posterior border of lateral fields extended behind the sacrum to encompass presacral lymph nodes. Intensity-modulated RT was not used. Prescription dose of whole-pelvis EBRT varied between 39.6 Gy and 50.4 Gy based on clinical factors and the preference of the treating radiation oncologist. Paraortic nodal irradiation and pelvic nodal or parametrial boost was performed at the discretion of the radiation oncologist who designed the EBRT treatment. EBRT fraction size of 1.7 or 1.8 Gy per fraction was prescribed for all patients.
Low-dose-rate (LDR) and high-dose-rate (HDR) brachytherapy procedures were performed according to previously published institutional techniques (4, 5). All patients from 1989–1999 were treated with HDR. Following 1999, choice of HDR or LDR brachytherapy was based on clinical factors at the discretion of the treating radiation oncologist. Prescription dose of HDR or LDR brachytherapy and brachytherapy applicator type varied based on clinical factors and the preference of the treating radiation oncologist. Relative to point A or point M prescription dose, dose to vaginal surface was consistently ≤140% in the pre-chemotherapy era (1989–1998) and ≤125% in the chemotherapy era (1999–2009), following institutional practice. In addition, International Commission on Radiation Units and Measurements (ICRU) (6) doses to bladder and rectal points were consistently ≤80% and ≤75% of point A or point M prescription dose, respectively.
For the purpose of this late toxicity analysis, prescription doses for pelvic EBRT and combined pelvic EBRT/brachytherapy dose were assessed by late effects model for biological equivalent dose in 2-Gy fractions (EQD2) assuming α/β = 3. For pelvic EBRT EQD2, whole-pelvis and pelvic nodal boost prescription doses were added. For combined pelvic EBRT/brachytherapy EQD2, whole-pelvis prescription dose and brachytherapy prescription dose to point A were added.
Chemotherapy
Most patients (96.6%) received cisplatin as their concomitant chemotherapeutic regimen, chosen at the discretion of their treating oncologist. Cisplatin was most commonly delivered weekly at a dose of 40 mg/m2 (maximum dose, 70 mg weekly).
Outcome assessment
Following institutional practice, oncologic surveillance was recommended every 3 months for 2 years after treatment, every 6 months for years 3 through 5 after treatment, and annually thereafter. All patients received at least part of their oncologic surveillance at the single academic medical center where this study was conducted (Appendix E1). The end point of SLT was defined using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE) (Table 1) (7). Imaging studies to diagnose pelvic fracture were obtained only if prompted by patient-reported pelvic pain. An SLT was required to occur at least 6 months from treatment completion and to predate any salvage therapy. Time to SLT was defined from the end of radiotherapy or chemoradiotherapy to the time of first documentation of SLT. Patients without documented SLT were censored at their date of last follow-up for a maximum of 8 years' follow-up.
Table 1.
Definition of SLT using CTCAE version 4.0
| SLT type | Type | Grade | CTCAE description |
|---|---|---|---|
| Vaginal | Vaginal stricture | Grade 3 | Vaginal narrowing and/or shortening interfering with the use of tampons, sexual activity or physical examination |
| Skeletal | Any pelvic fracture diagnosed by imaging, obtained only if prompted by patient-reported pelvic pain. | ||
| Urologic | Urinary fistula (including vesicovaginal fistula) | Grade 3 | Severe symptoms; elective operative intervention indicated |
| Grade 4 | Life-threatening consequences; urgent intervention indicated | ||
| Grade 5 | Death | ||
| Noninfective cystitis | Grade 3 | Gross hematuria; transfusion, IV medications or hospitalization indicated; elective endoscopic, radiologic or operative intervention indicated | |
| Grade 4 | Life-threatening consequences; urgent radiologic or operative intervention indicated | ||
| Grade 5 | Death | ||
| Urinary incontinence | Grade 3 | Intervention indicated (eg, clamp, collagen injections); operative intervention indicated; limited self care ADL | |
| Grade 4 | Not specified | ||
| Grade 5 | Not specified | ||
| Other | Grade 3 | Severe or medically significant, but not immediately life-threatening; hospitalization or prolongation of existing hospitalization indicated; disabling; limiting self-care ADL | |
| Grade 4 | Life-threatening consequences; urgent intervention indicated | ||
| Grade 5 | Death | ||
| Gastrointestinal | Colonic obstruction/stenosis | Grade 3 | Severely altered GI function; tube feeding or hospitalization indicated; elective operative intervention indicated |
| Grade 4 | Life-threatening consequences; urgent operative intervention indicated | ||
| Grade 5 | Death | ||
| Enterocolitis | Grade 3 | Severe or persistent abdominal pain; fever; ileus; peritoneal signs | |
| Grade 4 | Life-threatening consequences; urgent intervention indicated | ||
| Grade 5 | Death | ||
| Diarrhea | Grade 3 | Increase of ≥7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL | |
| Grade 4 | Life-threatening consequences; urgent intervention indicated | ||
| Grade 5 | Death | ||
| Proctitis | Grade 3 | Severe symptoms; fecal urgency or stool incontinence; limited self-care ADL | |
| Grade 4 | Life-threatening consequences; urgent intervention indicated | ||
| Grade 5 | Death | ||
| Rectal fistula (including rectovaginal fistula) | Grade 3 | Severe altered GI function; TPN or hospitalization indicated; elective operative intervention indicated | |
| Grade 4 | Life-threatening consequences; urgent intervention indicated | ||
| Grade 5 | Death | ||
| Small bowel obstruction | Grade 3 | Hospitalization indicated; elective operative intervention indicated; limiting self-care ADL; disabling | |
| Grade 4 | Life-threatening consequences; urgent operative intervention indicated | ||
| Grade 5 | Death | ||
| Other | Grade 3 | Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of existing hospitalization indicated; disabling; limiting self-care ADL | |
| Grade 4 | Life-threatening consequences | ||
| Grade 5 | Death | ||
Abbreviations: ADL = activities of daily living; SLT = severe late toxicity.
Definition of severe late toxicity (SLT) using CTCAE version 4.0. Vaginal SLT referred to grade 3 vaginal stricture. Skeletal SLT referred to any pelvic fracture diagnosed by imaging, obtained only if prompted by patient-reported pelvic pain. Urologic SLT referred to any toxicity of grade ≥3 of the urinary organ system, including vesicovaginal fistulae. Gastrointestinal SLT referred to any toxicity grade ≥3 of the gastrointestinal organ system, including rectovaginal fistulae. An SLT was required to occur at least 6 mos from treatment completion and to predate any salvage therapy.
Covariate factors
Covariate factors were assessed as categorical variables and are shown in Table 2. Dilator compliance was determined based on documentation of frequency of dilator use or sexual intercourse prior to the development of vaginal SLT. High dilator compliance was defined according to our institutional recommendations of vaginal penetration (either through dilation or sexual intercourse) 2 or more times per week for the first 2 years following treatment, with at least monthly use thereafter and no documented breaks in usage. Poor dilator compliance was defined as less than monthly use following treatment. Moderate dilator compliance defined remaining situations. According to institutional practice, all patients were provided with vaginal dilator and advised to initiate its use following treatment, unless medically contraindicated.
Table 2.
Chi-squared analysis comparing patients treated with radiotherapy alone and chemoradiotherapy
| Factor | Radiotherapy alone (n = 195) | Chemoradiotherapy (n = 179) | P value |
|---|---|---|---|
| Age | ≤50: 112 (57.4%) | ≤50: 87 (48.6%) | NS |
| >50: 83 (42.6%) | >50: 92 (51.4%) | ||
| Race | White: 182 (93.3%) | White: 160 (89.4%) | NS |
| African-American: 7 (3.6%) | African-American: 5 (2.8%) | ||
| Hispanic: 4 (2.1%) | Hispanic: 4 (2.2%) | ||
| Other: 2 (1.0%) | Other: 10 (5.6%) | ||
| Body mass index | ≤30: 124 (63.6%) | ≤30: 102 (57.0%) | NS |
| >30: 62 (31.8%) | >30: 73 (40.8%) | ||
| NA: 9 (4.6%) | NA: 4 (2.2%) | ||
| Pretreatment nodal dissection | Yes: 38 (19.5%) | Yes: 10 (5.6%) | <.001 |
| No: 157 (80.5%) | No: 157 (87.7%) | ||
| NA: 0 (0%) | NA: 12 (6.7%) | ||
| History of diabetes | Yes: 14 (7.2%) | Yes: 20 (11.2%) | NS |
| No: 180 (92.3%) | No: 159 (88.8%) | ||
| NA: 1 (0.5%) | NA: 0 (0%) | ||
| History of hypertension | Yes: 28 (14.4%) | Yes: 45 (25.1%) | .009 |
| No: 166 (85.1%) | No: 134 (74.9%) | ||
| NA: 1 (0.5%) | NA: 0 (0%) | ||
| History of intestinal disorder | Yes: 3 (1.5%) | Yes: 6 (3.4%) | NS |
| No: 191 (98.0%) | No: 173 (96.6%) | ||
| NA: 1 (0.5%) | NA: 0 (0%) | ||
| History of abdominopelvic surgery | Yes: 74 (38.0%) | Yes: 72 (40.2%) | NS |
| No: 120 (61.5%) | No: 107 (59.8%) | ||
| NA: 1 (0.5%) | NA: 0 (0%) | ||
| Cigarette smoking history | Previously/Currently smoking: 97 (49.7%) | Previously/Currently smoking: 96 (53.6%) | NS |
| Never smoking: 76 (39.0%) | Never smoking: 83 (46.4%) | ||
| NA: 22 (11.3%) | NA: 0 (0%) | ||
| Histology | Squamous cell carcinoma: 149 (76.4%) | Squamous cell carcinoma: 149 (83.2%) | NS |
| Nonsquamous: 46 (23.6%) | Nonsquamous: 30 (16.8%) | ||
| FIGO stage | IB1: 46 (23.6%) | IB1: 17(9.5%) | .001 |
| IB2: 31 (15.9%) | IB2: 33 (18.4%) | ||
| IIA: 6 (3.1%) | IIA: 15 (8.4%) | ||
| IIB: 64 (32.8%) | IIB: 79 (44.1%) | ||
| IIIA: 2 (1.0%) | IIIA: 4 (2.2%) | ||
| IIIB: 44 (22.6%) | IIIB: 27 (15.1%) | ||
| IVA: 2 (1.0%) | IVA: 4 (2.2%) | ||
| Pelvic EBRT EQD2 (Gy3)* | ≤50: 92 (47.2%) | ≤50: 98 (54.8%) | NS |
| >50: 101 (51.8%) | >50: 72 (40.2%) | ||
| NA: 2 (1.0%) | NA: 9 (5.0%) | ||
| Paraortic nodal EBRT | No: 178 (91.3%) | No: 143 (79.9%) | .036 |
| Yes: 17 (8.7%) | Yes: 27 (15.1%) | ||
| NA: 0 (0%) | NA: 9 (5.0%) | ||
| Brachytherapy dose rate | HDR: 193 (99.0%) | HDR: 151 (84.3%) | <.001 |
| LDR: 2 (1.0%) | LDR: 25 (14.0%) | ||
| NA: 0 (0%) | NA: 3 (1.7%) | ||
| Brachytherapy applicator type | Tandem & Ovoids: 139 (71.3%) | Tandem & Ovoids: 153 (86.0%) | <.001 |
| Tandem & Cylinders or Ring†: 50 (25.6%) | Tandem & Cylinders or Ring†: 4 (2.2%) | ||
| Interstitial†: 6 (3.1%) | Interstitial: 21 (11.8%) | ||
| Combined pelvic EBRT/brachytherapy EQD2 (Gy3)* | ≤105:161 (82.6%) | ≤105: 178 (99.4%) | <.001 |
| >105: 34 (17.4%) | >105: 1 (0.6%) | ||
| Dilator compliance | High: 39 (20.0%) | High: 91 (50.8%) | <.001 |
| Moderate: 38 (19.5%) | Moderate: 53 (29.6%) | ||
| Poor: 40 (20.5%) | Poor: 17 (9.5%) | ||
| NA: 78 (40.0%) | NA: 18 (10.1%) |
Abbreviations: EBRT = external-beam radiotherapy; EQD2 = biological equivalent dose in 2-Gy fractions assuming α/β = 3; FIGO = International Federation of Gynecology and Oncology; HDR = high-dose-rate; LDR = low-dose-rate; NA = not available; NS = nonsignificant.
Numbers presented in bold are statistically significant.
Assessed by late effects model for biological EQD2 (assuming α/β = 3).
Includes the use of vaginal cylinders (with tandem placement), vaginal ring (with tandem placement), or interstitial brachytherapy alone or in combination with tandem and ovoid placement.
Statistical considerations
Chi-squared analysis of covariate factors was used to compare RT and CRT cohorts. Comparison of follow-up times used the nonparametric Mann-Whitney test. Time to SLT was estimated with Kaplan-Meier analysis, and log-rank statistical analysis was used to make univariate comparisons. Using the above covariates, a stepwise selection procedure was used to build a multivariate Cox proportional hazards model with entry criterion set at a P value of <.20 on univariate log rank analysis. In addition, to confirm association between covariate and SLT, we built a separate Cox proportional hazards model with all covariates entered. All statistical analyses were performed using SPSS Statistics version 19 (SPSS Inc, Chicago, IL).
Results
Baseline differences between cohorts
After we excluded 20 patients (RT, n = 19; CRT, n = 1) who had relapsed or died prior to 6 months' follow-up and 86 patients (RT, n = 65; CRT, n = 21) who were lost to follow-up, the final data set included 374 assessable patients, consisting of 195 RT patients and 179 CRT patients (Table 2). A total of 91.8% of the RT cohort was treated from 1989–1998, prior to the introduction of CRT at the academic medical center where this study was conducted. All CRT patients were treated from 1999–2009. Median follow-up was 35.5 months overall, with longer follow-up in the RT cohort (45.7 months; range, 6.0–96.0 months) than in the CRT cohort (29.3 months; range, 6.6–96.0 months; P=.031).
Vaginal SLT outcomes
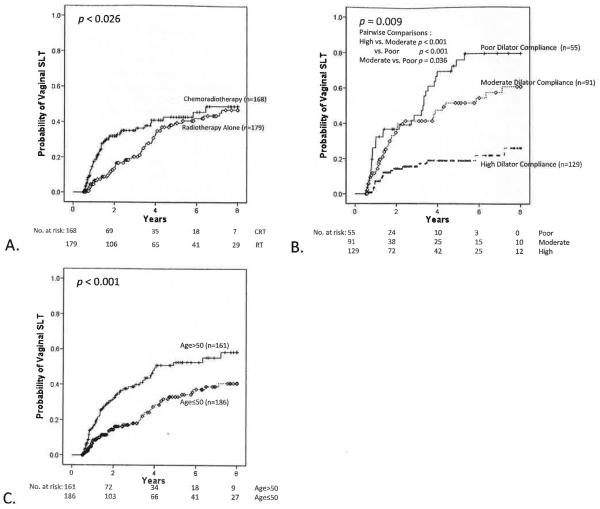
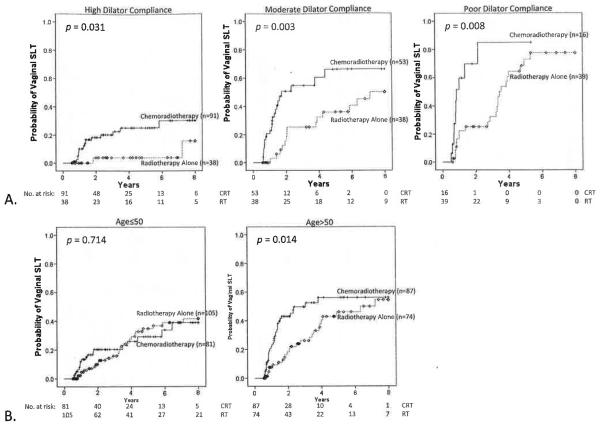
A total of 106 patients, 51 in the RT group and 55 in the CRT cohort, developed vaginal SLT. At 3 years, probability of developing vaginal SLT was 20.2% for RT (95% CI, 13.3%–27.1%) and 35.1% for CRT (95% CI, 26.9%–43.3%; P=.026) patients (Fig. 1A). At 3 years, probability of developing vaginal SLT was 15.6% (95% CI, 8.5%–22.7%) for patients with high dilator compliance; 41.6% (95% CI, 29.8%–53.4%; P<.001 compared to high compliance) for patients with moderate compliance; and 44.9% (95% CI, 30.2%–59.6%; P<.001 compared to high compliance, P=.036 compared to moderate compliance) for patients with poor compliance (Fig. 1B). At 3 years, probability of developing vaginal SLT was 18.0% for patients aged ≤50 (95% CI. 11.5%–24.5%) and 38.7% for those aged >50 (95% CI, 29.9%–47.5%; P<.001) (Fig. 1C). When patients were stratified by dilator compliance, CRT demonstrated higher probability of developing vaginal SLT in the setting of high (P=.031), moderate (P=.003), or poor (P=.008) dilator compliance (Fig. 2A). Stratified by age group, CRT patients demonstrated higher probability of developing vaginal SLT for those aged >50 (P=.014) but not for those aged ≤50 (P=.714), but the test for interaction showed this to be nonsignificant (P=.190) (Fig. 2B). Stratification by CRT or RT cohort showed that treatment year was not associated with probability of developing vaginal SLT (P=.084).
Fig. 1.
Probability of vaginal SLT. (A) CRT vs RT alone (P=.026); (B) Dilator compliance (high vs moderate vs low) (P=.009); and, (C) age (≤50 vs >50; P<.001). Pairwise comparisons were significant for high compliance vs moderate compliance (P<.001) and vs poor compliance (P<.001) and for moderate compliance vs poor compliance (P=.036). Vaginal SLT was defined as grade 3 vaginal stricture, using CTCAE version 4.0, occurring ≥6 months from treatment completion and predating any salvage therapy.
Fig. 2.
Probability of vaginal SLT for CRT vs RT alone. Stratified by (A) dilator compliance (P=.031 for high compliance; P=.003 for moderate compliance, and P=.008 for poor compliance; all strata, P<.001) and (B) age (P=.714 for age ≤50 and P=.014 for age >50; all strata, P=.035; test for interaction, P=.190). Vaginal SLT was defined as grade 3 vaginal stricture, using CTCAE version 4.0, occurring ≥6 months from treatment completion and predating any salvage therapy.
Brachytherapy dose rate and applicator type were not associated with vaginal SLT. After we adjusted for covariates potentially associated with vaginal SLT, CRT remained associated with a higher risk of vaginal SLT than radiotherapy alone (hazard ratio [HR], 3.0; 95% CI, 1.7%–5.2%; P<.001); moderate (HR, 3.6; 95% CI, 2.0%–6.5%; P<.001) and poor (HR, 8.5; 95% CI, 4.3%–16.9%; P<.001) dilator compliance remained associated with a higher risk of vaginal SLT than high dilator compliance; and, patient age >50 years remained associated with a higher risk of vaginal SLT than age ≤50 years (HR, 1.8; 95% CI, 1.1%–3.0%; P=.013) (Table 3). Associations between increased risk of vaginal SLT and CRT, moderate and poor dilator compliance, and age >50 retained significance after adjustments were made for all covariates (P<.001, P<.001, and P=.031, respectively; data not shown). Sensitivity analyses that excluded patients who received LDR brachytherapy and that excluded patients treated with vaginal cylinders or ring did not alter these findings (data not shown). In addition, dilator compliance rates were similar between age >50 and age ≤50 cohorts (P=.105).
Table 3.
Unadjusted and adjusted analyses
| Vaginal SLT |
Skeletal SLT |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||
| Parameter | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Treatment | .027 | <.001 | .023 | .016 | ||||
| CRT vs RT | 1.5 (1.1–2.3) | 3.0 (1.7–5.2) | 5.83 (1.28–26.7) | 7.0 (1.4–34.1) | ||||
| Dilator compliance | <.001 | <.001 | .709 | NA | ||||
| High | RG | RG | ||||||
| Moderate | 3.0 (1.8–5.2) | 3.6 (2.0–6.5) | <.001 | |||||
| Poor | 5.0 (2.9–8.7) | 8.5 (4.3–16.9) | <.001 | |||||
| Age | .001 | .013 | .049 | .028 | ||||
| >50 vs ≤50 | 2.0 (1.3–2.9) | 1.8 (1.1–3.0) | 3.7 (1.01–13.7) | 5.7 (1.2–27.0) | ||||
Abbreviations: CI = confidence interval; HR = hazard ratio; NA = not applicable; RG = reference group.
Unadjusted analysis used Cox proportional hazards model. Adjusted analysis used Cox proportional hazards model built with stepwise selection procedure. Covariate factors included histology, body mass index, race, cigarette smoking history, history of hypertension, history of diabetes, history of intestinal disorder, history of abdominopelvic surgery, pre-radiotherapy nodal dissection, FIGO stage, pelvic EBRT biologically equivalent dose in 2-Gy fractions (EQD2) (Gy3), paraortic nodal EBRT, brachytherapy dose rate, brachytherapy applicator type, pelvic EBRT/brachytherapy EQD2 (Gy3). Entry criterion was set at a P value <.20 on unadjusted log-rank analysis. All statistically significant P values on multivariate analysis were confirmed with Cox proportional hazards model with all covariates entered (data not shown).
Skeletal SLT outcomes
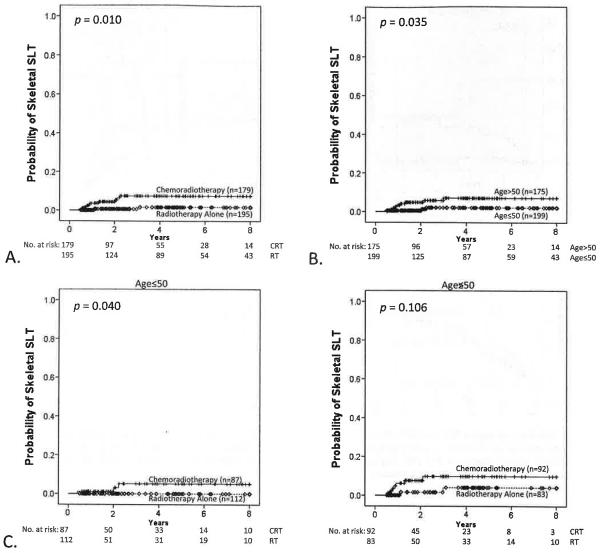
A total of 12 patients, 2 in the RT group and 10 in the CRT cohort, developed skeletal SLT. Skeletal SLT was diagnosed by magnetic resonance imaging in 10 patients and computed tomography in 2 patients. At 3 years, the probability of developing skeletal SLT was 1.6% for RT patients (95% CI, 0.0%–4.0%) and 7.5% for CRT patients (95% CI, 2.8%–12.2%; P = .010) (Fig. 3A). At 3 years, the probability of developing skeletal SLT was 2.1% for patients age ≤50 (95% CI, 0.0%–4.6%) and 7.1% for patients age >50 (95% CI, 2.4%–11.8%; P = .035) (Fig. 3B). Results stratified by age group showed that CRT demonstrated higher probability of developing skeletal SLT for patients age ≤50 (P = .040) but not those age >50 (P = .106), but a test for interaction showed this to be nonsignificant (P = .497) (Fig. 3C). Stratified by CRT or RT cohort, treatment year was not associated with probability of developing skeletal SLT (P = .211).
Fig. 3.
Probability of skeletal SLT. (A) CRT vs RT alone (P=.010); (B) age (≤50 vs >50, P=.035); and (C) CRT vs RT alone, stratified by age (age ≤50; P=.040; age >50, P=.106; all strata, P=.015; test for interaction, P=.497). Skeletal SLT was defined as any pelvic fracture occurring ≥6 months from treatment completion and predating any salvage therapy.
After adjustment was made for covariates potentially associated with skeletal SLT, CRT (HR, 7.0; 95% CI, 1.4%–34.1%; P = .016) and age >50 (HR, 5.7; 95% CI, 1.2%–27.0%; P = .028) continued to be associated with an increased risk of skeletal SLT compared to RT and age ≤50, respectively (Table 3). These associations retained statistical significance after we adjusted for all covariates (P = .010 for CRT vs RT; P = .017 for age >50 vs age ≤50; data not shown).
Gastrointestinal SLT outcomes
A total of 44 patients developed gastrointestinal SLT. Univariate analyses showed no difference in gastrointestinal SLT between CRT and RT cohorts (3-year rate of gastrointestinal SLT for RT was 11.7% vs 13.1% for CRT; P = .886). Multivariate analysis of covariate factors potentially associated with gastrointestinal SLT demonstrated statistical significance for FIGO stage (P = .022) and cigarette smoking history (HR, 2.2; 95% CI, 1.1%–4.1%; P = .019). However, these factors lost significance after we adjusted for all covariates (P = .212 and P = .109, respectively).
Urologic SLT outcomes
A total of 14 patients developed urologic SLT. Univariate analyses showed a trend toward a higher rate of urologic SLT with CRT (3-year rate of urologic SLT for RT was 1.1% vs 3.7% for CRT; P = .053). After adjustment for covariate factors potentially associated with urologic SLT, CRT lost statistical significance (P = .063). Multivariate analysis of covariate factors potentially associated with urologic SLT demonstrated statistical significance for cigarette smoking history (HR, 10.7; 95% CI, 1.4%–84.1%; P = .024). However, this factor lost statistical significance after adjusting for all covariates (P = .131).
Discussion
Our study is the first prospective or retrospective clinical series in cervical cancer to observe an association between the administration of concomitant chemotherapy and subsequent SLT. After adjusting for case mix differences, we observed a higher rate of severe vaginal and skeletal late toxicity following CRT than RT for cervical cancer. In addition, we observed independent effects of vaginal dilator compliance on severe vaginal toxicity and age on severe vaginal and skeletal toxicity. Although the study is retrospective in nature, these findings can be used in counseling patients about potential treatment-related late toxicities.
The original purpose of documenting dilator compliance was to serve as a potentially confounding factor in assessing vaginal SLT. However, stratified results from univariate and multivariate analyses demonstrated that CRT and moderate/poor dilator usage represent independent risk factors for vaginal SLT. Existing data for effects of vaginal dilation in preventing vaginal stricture are limited (8). To our knowledge, ours is the largest study to document the effect of dilator compliance on severe vaginal late toxicity. To elucidate the cause-and-effect relationship between dilator usage and vaginal SLT, we assessed vaginal dilator usage only during follow-up visits occurring prior to the identification of vaginal SLT. However, it is possible that grade ≤2 vaginal stricture causing pain and/or discomfort may have reduced vaginal dilator compliance and contributed, at least in part, to the subsequent development of vaginal SLT. In addition, data on systemic or vaginal estrogen use was not collected and could not be adjusted for in this analysis. We also observed age >50 to be associated with a significantly increased risk of vaginal and skeletal SLT. These results are consistent with previous reports demonstrating the importance of age in the development of vaginal stenosis and pelvic fractures after definitive radiotherapy for cervical cancer (9–11). Interestingly, in our study, we observed CRT and age >50 to be independent risk factors in stratified univariate and multivariate analyses.
Estimation of late toxicity rates can be significantly impacted by the definition used. In our analysis, we opted to use the most recent version (version 4.0) of CTCAE (Table 1) (7). An important change from version 3.0 to version 4.0 was the definition of grade 3 vaginal stricture, now inclusive of situations where vaginal narrowing interferes with sexual function, tampon use, and physical examination. This contrasts with version 3.0, where grade 3 vaginal stricture (termed “vaginal stenosis”) was defined as surgically uncorrectable complete obliteration of the vaginal vault. A more encompassing definition of grade 3 vaginal stricture may explain in part why our rates of vaginal SLT are higher than those reported in previous retrospective series. An advantage of the CTCAE version 4.0 definition of grade 3 vaginal stricture is its reliance on criteria of significant function (interference with sexual function and tampon use) and disease surveillance (interference with physical examination) implications.
In this study, a rigorous analysis of medical records at the academic medical center where this study was conducted was used to assign dates to the first development of late toxicity so that actuarial estimates of late toxicity could be determined. Such methodology was used based on previous reports emphasizing the importance of actuarially assessing late complications rather than simply providing crude rates. Eifel et al (12) reported on late complications of patients treated with radiotherapy alone for FIGO stage IB cervical cancer and observed a small but continuous risk per year of follow-up for up to 20 years after treatment. Similarly, we observed 5-year rates of vaginal SLT in the overall population to be 42.0% at 5 years, with a subsequent continuous risk of approximately 2.3% per year, resulting in an actuarial risk of vaginal SLT of 48.8% at 8 years.
Eifel et al (13) previously demonstrated a strong correlation between cigarette smoking history and urologic and gastrointestinal complications following RT alone. In our analysis of patients treated with RT or CRT, a similar association was also observed. However, to account for potential bias inherent in a retrospective analysis, we established a priori that a factor must remain statistically significant on multivariate analysis built with all covariates entered for it to be considered predictive of SLT. In such multivariate modeling, the association between smoking history and urologic and gastrointestinal SLT lost statistical significance, potentially due to limited number of events. However, these data do further emphasize the importance of cigarette smoking history on urologic or gastrointestinal SLT.
Given its retrospective design, this study has a number of important limitations. First, for SLT identification, this study relied on the rigorous review of medical records, which has the potential to introduce bias. For instance, a higher percentage (40%) of patients in the RT-alone cohort had missing data with respect to dilator compliance. Second, assessing the role of radiation dose on risk of SLT is limited by the absence of 3-dimensional treatment planning in all patients. As a result, exact vaginal and sacral dosimetric analysis is not feasible. Third, this analysis adjusted for 17 covariate factors in addition to the use of concomitant chemotherapy. However, it is possible that the SLT associations observed in this study could be attributable to confounding covariates not captured in this retrospective analysis. For instance, although published institutional practice permitted a higher vaginal surface dose during brachytherapy during the pre-chemotherapy (1989–1998) than in the chemotherapy (1999–2009) era, sufficient data were not available for statistical analysis of vaginal surface dose as a potential predictor of vaginal SLT. This limitation also applies to International Commission on Radiation Units and Measurements bladder and rectal point dosimetry as potential predictors of urologic and gastrointestinal SLT, respectively.
Of the 12 patients with skeletal SLT, only 1 patient came from the pre-chemotherapy (ie, 1989–98) era, and 10 patients were diagnosed with magnetic resonance imaging. Although our approach to identifying skeletal SLT was prompted by patient reports of pelvic pain rather than routine imaging surveillance, we were concerned that the difference in skeletal SLT between CRT and RT cohorts might be due to evolving year-to-year differences in evaluation for pelvic fracture. To assess this possibility, we evaluated year of treatment as a continuous variable, stratified by treatment cohort, and found no significant association with skeletal SLT.
Finally, despite censoring patients free of SLT at a maximum of 8 years follow-up, the RT cohort had significantly longer follow-up than the CRT cohort, because of the different eras of treatment. The longer time during which RT patients were at risk to manifest SLT suggests that the effect of CRT on SLTs may actually be larger than is estimated by this study.
Acknowledgments
This project was supported by a University of Wisconsin Department of Human Oncology seed grant.
Footnotes
Presented in abstract form at the Annual Meeting for the American Society of Radiation Oncology. 2010.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 2.Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966–972. doi: 10.1200/JCO.2002.20.4.966. [DOI] [PubMed] [Google Scholar]
- 3.Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomadsen BR, Shahabi S, Stitt JA, et al. High dose rate intracavitary brachytherapy for carcinoma of the cervix: the Madison system: II. Procedural and physical considerations. Int J Radiat Oncol Biol Phys. 1992;24:349–357. doi: 10.1016/0360-3016(92)90691-a. [DOI] [PubMed] [Google Scholar]
- 5.Stitt JA, Fowler JF, Thomadsen BR, et al. High dose rate intracavitary brachytherapy for carcinoma of the cervix: the Madison system: I. Clinical and radiobiological considerations. Int J Radiat Oncol Biol Phys. 1992;24:335–348. doi: 10.1016/0360-3016(92)90690-j. [DOI] [PubMed] [Google Scholar]
- 6.International Commission on Radiation Units and Measurements . ICRU Report No. 38. International Commission on Radiation Units and Measurements; Bethesda, MD: 1985. Dose and volume specification for reporting intracavitary therapy in gynecology. [Google Scholar]
- 7.National Cancer Institute . Common terminology criteria for adverse events (CTCAE) US Department of Health and Human Services; National Cancer Institute; 2010. [Google Scholar]
- 8.Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2010;9:CD007291. doi: 10.1002/14651858.CD007291.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer. 2006;16:288–293. doi: 10.1111/j.1525-1438.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmeler KM, Jhingran A, Iyer RB, et al. Pelvic fractures after radiotherapy for cervical cancer: implications for survivors. Cancer. 116:625–630. doi: 10.1002/cncr.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh D, Huh SJ, Nam H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: analysis of risk factors. Int J Radiat Oncol Biol Phys. 2008;70:1183–1188. doi: 10.1016/j.ijrobp.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Eifel PJ, Levenback C, Wharton JT, et al. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1995;32:1289–1300. doi: 10.1016/0360-3016(95)00118-I. [DOI] [PubMed] [Google Scholar]
- 13.Eifel PJ, Jhingran A, Bodurka DC, et al. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]