Abstract
Introduction
There are major new advancements in the fields of stem cell biology, developmental biology, regenerative hair cycling, and tissue engineering. The time is ripe to integrate, translate and apply these findings to tissue engineering and regenerative medicine. Readers will learn about new progress in cellular and molecular aspects of hair follicle development, regeneration and potential therapeutic opportunities these advances may offer.
Areas covered
Here we use hair follicle formation to illustrate this progress and to identify targets for potential strategies in therapeutics. Hair regeneration is discussed in four different categories. (1) Intra-follicle regeneration (or renewal) is the basic production of hair fibers from hair stem cells and dermal papillae in existing follicles. (2) Chimeric follicles via epithelial-mesenchymal recombination to identify stem cells and signaling centers. (3) Extra-follicular factors including local dermal and systemic factors can modulate the regenerative behavior of hair follicles, and may be relatively easy therapeutic targets. (4) Follicular neogenesis means the de novo formation of new follicles. In addition, scientists are working to engineer hair follicles, which require hair forming competent epidermal cells and hair inducing dermal cells.
Expert opinion
Ideally self-organizing processes similar to those occurring during embryonic development should be elicited with some help from biomaterials.
Keywords: regenerative medicine, tissue engineering, alopecia, wound healing, dermal papilla, stem cells, wound, biomaterials
1. Introduction: Current issues in hair loss related disorders
Hair loss or alopecia can result either from a failure to regrow hair fibers from existing hair follicles (HFs), from extrafollicular environmental factors that affect follicular stem cell activity, or alternatively from the loss of HFs themselves (Fig. 1). Different therapeutic strategies are required for each condition.
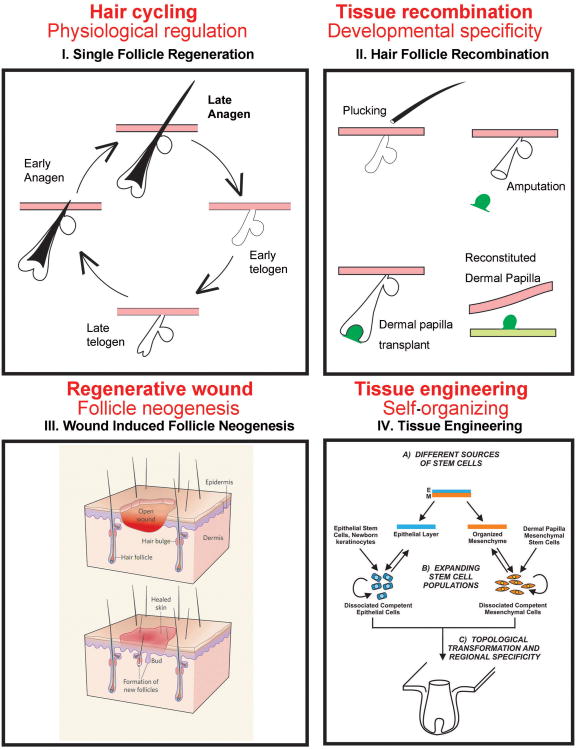
Fig. 1. Categories of hair regeneration.
I. Hair cycle activation. This is regeneration within the same follicle and some named it as “renewal”. A single HF cycles through anagen, catagen, telogen and exogen phases in the normal hair cycle. Regeneration can be under physiological control or regenerate after hair plucking, which inflicts a micro-injury. The progression of cycling is modulated by stem cell niche, which is affected by micro- and macro- environmental factors (please see Fig. 2). II. Chimeric follicles. The epithelial vs mesenchymal contributions to the hair cycle can be analyzed by epithelial: mesenchymal recombination. III. Wound induced follicle neogenesis. New HF formation after large wounding. This also occurs physiologically such as after the shedding of deer antlers (from 67). Please see Fig. 4. IV. Tissue engineering based follicle neogenesis. Reconstitution of new HF from dissociated epidermal stem cells and hair inducing dermal cells. Please see Fig. 5.
Hair loss is most frequently caused by a failure to activate existing hair stem cells during hair cycling and may be associated with aging in both males and females. This condition may be rescued if the general follicular structure is preserved and the causative factor is removed. In androgenetic alopecia (AGA), hair fibers become progressively thinner. AGA is reversible at early stages but may become irreversible after continued disease progression. Hair stem cells in AGA seem to be normal, but activation to form hair germs is defective 1 due to the micro-environment within the HF, or macro-environment outside the HF. We will discuss the progress and potential therapeutic strategies for this category of disease (Fig. 1, 2, 3). Since basic follicle architecture remains and previous hair stem cells and dermal papilla cells remain, some, such as those in the tooth field call this process “renewal” not regeneration.
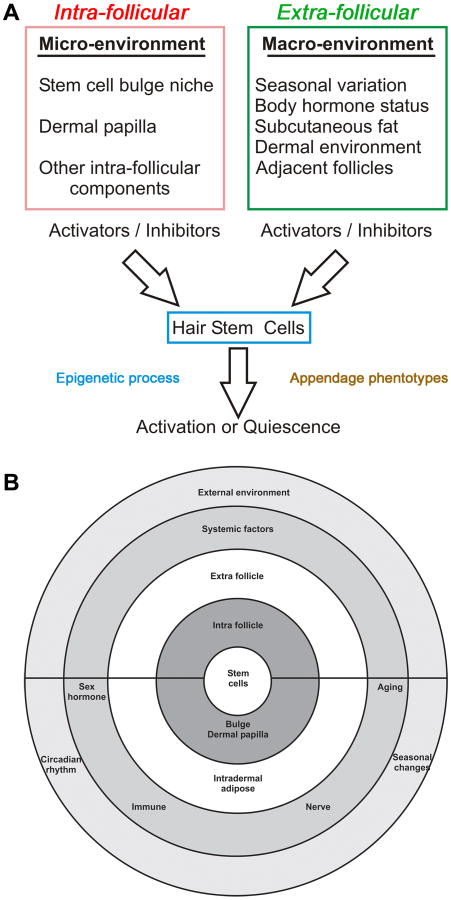
Fig. 2. Concept chart showing multi-layered environmental regulation on hair regeneration.
A. HF activation or quiescence is regulated by factors within the intra-follicular microenvironment and extra-follicular macro-environment. Hair stem cells sum up the positive and negative input and “decide” to get activated or remain quiescence (from 25). The many layers of regulation can be illustrated with concentric rings (modified from 30). They also show the potential targets for diseases and therapeutic strategies.
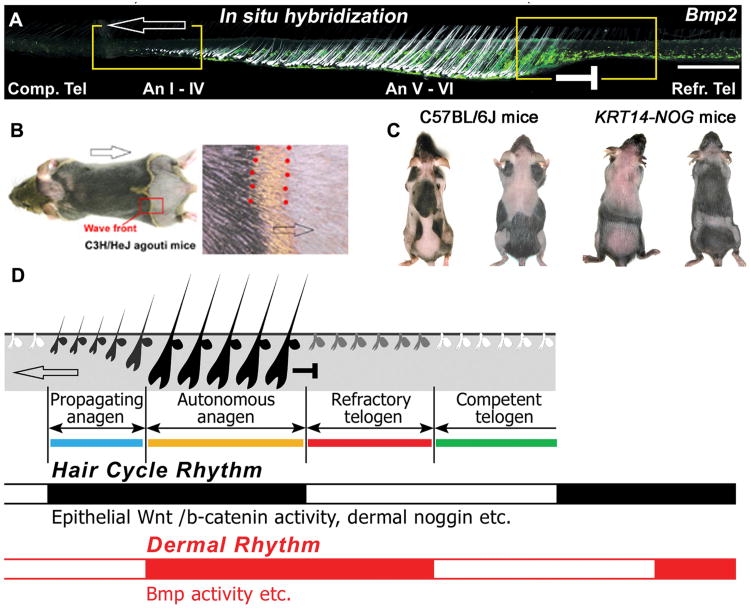
Fig. 3. Effects of macro-environmental factors on the regenerative hair wave.
A. Different temporal stages laid out spatially across a skin strip shows HFs and BMP2 in situ hybridization (white speckles). B. Visualization of hair molting by observing changes in hair pigmentation. C. Control and KRT14-NOG mice. Hair cycle domains in two different stages show domain boundaries. D. Schematic summary of the hair cycle rhythm (black) and dermal rhythm (gray) which together define 4 new functional stages. Catagen is omitted for simplification. Panel B is from 98. All other panels are from 26.
Another category of hair loss is due to severe wounding. This can be caused by burns, accidents, or major surgery in which patients suffer from loss of skin in a large region. Epidermal transplantation from other regions of the body or foreskin grafts have been used to help save patients' lives 2, 3. However, patients heal their skin via repair type wound healing. The scars which form provide a protective cover to prevent infection and fluid loss. But this skin does not look, feel, or function normally. Much of the reason for this is that scar tissue does not contain skin appendages such as hair, sebaceous glands, or sweat glands, etc. Glands lubricate the skin and allow for thermal regulation. Hair, while no longer essential for maintaining endothermy in humans, still plays a major role in a person's appearance. The formation of skin appendages requires regenerative wound healing (i.e., the replacement of an injured area not only with reparative connective tissues and re-epithelialized epidermis but with normal functional components). We will discuss the possible reprogramming of cells to form new HFs (Fig. 1, 4) or to develop tissue engineering methods to generate hair germs from stem cells. We will also explore the role of extra-cellular matrices and the aid of biomaterials in this process (Fig. 5). However, to succeed in tissue engineering, we must first familiarize ourselves with the basic biology of HF development and regeneration. We can then mimic these principles and guide stem cells to do what we wish them to do in regenerative medicine.
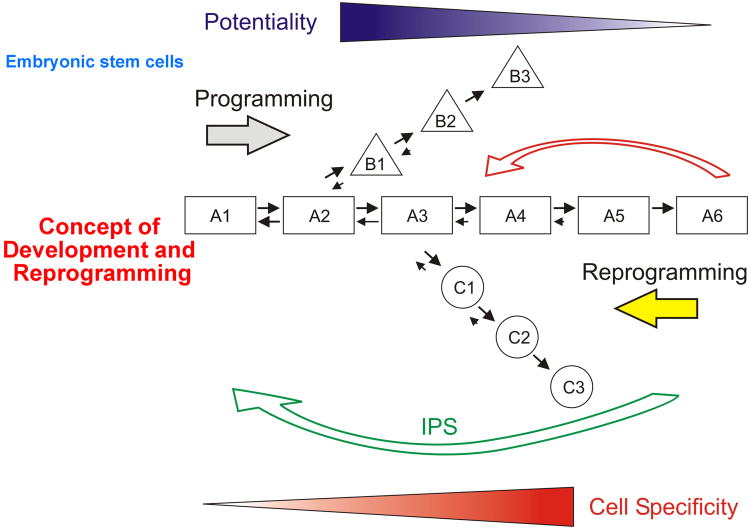
Fig. 4. Programming and reprogramming in development and regeneration.
Cells with high developmental potential progressively become progressively programmed toward differentiation (black triangle) as they move from A1 to A6. At early steps along this pathway cells can move equally in both directions (towards differentiation and de-differentiation). As they progress, their propensity to de-differentiate decreases until they become terminally differentiated cells (A6). Terminally differentiated cells have the highest cell specificity (gray triangle). At certain points along the differentiation cascade, cells can move into other lineages (B1 to B3 or C1 to C3). Recently progress has been made in reprogramming (straight arrows). Just a few factors were required to engineer these iPS cells (long curved arrow). We wish to reprogram committed adult cells back to an earlier stage of their progression towards differentiation (short curved arrow).
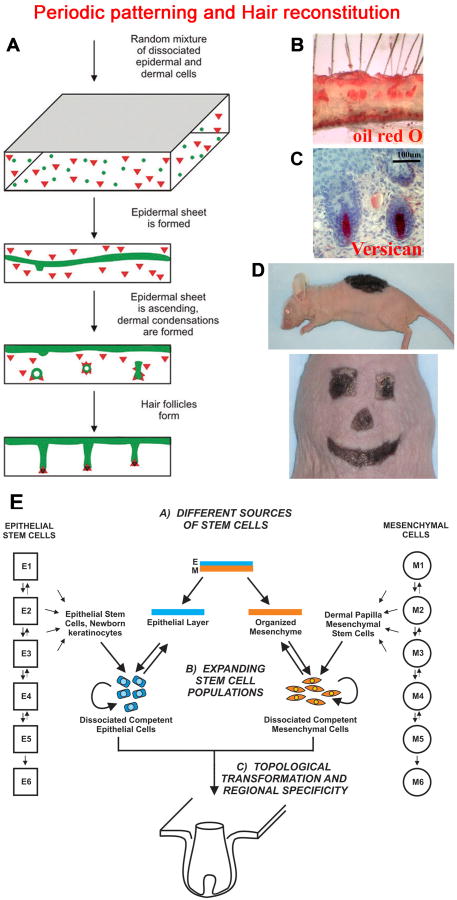
Fig. 5. Tissue engineering of new hairs.
A. Schematic drawing illustrating the hair reconstitution process 88. Epidermal cells (round) and dermal cells (triangle) are randomly mixed in a three dimensional matrix. These cells self-organize and form periodic hair germs which grow into HFs. B. Oil red O stains sebaceous glands and subcutaneous adipose tissue. C. Anti-Versican antibody highlights DP. C. Schematic showing the hair reconstitution process. D. Hairs grow from the grafted region by 21 days. Reconstitution of hairs on a stiff matrix enables us to create specific shapes and sizes for cosmetic applications. (From 88). E. The strategy to engineer new hairs with the generation of hair forming epidermal stem cells and hair inducing dermal cells via reprogramming.
In some inherited forms of alopecia, hair loss is due to genetic mutations in molecules involved in hair keratin architecture or failure to differentiate properly 4. These are difficult to correct. In contrast, acquired alopecia is commonly classified into non-scarring alopecia and scarring/cicatricial alopecia. In cicatricial alopecia, HF structure is destroyed by inflammation of various etiologies and replaced by fibrosis with the HF permanently lost. These defects are hard to correct and will not be discussed further here.
2. Basic biology of hair follicles
Human HFs develop through complex morphogenetic processes resulting from reciprocal molecular interactions between epithelium and underlying mesenchyme during embryonic development 5-8. It is generally believed that no new HFs form after birth in humans though this general assumption was challenged more than half a century ago 9. Each HF goes through regenerative cycling. The hair cycle consists of phases of growth (anagen), degeneration (catagen) and rest (telogen). In catagen, hair follicle stem cells are maintained in the bulge. Then the resting follicle re-enters anagen (regeneration) when proper molecular signals are provided. During late telogen to early anagen transition, signals from the dermal papilla (DP) stimulate the hair germ and quiescent bulge stem cells to become activated 10. In anagen, stem cells in the bulge give rise to hair germs, then the transient amplifying cells in the matrix of the new follicle proliferate rapidly to form a new hair filament 11. After catagen, follicles undergo apoptosis. The hair filament remains in the telogen follicle to become a club hair, which later is detached during exogen 12. These regenerative cycles continue repetitively throughout the lifetime of an organism 12, 13.
Several molecules have been implicated the regulation of phase transition during hair cycling. Many of these molecules were explored using a gene deletion strategy. For example, the skin of FGF18 conditional knockout mice (K5creFGF18flox) utilizing the Keratin 5 (KRT5) promoter precociously enters anagen via a shortened telogen 14. Knockout of Tcl1, which is highly expressed in the secondary hair germ and bulge cells during the catagen-telogen transition, results in a loss of the bulge stem cell surface marker CD34 and disturbs HF homeostasis 15. The role of other molecules in hair cycling were demonstrated by exogenous gene delivery. For example, adenovirus mediated Shh delivery induced anagen re-entry 16. These approaches were used to show that the bulge and hair germ are kept in quiescence by BMPs, NFAT, and FGF18 signaling. Wnts, FGF7, SHH and neurotrophins exert activation signaling and stimulate the hair germ for anagen re-entry 17. FGFs, SHH, TGF-βs, Wnts, IGFs, EGFs and HGFs favor anagen growth 18, while their down-regulation signals the end of anagen 12. Knowledge of these molecular targets will help us to identify potential therapeutic molecular pathways to explore.
The development and regeneration of HFs results from the delicate molecular balance as well as from reciprocal and sequential interactions between the follicular epithelium and mesenchymal DP 5. The DP, located at the base of the HF, is a group of specialized dermal fibroblast cells that can induce new hair formation 19. Earlier work showed that hair stem cells are slow cycling, enriched in the hair bulge region, and can give rise to HFs when isolated from adult follicles 20, 21. They are kept in a quiescent state in telogen, but get activated by the DP and others to enter hair germ and hair matrix states in anagen 10. Wnt, BMP, and Shh signaling are critical for DP function 17, 22, 23. These molecular pathways play similar roles in regulating the reciprocal epidermal and mesenchymal interactions. Furthermore, scientists have recently found that molecules such as Sox-2 in the DP may modulate hair subtypes 24.
Molecules regulating interactions between epidermal hair stem cells and DP cells have recently been reviewed. Due to space limitations, we refer interested readers to these reviews which focus on how to regulate hair cycling within a single HF 11, 17. Here, we will focus on a potential strategy to alter HF cycling states based on extra-follicular modulation.
3. Physiological regeneration: Modulating the regeneration of existing hair follicles by the extra-hair follicle environment and systemic hormone factors
3.1. Dermal macro-environment including intra-dermal adipose tissue can modulate hair regeneration
HF stem cells must be released from a quiescent state to an activated state to initiate a new anagen phase of the hair cycle. The duration of hair cycle phases can be modulated by different physiological conditions in the same individual 25. Although the intrinsic molecular rhythm within the HF organ micro-environment that regulates HF cycling is poorly understood, there are clues that macro-environmental factors from adjacent dermis/adipose tissue or systemic hormones can regulate the HF cycles (Fig. 3, 26, 27).
The local macro-environment can also regulate HF stem cell activity 30. We found that BMPs from the local dermal and adipose tissue play an important role in regulating physiological murine hair cycles 26. BMPs serve as inhibitors that prevent entry into anagen. Such inhibitory factors from the local macro-environment have not been investigated under physiological and pathological conditions. Some adipocyte derived PDGF can stimulate hair growth27. More intra-dermal adipocyte layer factors are being characterized for their ability to enhance or suppress hair regeneration26, 27. 96.
Circulating hormones associated with pregnancy were found to act as systemic factors that can modulate murine hair cycling. During pregnancy, hairs are held in telogen, and entry into anagen is not reinitiated until after lactation 26. Murine DPs express the estrogen receptor during telogen and exogenous estrogen can keep HFs from entering a new anagen 28. However, which systemic pregnancy associated hormone arrests HFs in telogen remains to be clarified. Interestingly, in contrast to the mouse, human hairs are induced to enter a prolonged anagen phase upon hormonal stimulation associated with pregnancy 29. About 3 to 6 months after labor, hair loss in the form of telogen effluvium can often be seen.
One practical note here. Scientists have used mouse skin to screen for small molecules or drug candidates for effects on hair growth, tumor formation, skin homeostasis, etc. Experimental data show the mouse skin goes through different phases of hair cycling. In the first month, they are synchronized. As intradermal adipose tissue develops, the hair cycles become asynchronized in different parts of the mouse skin 26, 96, 97. To obtain more consistent experimental results, one should take this into consideration. Otherwise, it is possible to produce a synchronized region by large scale waxing of the skin.
3.2 Effects of sex hormones and other systemic factors on hair regeneration diseases such as androgenic alopecia
In addition to physiological changes in hair cycle and regeneration, systemic factors also lead to pathological changes in susceptible patients. Testosterone can induce the growth of axillary, facial and pubic hair. Hirsutism is a hair disorder of women presenting with unwanted excessive male-pattern terminal hair growth 31, 32. In androgen-dependent regions such as the face, chest or lower abdomen, vellus hairs are turned into coarse terminal pigmented hairs in which anagen phase is lengthened and pigmentation is induced due either to stimulation by excessive circulating androgen levels 33, enhanced local androgen production or possibly increased local androgen sensitivity.
In striking contrast to this type of androgen-dependent hair growth promotion, in AGA androgen stimulation can lead to a patterned distribution of miniaturized hairs which have prolonged telogen, and shortened anagen 34, 35. DPs from balding scalp have both higher levels of androgen receptor (AR) 36 and type II 5-alpha reductase which converts testosterone to dihydrotestosterone (DHT). DHT can induce DPs to secrete factors including TGF- beta1 and DKK-1 that inhibit keratinocyte growth 37, 38. Hence, the locally high levels of DHT and ARs in DPs from balding scalp may explain the patterned distribution of alopecia. It also explains the clinical response of AGA to Finasteride, a potent type II 5-alpha reductase inhibitor that reduces the conversion of testosterone to DHT.
DP volume shrinks and DP cells adopt a senescent phenotype in balding follicles 39, 40. This may be because high levels of DHT can be cytotoxic and induce DP cell apoptosis 41. DPs from balding scalp also can secrete inhibitory autocrine factors 42. These results may help explain the reduced size of DPs in AGA, but how DP cells become senescent and the effect of senescent DP on HF biology still needs to be resolved.
The close association of AGA with androgen, especially DHT, can also be illustrated by the absence of male pattern baldness in individuals lacking androgens (such as eunuchs), functional AR, or 5 alpha reductase 43-45. Balding scalp regions also tend to have altered blood flow and a lower rate of oxygen delivery than that found in non-balding scalp 46.
The causes of AGA are assumed to be polygenic. Genome-wide association studies demonstrated polyglycine repeats in exon 1 of the AR increases the propensity towards AGA 47. The finding that modifications of the AR are involved in AGA is not surprising. AR is x-linked and suggests that it is inherited through a matrilineal lineage. An SNP in a region on Chromosome 20 (20p11) is associated with AGA in a study of German males 48. There is no apparent link with this locus and either the AR or hormones and the nature of its function in AGA remains to be identified.
The logical therapeutic approach for AGA treatment addressing the underlying pathology should be complete reversal of follicle miniaturization and de-pigmentation either by suppression of testosterone to DHT conversion, or by blockage of ARs. However, drug treatment involving increasing blood flow (Minoxidil) or decreasing androgen formation (Finasteride) did not effectively serve these purposes. Recently, it was found that the scalp of male AGA patients retain normal number of HF stem cells but the progression from stem cells to progenitors cells is severely blocked 1. This stem cell inactivation coincides with the known phenomenon of progressive follicle miniaturization during hair cycling.
4. Regeneration of hair fibers from existing follicles after hair plucking
Plucking of a hair fiber is an injury, albeit minor. As long as stem cells and the DP remain, the existing HFs can respond to regenerate a new hair filament. Though the molecular mechanism underlying plucking-regeneration is not fully understood, research in cell dynamics demonstrated that cell death takes place in the dermal and epithelial remnant, followed by cell proliferation which leads to synchronized anagen in the plucked area 49-51. This observation stresses the complimentary and coordinated roles of degeneration and regeneration in tissue and organ repair and regeneration. In contrast to physiological cycling, the cell dynamics of HF stem cells during this process is less clear. The label-retaining stem cells in the follicle bulge are believed to remain intact after plucking to serve its role in future regeneration 52-54. However, evidence also suggests that HF stem cells are susceptible to apoptosis after plucking, and some remaining label-retaining hair germ cells will migrate to the damaged stem cell region and reconstruct the bulge 55. Very recently, label retention and lineage tracing experiments demonstrated that in telogen a “new bulge” forms and coexists with the “old bulge” to which the club hair anchors 11. The new bulge contains a certain number of label retaining cells. As a terminally differentiated companion layer marker, KRT6+ cells in the well formed telogen bulge is ultrastructurally similar to CD34+ bulge stem cells. Lineage tracing with Lgr5-CreER/Rosa-LacZ mice, showed KRT6+ cells are derived from actively cycling cells in the lower outer root sheath 11. However, for normal hair homeostasis, it is the outer bulge CD34+ slow-cycling stem cells that contribute to wound healing and hair regeneration and not the KRT6+ cells located in the inner bulge. The CD34+ outer bulge cells are the initial source for new HF down growth during regeneration. Proliferative signals are only detected at the outer bulge and hair germ during wound healing, indicating the wound healing response originates from CD34+ cells. Under more extreme conditions when the CD34+ stem cell reservoir is depleted, KRT6+ cells do not respond to this CD34+ bulge cell ablation. This suggests CD34+ bulge cells are functional differently than KRT6+ cells even though they are located in the same niche. K6+ cells keep the bulge more quiescent, loss of which will trigger a precocious anagen.11, 55. The origin and homeostasis of the bulge stem cells after hair plucking should be further clarified.
5. Regeneration of chimeric hair follicles following tissue recombination
Given the inductive capacity of DP cells and reciprocal epithelial–mesenchymal interactions for hair regeneration, much effort has been invested on experimental manipulation of dermal and epidermal components to study follicle development and growth (Fig. 1, see detailed review 19, 56). Different in vivo tissue and cellular recombination assays with same-species (allograft) 57-60 or trans-species (xenograft) 61-63 models have been employed to probe hair follicle regeneration. In these bioassays, the inductive component of intact freshly dissected or cultured DP or dermal sheath (DS) cells from rodents or humans were recombined with its receptive epidermal counterpart. These dermal and epidermal components come from the same or different body sites such as vibrissa, ear, scalp and forearm and are also derived from tissues at different ages (ie., neonatal vs adult).
Results from these different approaches vary in producing a new DP or regenerating a hair follicle and its components. In same–species studies, for instance, the cultured rodent vibrissa DP was implanted beneath the upper half of the transected host rodent vibrissa follicle, and active HF induction and hair fiber growth were observed 57. This pioneering study incited continuous exploration on tissue engineering of HF regeneration using inductive DP tissue. The same research group micro-dissected the lower dermal sheath (DS) or DP from the scalp of a human male donor and transplanted (allograft) them onto shallow skin wounds on the inner forearm of an immunologically incompatible and genetically unrelated female recipient 59. Strikingly, such trans-gender and trans-region implantation of a grafted DS generated a new DP! This induced overt larger, thicker and often pigmented hairs against small, thin and unpigmented arm hairs of the host, and the results displayed the immunological privilege of the donor HF 64, 65. However, the failure of the implanted DP to induce a new HF is probably due to its inability to anchor appropriately in the shallow and structurally loose wound environment, and to its weaker immuno-tolerance than that of a DS 62. To further evaluate the inductive capacity of isolated human DPs, a trans-species (xenograft) study using athymic nude mice as hosts was performed by the same research group 62. A nude mouse vibrissae DP was exchanged for a human DP. The isolated DPs from groin hair or occipital hair of female and male donors were exchanged for DPs in isolated nude mouse vibrissa follicles. The recombinant constructs were then grafted into nude mice, either in the kidney capsule or in a subcutaneous graft pocket (created by implanting a glass disk) of well vascularized granulation tissue in the lower dorsum. These implanted recombined associations resulted in induction of a new bulb and fiber-producing follicle through the interaction of a human DP with a directly and well-attached mouse upper whisker epithelium.
These allograft and xenograft recombination studies display several significant findings including (1) The maintenance of inductive capacity of human DP and DS even under trans-species (xenograft), trans-gender (allograft) and across- body site (autograft) scenarios, but the immuno-tolerance issue must be further investigated and resolved; (2) The demonstration of trans-species similarities in epithelial-mesenchymal signaling and recognition properties between humans and rodents will give researchers more tools for in-depth understanding of the mechanism of human follicle regeneration; (3) The availability of diverse combinations of epidermal-dermal components and reconstitution methods gives flexibility for future clinical treatment approaches. The formed chimeric follicles may not show all the characteristics of HFs. These findings support prospective studies and inspire future biological therapies for hair disorders.
The basic criteria of HFs is that they should show basic follicular architecture, have stem cells, DP, transient amplifying cells as well as differentiating hair shafts and sebaceous glands. They also must show an ability to do repetitive regenerative cycling, and respond to plucking to regenerate new hair filaments (Table 1).
Table 1.
Defining engineered hair follicles.
|
Adopted from Chuong CM, Cotsarelis, G, and Stenn, K. Defining hair follicles in the age of stem cell bioengineering J. Invest. Dermatol. 2007.
6. Wound induced hair follicle neogenesis in the adult
Another major advance in wound healing is the finding of de novo hair formation in adult rabbit and mouse dorsal skin when the initial wound bed is greater than 1cm in diameter 66. This represents a physiological reprogramming of endogenous cells, since no external cells, or molecules were introduced. While the origin of these cells has not been fully established, it is remarkable that hair growth can occur in this way. These findings imply that we should be able to facilitate this process. It is also interesting to note that new hairs emerge only from the center of these large wounds, not from the zone adjacent to the wound margin (about 300-400 um distance). This led us to suggest that repair and regeneration are in competition and the wound margin may secrete some molecules that suppress regeneration, enabling repair. Whereas cells at the wound center, far away from the wound margin, are permitted to be reprogrammed successfully to regenerate new hair (Fig. 1, 67). This further suggests that it should be possible for a reprogramming strategy to work in organized tissues in vivo.
Interestingly, recently the African spiny mouse (Acomys) was found to shed its skin in response to predation as a means of escape. These mice can rapidly re-epithelialize the wounds and regenerate hair follicles, sebaceous glands and dermis. The authors found the extracellular matrix was less organized and tension may play a role in wound healing 95. Learning how wild animals do regenerative skin wound healing can inspire us to apply their mechanism to regenerative medicine in the context of biomimetics.
One important fact to notice is that this process is novel and there are many factors scientists are still studying. For example, the prostaglandin pathway is involved in the efficiency of this process 96. Practically, authors found different strains have different efficacy in producing new follicles, with a mixed strain being of higher efficiency 96 . For example, C57Bl6 mice have a moderateresponse. For now, scientists who study this should use the same inbred strain. This sometimes poses a problem when transgenic mice of a particular strain are used for analyses.
6.1 Source of epidermal cells
Normally, the wound healing process after injury fails to regenerate lost appendages such as HFs and sebaceous glands. In the past, certain de novo hair regeneration after wounding was observed in rabbits, mice and humans 9, 68-70. These observations were not seriously recognized due to a lack of conclusive evidence. These early studies were limited by relatively primitive research techniques and tools, and the well-accepted conventional dogma of the impossibility of hair re-growth for adult mammals. In this recent re-discovery it was found that new unpigmented hair follicles formed at the center of re-epithelialized wounds on the mouse's back. The new follicles behave like normal ones as they exhibit epithelial and mesenchymal cell differentiation and proliferation, sebaceous glands, and hair shaft formation, as well as successive hair cycling indicative of the presence and function of stem cells 66 . The investigators noted the de novo folliculogenesis resembled embryonic follicle development at morphogenic and molecular levels, with formation of epidermis and dermis, and subsequent down growths of epidermis into the underlying dermis. It was believed that the substantial wound size and the relatively long healing time on the adult mouse dorsum activated the Wnt-mediated signaling pathways 66 that control embryonic follicle development and hair cycling 71. The study indicated suppression of such pathways by the Wnt antagonist DKK1 blocked folliculogenesis after wound closure and regenerated hair numbers increased considerably for mice with enhanced Wnt activity in its epidermis 66.
Wound healing is a complex process and the active participation of epidermis and HF dermis with intact HFs is important. This is illustrated by the observation of faster wound healing in hair-bearing regions of humans 72, 73 and the delayed acute wound healing in the hair-less tail epidermis of mutant mice with an impaired Eda receptor 74.
In response to skin damage, keratinocytes from the upper isthmus (the middle HF segment) or from the infundibulum (the upper HF segment) of remnant follicles, contain high proliferative capability that can regenerate and permanently replenish the epidermis 75. On the other hand, the bulge stem cells respond promptly and efficiently by migrating upwards from the lower isthmus to interfollicular epidermis of the wound site at the surface, and by recruiting and mobilizing its progeny into the injured site for acute repair 76-78. However, the recent discovery of de novo hair regeneration in healing wounds of mice implied that inter-follicular epidermal cells, with a HF stem cell phenotype in the wound and not the existing HF bulge stem cells in the neighboring skin, play a significant role in folliculogenesis after wound repair 76-78. But the question of whether the origin of the new hair follicle is due to epidermal stem cells or infundibular cells requires further clarification by efficient markers.
6.2. Source of dermal cells
During wound healing and de novo follicle neogenesis, the replenished epidermis will proceed to interact with its underlying dermal component. However, the origin of de novo regenerated follicular dermal cells is unknown. They could be generated from mesenchymal stem cells. This issue awaits related investigation by lineage tracing analysis and other tools to identify and delineate the nature of multipotent adult skin-derived precursor cells from the mesenchymal niche.
Despite limited knowledge and premature understanding on precise cellular and molecular mechanisms or signaling pathways, such intriguing findings like the de novo hair regeneration of adult mice during skin wound healing, would prompt development of optional hair loss treatment strategies. These strategies would take advantage of the principle of a natural re-epithelialization process, and if the true underlying mechanism and signaling molecules can be clearly identified, then these can be re-established and modulated effectively and safely in the future clinical setting.
Recently, skin derived progenitor cells (SKPs) have been expanded in vitro from the dermis and share certain characteristics of DP cells 21, 79, 80. SKPs are highly plastic even after long-term expansion in vitro and can differentiate into multiple lineages from different germ layers including neuron, adipocytes, sebaceous glands, etc. It was later demonstrated that SKPs can be more effectively cultivated from DP cells. Surprisingly, SKPs are also able to induce HF formation in a way similar to cultured low passage DP cells. It is not clear if the hair inducing ability is an epigenetic memory of the follicular cell origin, or if the SKPs derived from hairless dermis are also endowed with this ability. Since SKPs can be serially expanded in vitro, they can be an ample source of inductive dermal cells for HF regeneration.
In addition to these specified cells, we speculate that local dermal fibroblasts can also be induced to generate inductive dermal cells. The most direct way can be specific reprogramming of dermal fibroblasts into DP cells, an approach similar to the generation of induced pluripotent stem (iPS) cells. This approach has been used successfully to reprogram fibroblasts into neurons and cardiomyocytes 81, 82. In this process, a promoter specific to DP cells is needed to drive a reporter transgene for high throughput screening of cocktails of transcriptional factors. Though versican and corin have been reported to be highly expressed in DP cells relative to dermal fibroblasts 83, 84, they are not specific to the DP. Until a specific promoter for DP cells is available, using the versican and corin promoters to drive 2 different reporter transgenes (such as GFP and YFP) is a good alternative choice for screening purposes.
In addition to direct reprogramming via transcriptional factors, indirect reprogramming by environmental cues or paracrine factors is another approach. The observation of neogenesis of HFs after wounding 66 and de novo generation of DP cells 85 during hair cycles, suggests that local fibroblasts can be converted to a DP fate. However, the paracrine factors required to reprogram fibroblasts into DP cells need to be defined and characterized.
7. Tissue engineering based follicle neogenesis: Reconstitution of dissociated epidermal stem cells and inducing dermal cells to form hair follicles
7.1 A simple planar hair forming procedure
Our overall goal is to apply stem cell engineering technology to form a reconstituted skin replacement that functions close to normal for these patients. This reconstituted skin must contain the appropriate components in the right ratio with the correct architecture. Here we focus on using tissue engineering to produce reconstituted skin that can grow hair. Hair grows on the surface of the skin making it easy to measure HF density, as well as the thickness and length of the hair shaft.
One of the major objectives of tissue engineering is to reconstitute skin from stem cells. This requires multi-potent skin stem cells and the ability to guide these cells to form a piece of skin with proper architecture and skin appendages. Scientists have made some progress in animal models that lead to hair formation. Notable progress along these lines comes from Lichti's grafting chamber assay and Zheng's patch assay 60, 86, 87. However, the former is cumbersome and not practical for clinical use. The latter forms nice single hairs, but the hair pattern is disrupted and HFs form an entangled hair cyst and do not form the desired outcome. Yet, these methods provide a baseline for improvement.
In order to achieve a clinically relevant means of regenerating functional skin for wound and trauma patients, we recently developed a much improved hair reconstitution procedure. The hair precursor cells are placed upon a supportive matrix scaffold prior to their application to the wound site (Fig. 5, 88). This new method is simple to set up and produces reconstituted hairs arranged along a single plane with a common orientation. In this planar hair forming procedure, newborn mouse cells are used. Dissociated epidermal and dermal cells in high density suspension are allowed to reconstitute in vitro to generate their own matrix, or seeded into a scaffold-like matrix already used clinically. These cells self-organize and form a reconstituted skin with proper proportions and topological organization of different components. Large numbers of HFs form. The cellular and molecular events are characterized, showing a distinct but parallel morphogenetic process compared to those occurring in embryonic development. The formed HFs can cycle and regenerate, and the reconstituted skin can heal after injury. The reconstituted skin tissue remains in good condition one year after transplant to the mouse. This procedure also enables flexibility in producing an appropriately sized and shaped reconstituted skin.
This is a promising procedure for the high throughput screening of therapeutic agents. This procedure also produces topologically correct hairs with a clinically acceptable appearance. Clinical applications can be envisioned for the future when large numbers of multi-potential skin stem cells become available.
7.2. Integration of biomaterials
In terms of clinical application, thousands of new follicles are expected to be regenerated for a single patient to achieve a good cosmetic appearance. Hence, the procured DP cells need to be expanded. DP cells grow very slowly in vitro and their HF inducing ability is quickly lost. There has been progress in tackling this drawback by refining culture conditions 22, 89-91. In addition to growth factors for cell expansion, the intercellular organization of DP cells also affects the HF inducing ability. HF inducing ability is better preserved when DP cells are transplanted as cell aggregates 90.
To tackle the issue of efficiency and HF inductivity, and to facilitate clinical transplantation, we proposed three key steps to engineering HFs; first, in vitro expansion of DP cells, second, generation of injectable DP cell aggregates by self-assembly in a bioreactor and third, transplantation of DP cell aggregates 92, 93. We found that relatively low adhesivity of the biomaterials surface, such as poly (ethylene-co-vinyl alcohol), is able to maintain DP cells in high motility and can promote the spontaneous assembly of dissociated DP cells into thousands of aggregated inductive DP cell aggregates within 3 days after one single seeding 92. Fibronectin can further enhance the DP self-aggregation process by enhancing the cell substrate adhesiveness while maintaining high cell motility 93. Such biomaterials surfaces can be further developed into a bioreactor that is able to generate inductive DP cell aggregates for clinical transplantation with very high efficiency.
In addition to homotypic self-aggregation of DP cells, an appropriate biomaterial surface is able to guide the self-assembly of heterotypic dissociated adult cultured DP cells and epidermal keratinocytes. These aggregates form thousands of cell aggregates with a hair germ-like layered structure; a core of DP cells surrounded by a shell of keratinocytes 94. In addition to a structural similarity to the hair bulb, adult epidermal keratinocytes also start to differentiate toward a follicular fate in such cell aggregates. Compared to dissociated adult keratinocytes and DP cells that are unable to grow into HF in vivo, these hair germ-like cell aggregates are able to grow into new HFs after transplantation. The result indicates that a structural cue between heterotypic cells conferred by interactions on biomaterials can enhance the epithelial-mesenchymal interaction, thereby promoting the trichogenesis from adult keratinocytes and DP cells.
8. Expert Opinion
In the study of hair formation (Fig. 1), we have to realize hair follicles are two component organs, made of epithelium and mesenchyme. Also, they undergo regenerative cycling under physiological conditions 25. So it is best to take advantage of this knowledge and modulate hair growth.
To modulate the progression of hair cycling, one will want to change the duration of anagen for longer hairs or shorter hairs, or to change the duration of telogen for frequency of new hair growth. Since the original HF stem cells and signaling center (DP) are still there, this should be relatively easy to achieve (Fig. 1, 2). Recent work demonstrated that in AGA, human hair stem cells are present but cannot get activated to form the hair germ 1, 95. Our work in the mouse demonstrates that altering the dermal environment is sufficient to modulate hair growth (Fig. 3, 26, 27, 97). So it is optimistic that investigators may come up with more small molecules that can modulate hair growth and help to produce more or less hairs as one may desire. In addition to perturbing signaling molecules such as BMP, Wnt, FGF activity, recent work on human alopecia shows that the prostaglandin pathway is also involved in modulating hair growth 96, adding another layer of possibilities.
For the de novo formation of HFs after severe injury, it is more challenging. Unlike some organs where stem cells can progress with one cellular component, here we have to provide both epidermal and dermal progenitors. In the laboratory mouse, newborn skin happens to be of the right competent stage (Fig. 5). The exciting finding that the African spiny mouse can produce de novo hair regeneration under natural conditions suggests the epigenetic status can be arranged so even adult cells can become competent to regenerate hair follicles. This might make it possible to apply these findings to human hair follicles. For humans, one would need to induce embryonic stem cells,use iPS cells or reprogram adult cells to become competent. One will have to make a population of hair inducing dermal cells as well as this population of hair forming competent epidermal cells.
The choice of reprogramming factors will be a challenge. While the proof of principle was demonstrated in the reprogramming of skin fibroblasts into neurons 81 or hepatocytes 82, the search for the proper factors in skin progenitors remains to be found (Fig. 4). It is not sufficient to just make them into hair lineage cells. For example, if we produce a population of differentiated inner root sheath cells, it will not be useful. We need to produce cells at the stage that they can undergo self-organization and generate many new hair germs.
The remarkable example is the endogenous reprogramming observed after large wounding (Fig. 1; 66, 67). In this case, new hair germs are able to be formed de novo from the center of the large wound. We discussed this possibility in the above sections. The important message is that this new HF formation event can occur without the addition of exogenous molecular factors or cells. If we can understand this process more, we may find clues we can use for application purposes.
Thus, there has been good progress in our understanding of the molecular and cellular basis of new therapies for alopecia, hair regeneration and a better regenerative wound healing of the skin. While there is still much work to do, the logic and path are clearer than ever.
9. Conclusion
Regenerative medicine is at the forefront of 21st century medicine. In the skin field, scientists strive to develop new methods and to identify genes that can enhance the regenerative ability to replace skin damage due to injury or aging. We hope to make “regenerative wound healing” a reality for patients who suffer from burns and other trauma. We strive toward developing a reconstituted functional skin for high throughput analyses and toward a readiness for clinical applications; however, this is a major undertaking. For now we wish to focus on a major road block in current therapies for wound healing; the inability to form HFs . The goal is to produce skin with appendages that can help patients who suffer from severe burns, wounds and other forms of alopecia.
Acknowledgments
This work is supported by NIH NIAMS grant AR 60306 (CMC, TXJ); 43177 (CMC), 47364 (CMC, RW). Additional support is from the Industrial Technology Research Institute, Hsinchu, Taiwan (SCC, CCC, TXJ, LMW); and a collaborative grant between Taiwan University/Yang Ming University (SJL and CCC). SJL is supported by a physician scientist fellowship from NHRI, Taiwan when he was at USC. MXL is supported by a fellowship from the China Scholarship Council.
Footnotes
Disclosure: The following two provisional patents were filed via University of Southern California. Compositions and Methods to Modulate Hair Growth, US Patent application No 61/116,619, filed Nov 20, 2008, Markus Plikus and Cheng Ming Chuong. Compositions and Methods to Generate Pilosebaceous Units, US Patent application No 61/116,620, filed Nov 20, 2008, Lily Lee, Cheng Ming Chuong, Warren Garner. Ting Xin Jiang
References
- 1.Garza LA, Yang CC, Zhao T, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–22. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallico GG, 3rd, O'Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–51. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med. 2005;353:2578–88. doi: 10.1056/NEJMcp033496. [DOI] [PubMed] [Google Scholar]
- 4.Chamcheu JC, Siddiqui IA, Syed DN, et al. Keratin gene mutations in disorders of human skin and its appendages. Arch Biochem Biophys. 2011;508:123–37. doi: 10.1016/j.abb.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–61. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–42. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuong CM. Molecular biology intelligence unit 1. Austin, Tex: R.G. Landes; 1998. Molecular basis of epithelial appendage morphognesis. [Google Scholar]
- 9.Kligman AM, Strauss JS. The formation of vellus hair follicles from human adult epidermis. J Invest Dermatol. 1956;27:19–23. doi: 10.1038/jid.1956.71. [DOI] [PubMed] [Google Scholar]
- 10.Greco V, Chen T, Rendl M, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–69. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs E, Merrill BJ, Jamora C, et al. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Kimura-Ueki M, Oda Y, Oki J, et al. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J Invest Dermatol. 2012;132:1338–45. doi: 10.1038/jid.2011.490. [DOI] [PubMed] [Google Scholar]
- 15.Ragone G, Bresin A, Piermarini F, et al. The Tcl1 oncogene defines secondary hair germ cells differentiation at catagen-telogen transition and affects stem-cell marker CD34 expression. Oncogene. 2009;28:1329–38. doi: 10.1038/onc.2008.489. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest. 1999;104:855–64. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo WM, Oro AE. SnapShot: hair follicle stem cells. Cell. 2011;146:334–34 e2. doi: 10.1016/j.cell.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J Exp Zool B Mol Dev Evol. 2003;298:164–80. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- 19.Ohyama M, Zheng Y, Paus R, et al. The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp Dermatol. 2010;19:89–99. doi: 10.1111/j.1600-0625.2009.00935.x. [DOI] [PubMed] [Google Scholar]
- 20.Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–7. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 21.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Enshell-Seijffers D, Lindon C, Kashiwagi M, et al. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–42. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driskell RR, Giangreco A, Jensen KB, et al. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–23. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuong CM, Randall VA, Widelitz RB, et al. Physiological regeneration of skin appendages and implications for regenerative medicine. Physiology (Bethesda) 2012;27:61–72. doi: 10.1152/physiol.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–4. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–71. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh HS, Smart RC. An estrogen receptor pathway regulates the telogen-anagen hair follicle transition and influences epidermal cell proliferation. Proc Natl Acad Sci U S A. 1996;93:12525–30. doi: 10.1073/pnas.93.22.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace ML, Smoller BR. Estrogen and progesterone receptors in androgenic alopecia versus alopecia areata. Am J Dermatopathol. 1998;20:160–3. doi: 10.1097/00000372-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Chen CC, Chuong CM. Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci. 2012;66:3–11. doi: 10.1016/j.jdermsci.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courtois M, Loussouarn G, Hourseau C, et al. Hair cycle and alopecia. Skin Pharmacol. 1994;7:84–9. doi: 10.1159/000211279. [DOI] [PubMed] [Google Scholar]
- 32.Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev. 2000;21:347–62. doi: 10.1210/edrv.21.4.0401. [DOI] [PubMed] [Google Scholar]
- 33.Reingold SB, Rosenfield RL. The relationship of mild hirsutism or acne in women to androgens. Arch Dermatol. 1987;123:209–12. [PubMed] [Google Scholar]
- 34.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–7. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann R, Happle R. Current understanding of androgenetic alopecia. Part I: etiopathogenesis. Eur J Dermatol. 2000;10:319–27. [PubMed] [Google Scholar]
- 36.Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 37.Inui S, Fukuzato Y, Nakajima T, et al. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–9. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 38.Kwack MH, Sung YK, Chung EJ, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128:262–9. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- 39.Randall VA, Hibberts NA, Hamada K. A comparison of the culture and growth of dermal papilla cells from hair follicles from non-balding and balding (androgenetic alopecia) scalp. Br J Dermatol. 1996;134:437–44. [PubMed] [Google Scholar]
- 40.Bahta AW, Farjo N, Farjo B, et al. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J Invest Dermatol. 2008;128:1088–94. doi: 10.1038/sj.jid.5701147. [DOI] [PubMed] [Google Scholar]
- 41.Winiarska A, Mandt N, Kamp H, et al. Effect of 5alpha-dihydrotestosterone and testosterone on apoptosis in human dermal papilla cells. Skin Pharmacol Physiol. 2006;19:311–21. doi: 10.1159/000095251. [DOI] [PubMed] [Google Scholar]
- 42.Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. Br J Dermatol. 2006;154:609–18. doi: 10.1111/j.1365-2133.2006.07144.x. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton JB. Male hormone stimulation is a prerequisite and an incitant in common baldness. Am J Anat. 1942;71:451–80. [Google Scholar]
- 44.Imperato-McGinley J, Guerrero L, Gautier T, et al. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186:1213–5. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- 45.Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4:1–11. doi: 10.1017/S1462399402005112. [DOI] [PubMed] [Google Scholar]
- 46.Klemp P, Peters K, Hansted B. Subcutaneous blood flow in early male pattern baldness. J Invest Dermatol. 1989;92:725–6. doi: 10.1111/1523-1747.ep12721603. [DOI] [PubMed] [Google Scholar]
- 47.Hillmer AM, Hanneken S, Ritzmann S, et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet. 2005;77:140–8. doi: 10.1086/431425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillmer AM, Brockschmidt FF, Hanneken S, et al. Susceptibility variants for male-pattern baldness on chromosome 20p11. Nat Genet. 2008;40:1279–81. doi: 10.1038/ng.228. [DOI] [PubMed] [Google Scholar]
- 49.Paus R, Handjiski B, Eichmuller S, et al. Chemotherapy-induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol. 1994;144:719–34. [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuo K, Mori O, Hashimoto T. Plucking during telogen induces apoptosis in the lower part of hair follicles. Arch Dermatol Res. 2003;295:33–7. doi: 10.1007/s00403-003-0384-9. [DOI] [PubMed] [Google Scholar]
- 51.Silver AF, Chase HB, Arsenault CT. Early anagen initiated by plucking compared with early spontaneous anagen. Adv Biol Skin. 1967;9:265–86. [Google Scholar]
- 52.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 53.Lavker RM, Sun TT. Hair follicle stem cells: present concepts. J Invest Dermatol. 1995;104:38S–39S. doi: 10.1038/jid.1995.56. [DOI] [PubMed] [Google Scholar]
- 54.Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–5. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 55.Ito M, Kizawa K, Toyoda M, et al. Label-retaining cells in the bulge region are directed to cell death after plucking, followed by healing from the surviving hair germ. J Invest Dermatol. 2002;119:1310–6. doi: 10.1046/j.1523-1747.2002.19644.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–2. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 58.Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa-type fibres are specified. Development. 1992;115:1103–9. doi: 10.1242/dev.115.4.1103. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, et al. Trans-gender induction of hair follicles. Nature. 1999;402:33–4. doi: 10.1038/46938. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Y, Du X, Wang W, et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–76. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- 61.Weinberg WC, Goodman LV, George C, et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol. 1993;100:229–36. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 62.Jahoda CA, Oliver RF, Reynolds AJ, et al. Trans-species hair growth induction by human hair follicle dermal papillae. Exp Dermatol. 2001;10:229–37. doi: 10.1034/j.1600-0625.2001.100402.x. [DOI] [PubMed] [Google Scholar]
- 63.Ehama R, Ishimatsu-Tsuji Y, Iriyama S, et al. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. 2007;127:2106–15. doi: 10.1038/sj.jid.5700823. [DOI] [PubMed] [Google Scholar]
- 64.Gibson WT, Westgate GE, Craggs RI. Immunology of the hair follicle. Ann N Y Acad Sci. 1991;642:291–300. doi: 10.1111/j.1749-6632.1991.tb24395.x. [DOI] [PubMed] [Google Scholar]
- 65.Streilein JW. Unraveling immune privilege. Science. 1995;270:1158–9. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 66.Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 67.Chuong CM. Regenerative biology: new hair from healing wounds. Nature. 2007;447:265–6. doi: 10.1038/447265a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lacassagne A, Latarjet R. Action of methylcholanthrene on certain scars of the skin in mice. Cancer Res. 1946;6:183–8. [PubMed] [Google Scholar]
- 69.Breedis C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 1954;14:575–9. [PubMed] [Google Scholar]
- 70.Billingham RE, Russell PS. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits' skin. Nature. 1956;177:791–2. doi: 10.1038/177791b0. [DOI] [PubMed] [Google Scholar]
- 71.Davies GC, Thornton MJ, Jenner TJ, et al. Novel and established potassium channel openers stimulate hair growth in vitro: implications for their modes of action in hair follicles. J Invest Dermatol. 2005;124:686–94. doi: 10.1111/j.0022-202X.2005.23643.x. [DOI] [PubMed] [Google Scholar]
- 72.Bishop GH. Regeneration after experimental removal of skin in man. Am J Anat. 1945:153–81. [Google Scholar]
- 73.Martinot V, Mitchell V, Fevrier P, et al. Comparative study of split thickness skin grafts taken from the scalp and thigh in children. Burns. 1994;20:146–50. doi: 10.1016/s0305-4179(06)80012-4. [DOI] [PubMed] [Google Scholar]
- 74.Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128:1311–8. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- 75.Ito M, Cotsarelis G. Is the hair follicle necessary for normal wound healing? J Invest Dermatol. 2008;128:1059–61. doi: 10.1038/jid.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–4. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 77.Levy V, Lindon C, Zheng Y, et al. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–66. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 78.Nowak JA, Polak L, Pasolli HA, et al. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandes KJ, McKenzie IA, Mill P, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–93. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 80.Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 81.Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–9. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 83.Kishimoto J, Ehama R, Wu L, et al. Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc Natl Acad Sci U S A. 1999;96:7336–41. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–25. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chi WY, Enshell-Seijffers D, Morgan BA. De novo production of dermal papilla cells during the anagen phase of the hair cycle. J Invest Dermatol. 2010;130:2664–6. doi: 10.1038/jid.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lichti U, Weinberg WC, Goodman L, et al. In vivo regulation of murine hair growth: insights from grafting defined cell populations onto nude mice. J Invest Dermatol. 1993;101:124S–29S. doi: 10.1111/1523-1747.ep12363165. [DOI] [PubMed] [Google Scholar]
- 87.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee LF, Jiang TX, Garner W, et al. A simplified procedure to reconstitute hair-producing skin. Tissue Eng Part C Methods. 2011;17:391–400. doi: 10.1089/ten.tec.2010.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inamatsu M, Matsuzaki T, Iwanari H, et al. Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from afollicular skin. J Invest Dermatol. 1998;111:767–75. doi: 10.1046/j.1523-1747.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 90.Osada A, Iwabuchi T, Kishimoto J, et al. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007;13:975–82. doi: 10.1089/ten.2006.0304. [DOI] [PubMed] [Google Scholar]
- 91.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–57. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Young TH, Lee CY, Chiu HC, et al. Self-assembly of dermal papilla cells into inductive spheroidal microtissues on poly(ethylene-co-vinyl alcohol) membranes for hair follicle regeneration. Biomaterials. 2008;29:3521–30. doi: 10.1016/j.biomaterials.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 93.Young TH, Tu HR, Chan CC, et al. The enhancement of dermal papilla cell aggregation by extracellular matrix proteins through effects on cell-substratum adhesivity and cell motility. Biomaterials. 2009;30:5031–40. doi: 10.1016/j.biomaterials.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 94.Yen CM, Chan CC, Lin SJ. High-throughput reconstitution of epithelial-mesenchymal interaction in folliculoid microtissues by biomaterial-facilitated self-assembly of dissociated heterotypic adult cells. Biomaterials. 2010;31:4341–52. doi: 10.1016/j.biomaterials.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 95.Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–5. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garza LA, Liu Y, Yang Z, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4:126ra34. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plikus MV, Baker RE, Chen CC, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–9. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plikus MV, Widelitz RB, Maxson R, et al. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol. 2009;53:857–68. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
Annotated References
- 1.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–4. doi: 10.1038/nature06457. This paper examines the role of the extra-follicular environment in regulating the hair cycle. It demonstrates that hairs can communicate with one another which results in a hair wave. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–4. doi: 10.1038/nm1328. This paper describes how hair follicle neogenesis can be induced in the wound bed of large wounds. [DOI] [PubMed] [Google Scholar]
- 3.Lee LF, Jiang TX, Garner W, et al. A simplified procedure to reconstitute hair-producing skin. Tissue Eng Part C Methods. 2011;17:391–400. doi: 10.1089/ten.tec.2010.0477. This paper describes a method for the tissue engineering of hair follicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–5. doi: 10.1038/nature11499. This paper describes how mechanical tension may play a role in skin and hair regeneration in the African spiny mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]