Abstract
Alteration of the surface of human neutrophils with the nonpenetrating, protein-inactivating agent p-diazobenzenesulfonic acid (DASA) was found to prevent activation of the respiratory burst by some stimuli, but not others. Production of superoxide anion (O2-) stimulated by concanavalin A or the chemotactic peptide formyl-methionyl-leucyl-phenylalanine FMLP was inhibited by DASA pretreatment, whereas O2- production stimulated by phorbol myristate acetate (PMA), sodium fluoride. or the ionophore A23187 was not inhibited by DASA. Pretreatment with DASA inhibited oxygen uptake stimulated by FMLP, but not oxygen uptake stimulated by PMA. DASA reproducibly inhibited activities of two known surface enzymes Mg++-ATPase and alkaline phosphatase, by 45-55% and 60-70%, respectively. The inhibition by DASA of O2- production did not appear to be caused by interference with binding of the affected stimuli, since pretreatment with DASA did not inhibit release of the lysosomal enzymes lysozyme and myeloperoxidase induced by concanavalin A or FMLP. Membrane-rich particulate fractions from neutrophils have been shown to contain NADPH-dependent oxidative activity that is presumably responsible for the phagocytosis-associated respiratory burst of intact cells. The PMA-activated enzyme was susceptible to inhibition of directly exposed to DASA in this particulate fraction. These findings suggest that more than one mechanism exists for activation of the respiratory burst oxidase in human neutrophils, and that the neutrophil possesses at least one oxidase that is not an ectoenzyme.
Full text
PDF

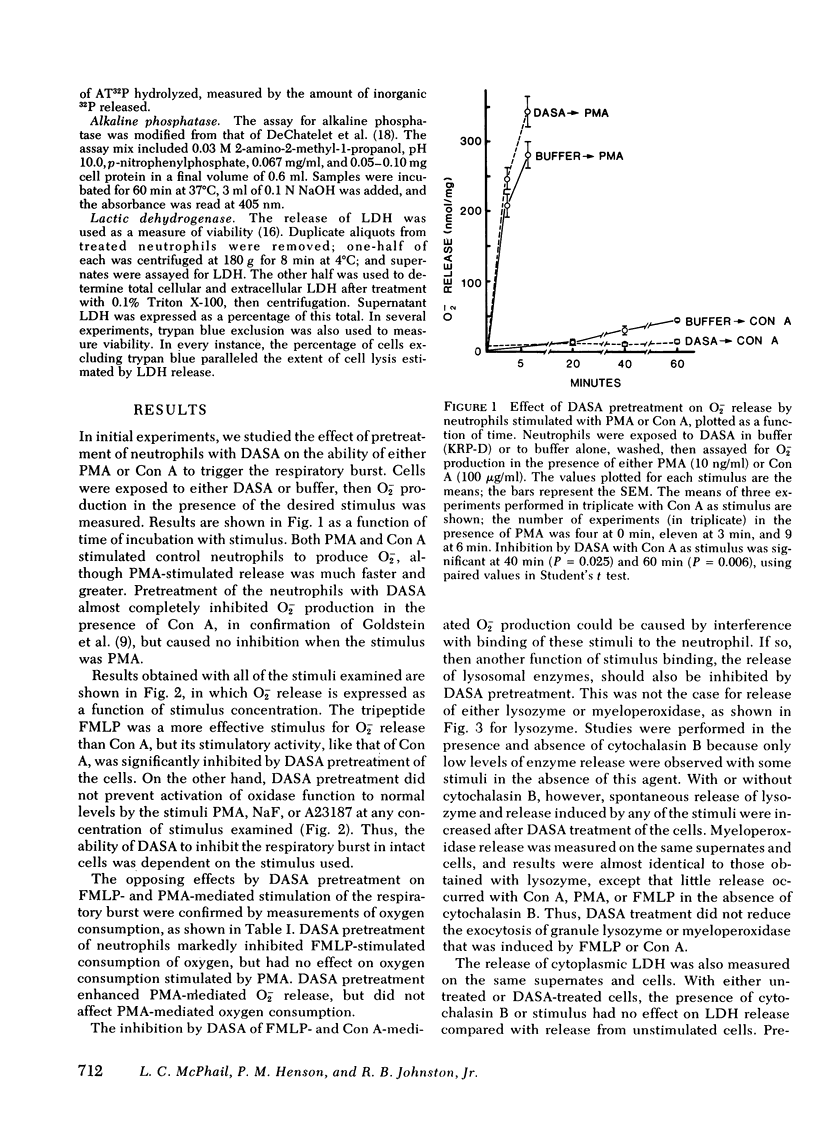

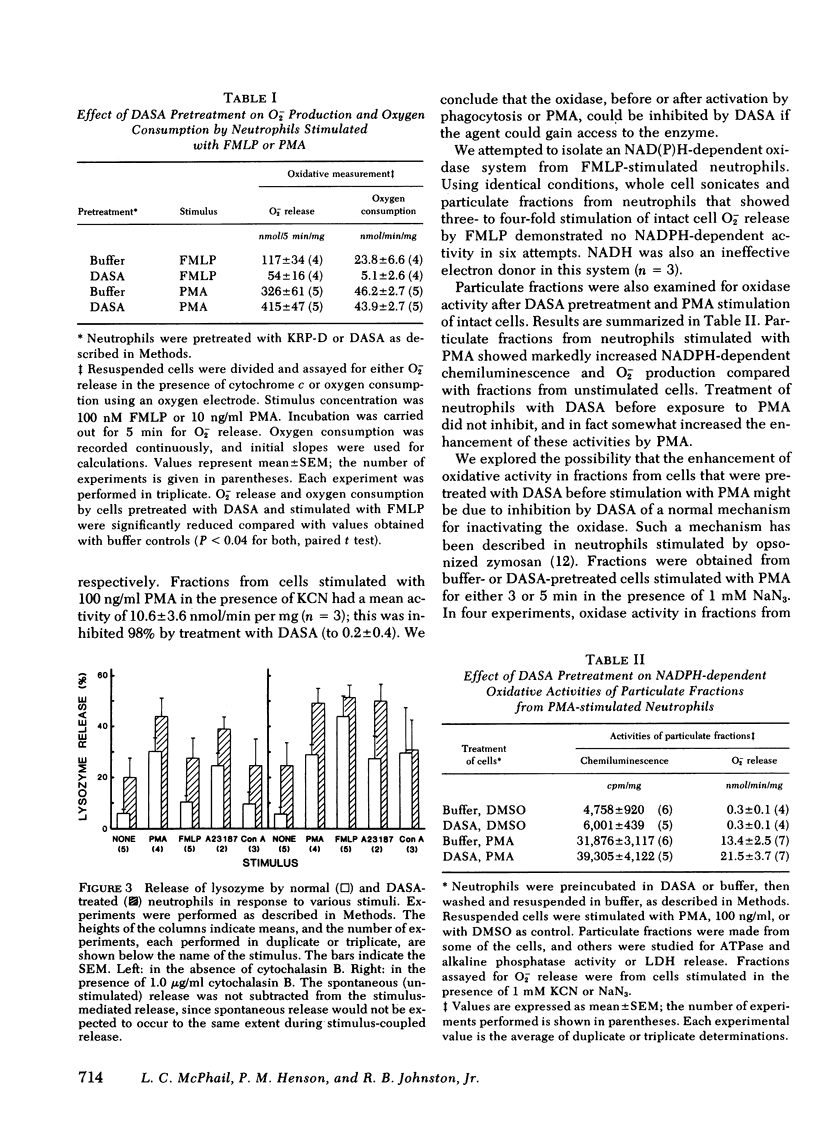


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Karnovsky M. L. The enzyme of granulocytes that produces superoxide and peroxide. An elusive Pimpernel. N Engl J Med. 1979 May 17;300(20):1157–1160. doi: 10.1056/NEJM197905173002009. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R. Initiation of the respiratory burst in human polymorphonuclear neutrophils: a critical review. J Reticuloendothel Soc. 1978 Jul;24(1):73–91. [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Mullikin D., McCall C. E. An isotopic assay for NADPH oxidase activity and some characteristics of the enzyme from human polymorphonuclear leukocytes. J Clin Invest. 1975 Apr;55(4):714–721. doi: 10.1172/JCI107981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Volk J. V., McCall C. E., Cooper M. R. Studies on leukocyte phosphatases. II. Inhibition of leukocyte alkaline phosphatase by amino acids and its reversal by zinc. Clin Chem. 1971 Mar;17(3):210–213. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J., DeChatelet L. R., Iverson D. B., McCall C. E. Magnesium-dependent adenosine triphosphatase as a marker enzyme for the plasma membrane of human polymorphonuclear leukocytes. Infect Immun. 1977 Feb;15(2):436–443. doi: 10.1128/iai.15.2.436-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Andersen B. R. Defective initiation of oxidative metabolism in polymorphonuclear leukocytes. N Engl J Med. 1979 May 17;300(20):1130–1135. doi: 10.1056/NEJM197905173002003. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson D. B., Wang-Iverson P., Spitznagel J. K., DeCHATELET L. R. Subcellular localization of NAD(P)H oxidase(s) in human neutrophilic polymorphonuclear leucocytes. Biochem J. 1978 Oct 15;176(1):175–178. doi: 10.1042/bj1760175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson D., DeChatelet L. R., Spitznagel J. K., Wang P. Comparison of NADH and NADPH oxidase activities in granules isolated from human polymorphonuclear leukocytes with a fluorometric assay. J Clin Invest. 1977 Feb;59(2):282–290. doi: 10.1172/JCI108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandl R. C., André-Schwartz J., Borges-DuBois L., Kipnes R. S., McMurrich B. J., Babior B. M. Termination of the respiratory burst in human neutrophils. J Clin Invest. 1978 May;61(5):1176–1185. doi: 10.1172/JCI109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma K., Boveris A., Chance B. H202 generation in subcellular fractions of leukocytes assayed by cytochrome c peroxidase method. FEBS Lett. 1977 Mar 1;74(2):295–299. doi: 10.1016/0014-5793(77)80868-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Johnston R. B., Jr Generation of chemiluminescence by a particulate fraction isolated from human neutrophils. Analysis of molecular events. J Clin Invest. 1979 Apr;63(4):648–655. doi: 10.1172/JCI109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Shirley P. S. Further characterization of NADPH oxidase activity of human polymorphonuclear leukocytes. J Clin Invest. 1976 Oct;58(4):774–780. doi: 10.1172/JCI108528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Dri P., Fant L., Basford R. E., Rossi F. NADPH oxidizing activity in rabbit polymorphonuclear leukocytes: localization in azurophilic granules. Biochem Biophys Res Commun. 1973 Aug 6;53(3):830–837. doi: 10.1016/0006-291x(73)90168-x. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Weening R. S., Roos D., Weemaes C. M., Homan-Müller J. W., van Schaik M. L. Defective initiation of the metabolic stimulation in phagocytizing granulocytes: a new congenital defect. J Lab Clin Med. 1976 Nov;88(5):757–768. [PubMed] [Google Scholar]


