Abstract
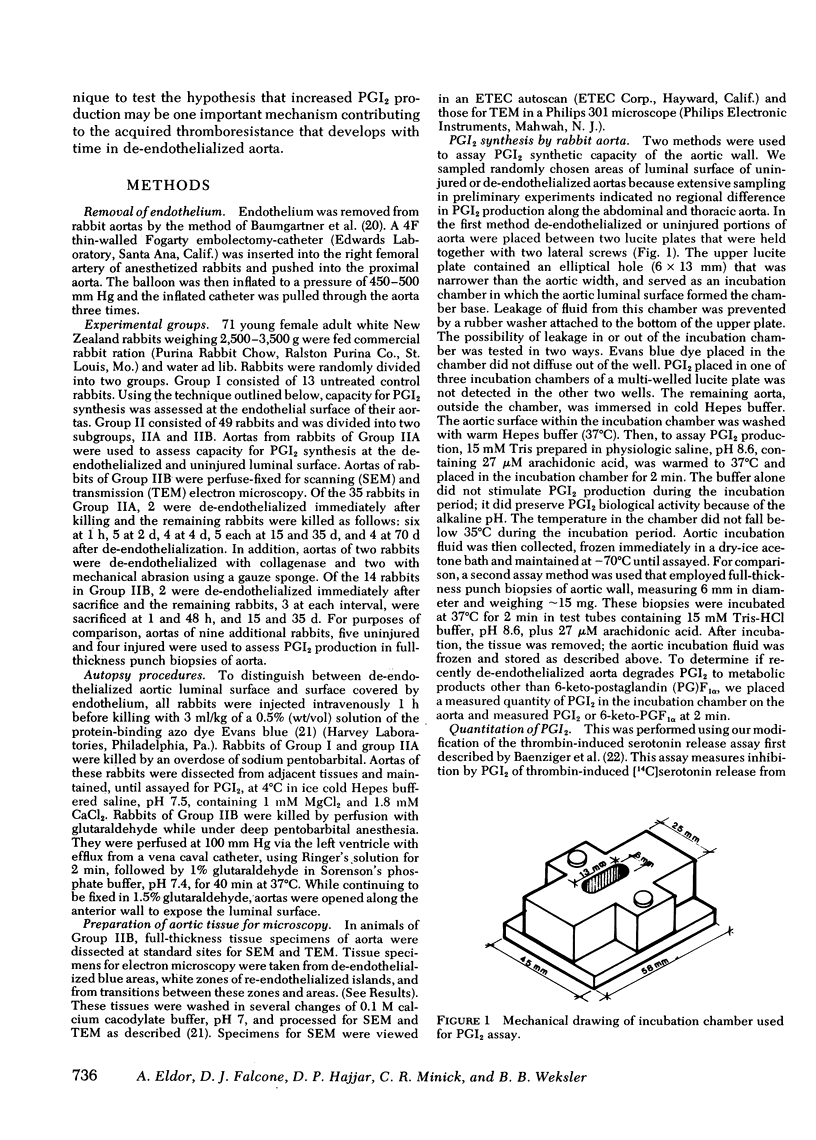
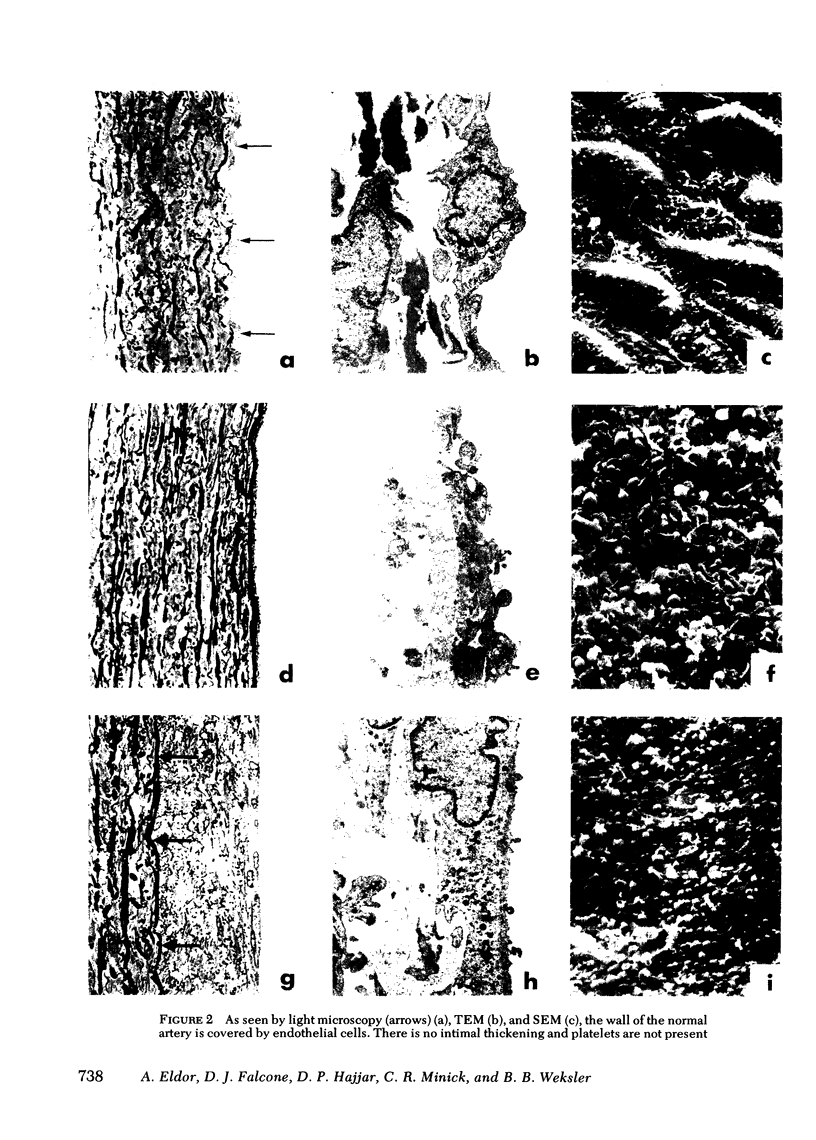
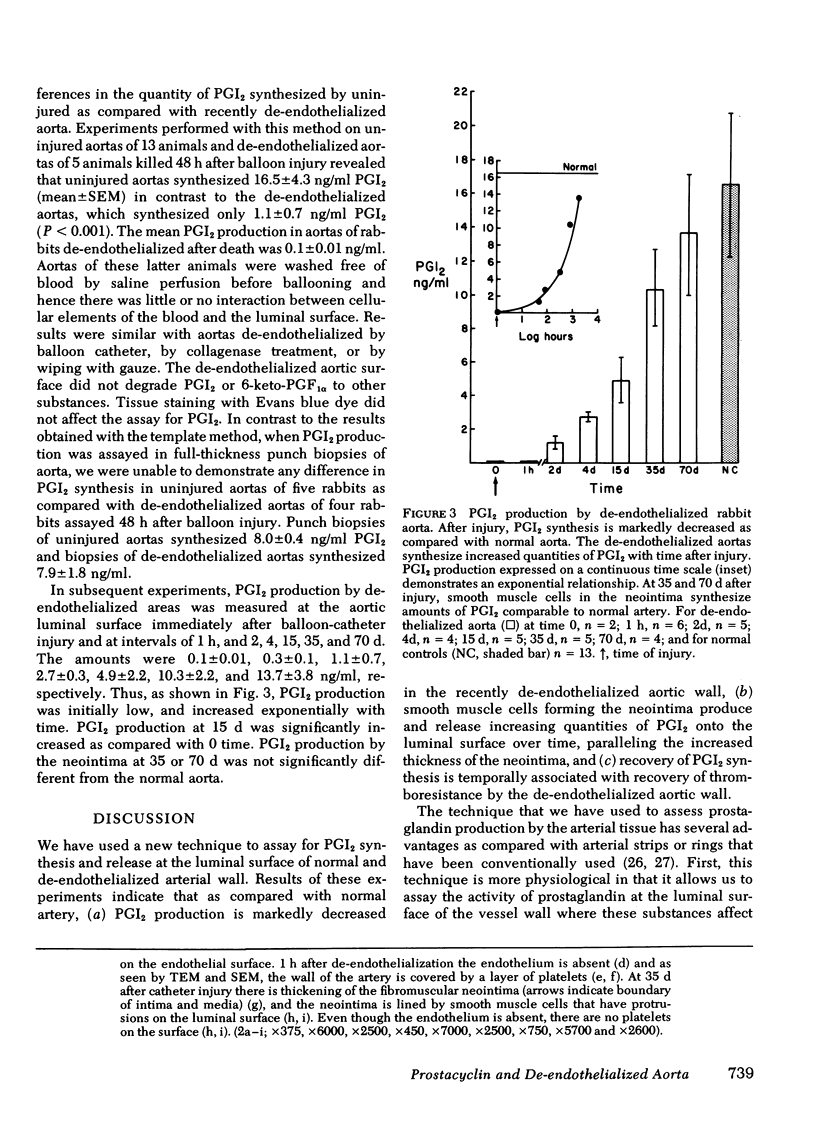
Prostacyclin (PGI2) synthetic capacity was assayed at the surface of aortas at various intervals after removal of endothelium with a balloon catheter. Results were correlated with morphologic changes in the vessel wall seen by light microscopy, scanning and transmission electron microscopy. To assay PGI2 synthetic capacity, we applied an incubation chamber to the luminal surface of the aortas; after arachidonic acid stimulation we assayed the PGI2 synthesized with a bioassay and radioimmunoassay. PGI2 synthesis in de-endothelialized aortas was determined immediately after balloon-catheter injury and at intervals of 1 h and 2, 4, 15, 35, and 70 d. PGI2 synthesis was low at 1 h and increased over time with levels at 35 and 70 d reaching that of normal artery. Scanning and transmission electron microscopy of de-endothelialized areas showed persistent absence of endothelium with formation of a neointima composed of smooth muscle cells. De-endothelialized aorta was covered with adherent platelets shortly after injury, however several days later only a few platelets adhered to the denuded surface. Results indicated that (a) endothelium is responsible for nearly all PGI2 production at the luminal surface of the normal aorta, (b) de-endothelialized muscular neointima synthesized increasing quantities of PGI2 with time after injury, and (c) increase of PGI2 production at the luminal surface of de-endothelialized aorta correlates with formation of a neointima and with the acquired thromboresistance of the aorta.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Dillender M. J., Majerus P. W. Cultured human skin fibroblasts and arterial cells produce a labile platelet-inhibitory prostaglandin. Biochem Biophys Res Commun. 1977 Sep 9;78(1):294–301. doi: 10.1016/0006-291x(77)91253-0. [DOI] [PubMed] [Google Scholar]
- Barnhart M. I., Baechler C. A. Endothelial cell physiology, perturbations and responses. Semin Thromb Hemost. 1978 Fall;5(2):50–86. doi: 10.1055/s-0028-1087146. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Stemerman M. B., Spaet T. H. Adhesion of blood platelets to subendothelial surface: distinct from adhesion to collagen. Experientia. 1971 Mar 15;27(3):283–285. doi: 10.1007/BF02138148. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Root M. Enzymatic degradation of heparin-related mucopolysaccharides from the surface of endothelial cell cultures. Biochim Biophys Acta. 1975 Mar 14;385(1):1–10. doi: 10.1016/0304-4165(75)90067-7. [DOI] [PubMed] [Google Scholar]
- Cazenave J. P., Dejana E., Kinlough-Rathbone R., Packham M. A., Mustard J. F. Platelet interactions with the endothelium and the subendothelium: the role of thrombin and prostacyclin. Haemostasis. 1979;8(3-5):183–192. doi: 10.1159/000214310. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Mauray F., Koerper M. A., Wiemer F., Heymann M. A., Rudolph A. M. Formation of prostacyclin (PGI2) by the ductus arteriosus of fetal lambs at different stages of gestation. Prostaglandins. 1978 Oct;16(4):633–642. doi: 10.1016/0090-6980(78)90193-4. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Hoak J. C., Fry G. L., Haycraft D. L. Use of a radioimmunoassay to study thrombin-induced release of PGI2 from cultured endothelium. Thromb Res. 1979;14(4-5):781–786. doi: 10.1016/0049-3848(79)90132-4. [DOI] [PubMed] [Google Scholar]
- Dejana E., Cazenave J. P., Groves H. M., Kinlough-Rathbone R. L., Richardson M., Packham M. A., Mustard J. F. The effect of aspirin inhibition of PGI2 production on platelet adherence to normal and damaged rabbit aortae. Thromb Res. 1980 Feb 1;17(3-4):453–464. doi: 10.1016/0049-3848(80)90080-8. [DOI] [PubMed] [Google Scholar]
- Dieterle Y., Ody C., Ehrensberger A., Stalder H., Junod A. F. Metabolism and uptake of adenosine triphosphate and adenosine by porcine aortic and pulmonary endothelial cells and fibroblasts in culture. Circ Res. 1978 Jun;42(6):869–876. doi: 10.1161/01.res.42.6.869. [DOI] [PubMed] [Google Scholar]
- Emeis J. J. The vascular wall and fibrinolysis. Haemostasis. 1979;8(3-5):332–339. doi: 10.1159/000214323. [DOI] [PubMed] [Google Scholar]
- Fry G. L., Czervionke R. L., Hoak J. C., Smith J. B., Haycraft D. L. Platelet adherence to cultured vascular cells: influence of prostacyclin (PGI2). Blood. 1980 Feb;55(2):271–275. [PubMed] [Google Scholar]
- Gamse G., Fromme H. G., Kresse H. Metabolism of sulfated glycosaminoglycans in cultured endothelial cells and smooth muscle cells from bovine aorta. Biochim Biophys Acta. 1978 Dec 18;544(3):514–528. doi: 10.1016/0304-4165(78)90326-4. [DOI] [PubMed] [Google Scholar]
- Groves H. M., Kinlough-Rathbone R. L., Richardson M., Moore S., Mustard J. F. Platelet interaction with damaged rabbit aorta. Lab Invest. 1979 Feb;40(2):194–200. [PubMed] [Google Scholar]
- Haudenschild C. C., Schwartz S. M. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- Higgs E. A., Moncada S., Vane J. R., Caen J. P., Michel H., Tobelem G. Effect of prostacyclin (PGI2) on platelet adhesion to rabbit arterial subendothelium. Prostaglandins. 1978 Jul;16(1):17–22. doi: 10.1016/0090-6980(78)90197-1. [DOI] [PubMed] [Google Scholar]
- Kelton J. G., Hirsh J., Carter C. J., Buchanan M. R. Thrombogenic effect of high-dose aspirin in rabbits. Relationship to inhibition of vessel wall synthesis of prostaglandin I2-like activity. J Clin Invest. 1978 Oct;62(4):892–895. doi: 10.1172/JCI109203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. G., Loskutoff D. J. Comparative studies of the fibrinolytic activity of cultured vascular cells. Thromb Res. 1979;15(5-6):869–878. doi: 10.1016/0049-3848(79)90195-6. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt F., Klöcking H. P. Heparin-induced release of plasminogen activator. Haemostasis. 1977;6(6):370–374. doi: 10.1159/000214203. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. B., Insull W., Jr Role of endothelium and hypercholesterolemia in intimal thickening and lipid accumulation. Am J Pathol. 1979 Apr;95(1):131–158. [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Carrara M. C., Rangaraj G., Nicolaou K. C. Enhanced formation of PGI2, a potent hypotensive substance, by aortic rings and homogenates of the spontaneously hypertensive rat. Prostaglandins. 1978 Jun;15(6):1005–1012. doi: 10.1016/0090-6980(78)90043-6. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- SAWYER P. N., PATE J. W., WELDON C. S. Relations of abnormal and injury electric potential differences to intravascular thrombosis. Am J Physiol. 1953 Oct;175(1):108–112. doi: 10.1152/ajplegacy.1953.175.1.108. [DOI] [PubMed] [Google Scholar]
- Spaet T. H., Stemerman M. B., Veith F. J., Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circ Res. 1975 Jan;36(1):58–70. doi: 10.1161/01.res.36.1.58. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B., Spaet T. H., Pitlick F., Cintron J., Lejnieks I., Tiell M. L. Intimal healing. The pattern of reendothelialization and intimal thickening. Am J Pathol. 1977 Apr;87(1):125–142. [PMC free article] [PubMed] [Google Scholar]
- Tansik R. L., Namm D. H., White H. L. Synthesis of prostaglandin 6-keto F1alpha by cultured aortic smooth muscle cells and stimulation of its formation in a coupled system with platelet lysates. Prostaglandins. 1978 Mar;15(3):399–408. doi: 10.1016/0090-6980(78)90123-5. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T. Prostacyclin (prostaglandin I2, PGI2) inhibits platelet adhesion and thrombus formation on subendothelium. Blood. 1979 Feb;53(2):244–250. [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]



