Abstract
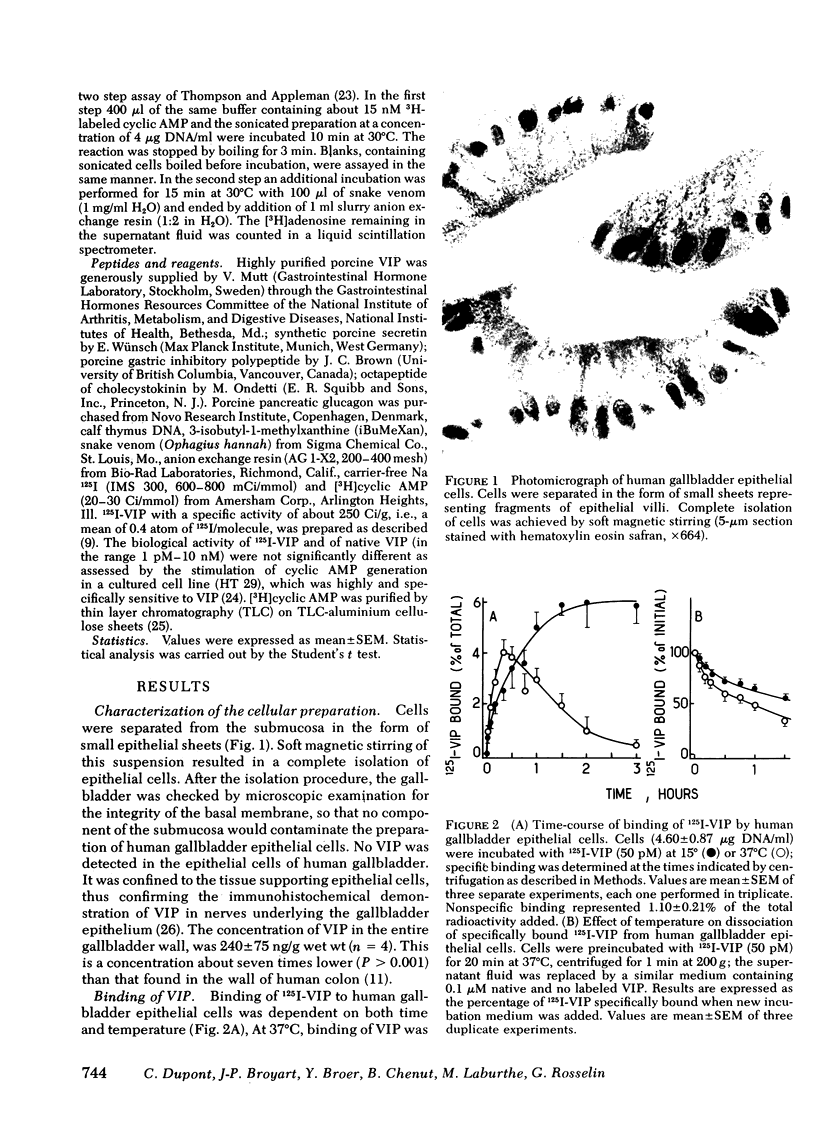
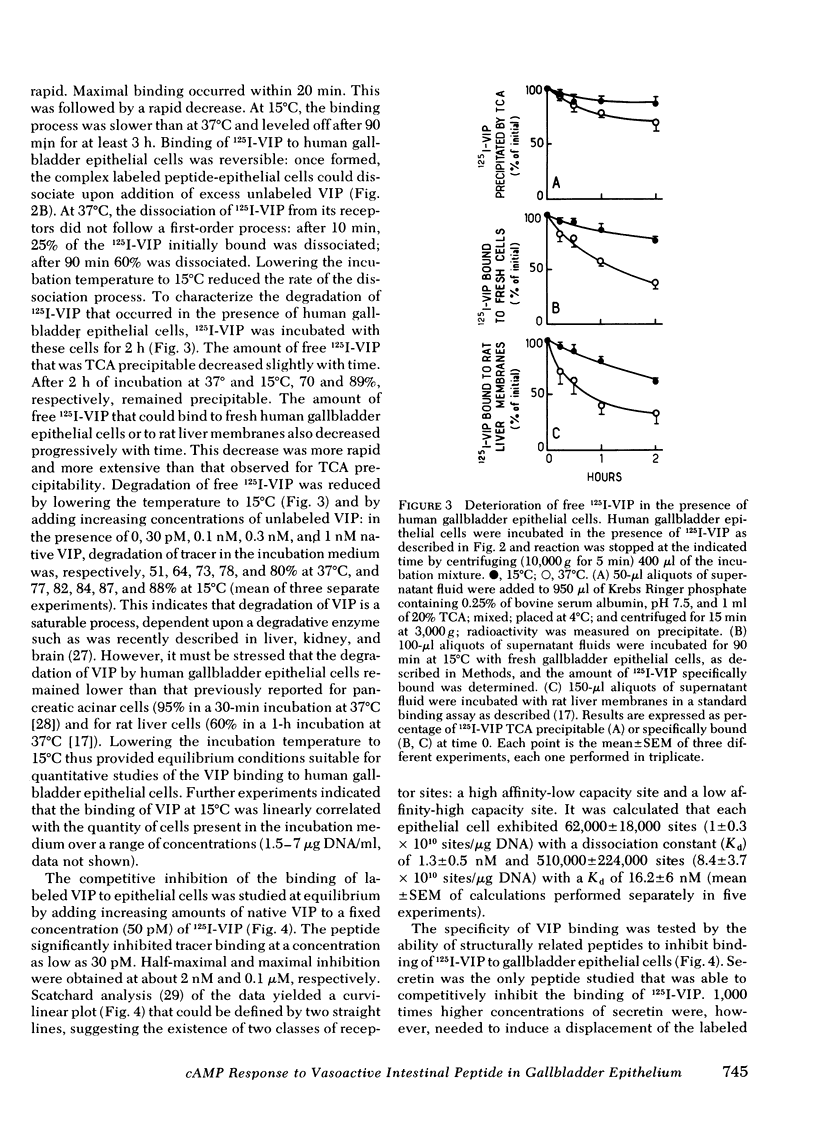
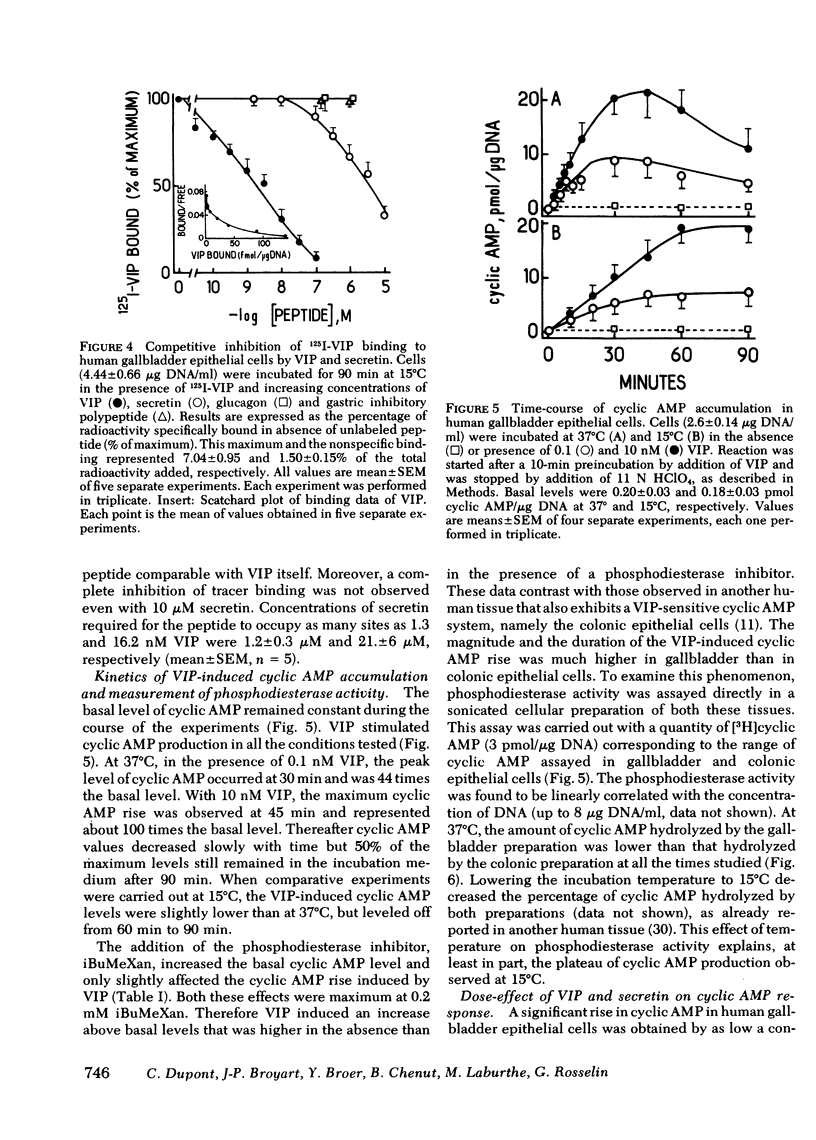
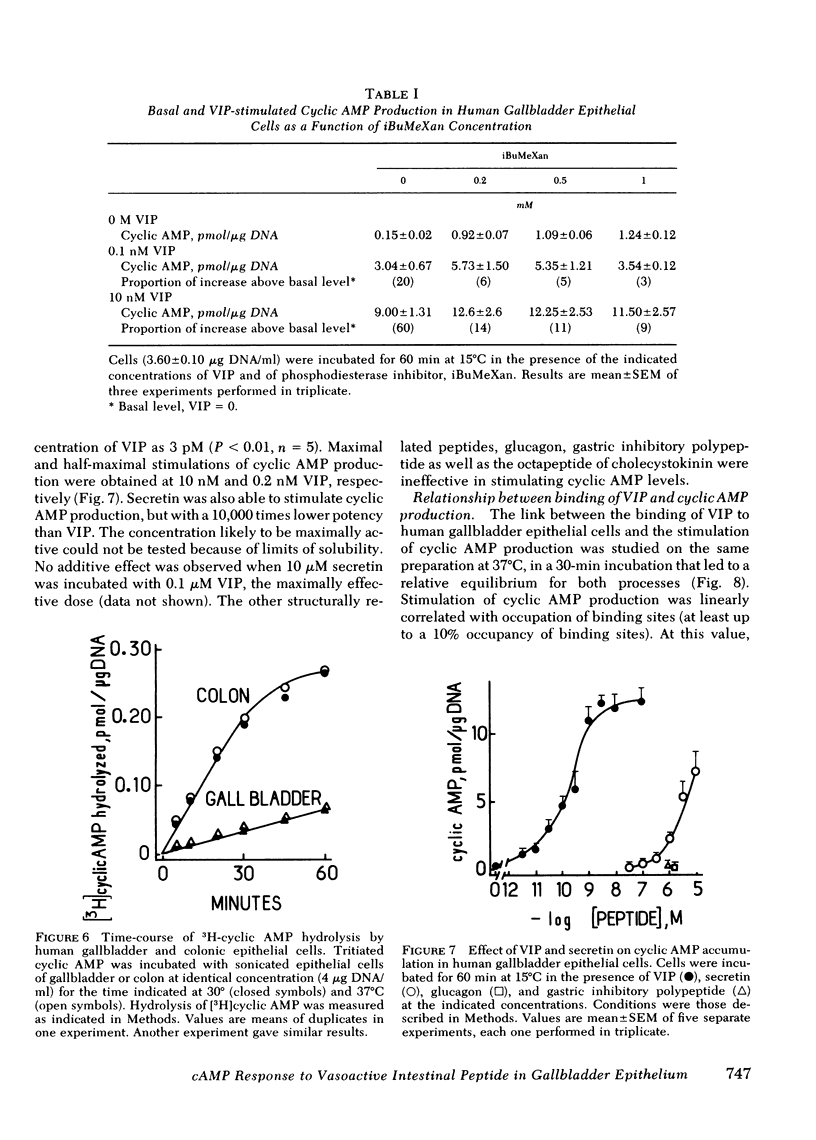
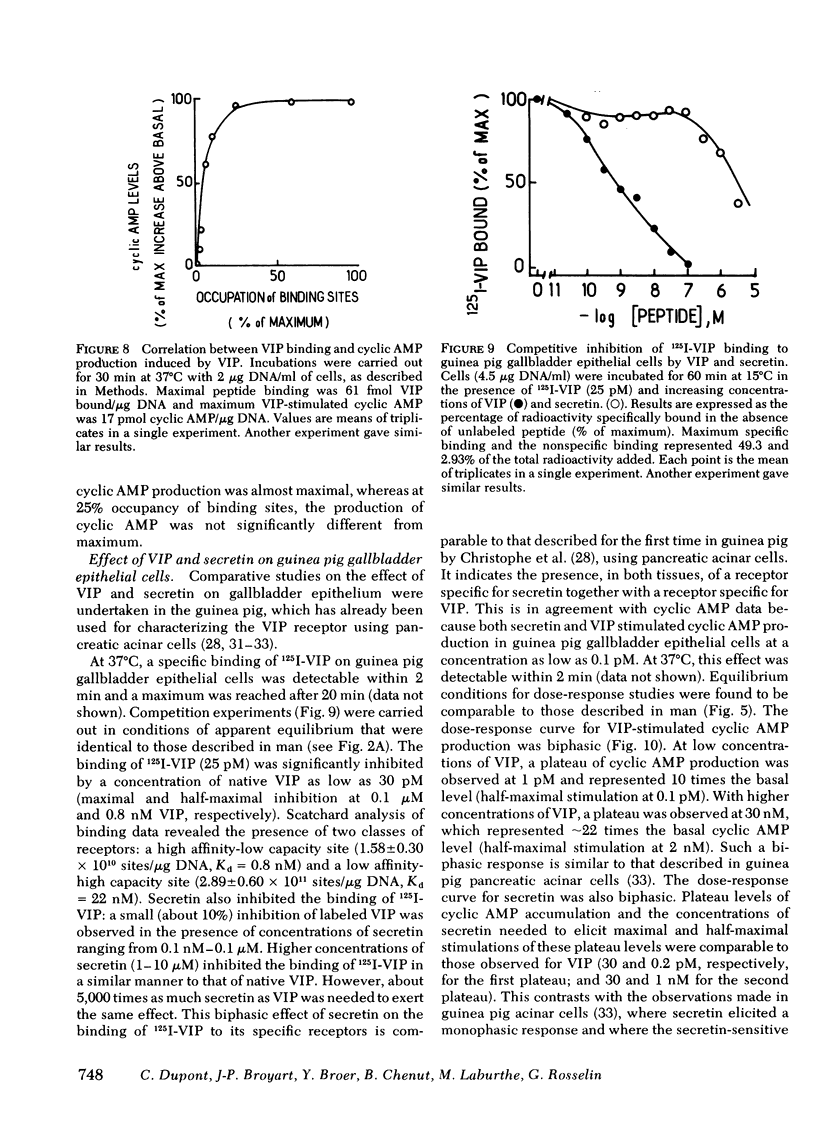
An EDTA procedure was used to prepare isolated epithelial cells of human gallbladder devoid of endogenous vasoactive intestinal peptide (VIP) as measured by radioimmunoassay. Specific binding sites for VIP were characterized in these cells. At 37°C, the binding of 125I-labeled VIP reached a peak within 20 min and then declined rapidly. At 15°C, binding was stable between 90 and 180 min of incubation. Binding of the labeled peptide was inhibited by concentrations of native VIP of 30 pM-0.1 μM. Half-maximal inhibition was observed at 2 nM. Scatchard analysis indicated two functionally independent classes of receptor sites: 62,000 high affinity sites/cell with a dissociation constant (Kd) of 1.3 nM, and 510,000 low affinity sites/cell with a Kd of 16.2 nM. Secretin inhibited tracer binding but with a 1,000 times lower potency than native VIP. VIP strongly stimulated adenosine 3′:5′ monophosphate (cyclic AMP) production in human gallbladder epithelial cells. At 37°C, 0.1 nM and 10 nM VIP raised cyclic AMP levels 44 and 100 times above the basal level, respectively. Maximal values remained constant between 60 and 90 min at 15°C. The importance of the VIP-induced cyclic AMP rise was related, at least in part, to a low phosphodiesterase activity in human gallbladder epithelial cells. At equilibrium, during a 60-min incubation at 15°C, cyclic AMP production was noted at concentrations of VIP as low as 3 pM. Maximal and half-maximal stimulations were observed at 10 nM and 0.2 nM VIP, respectively. Secretin also stimulated cyclic AMP production but with a 10,000 lower potency than VIP.
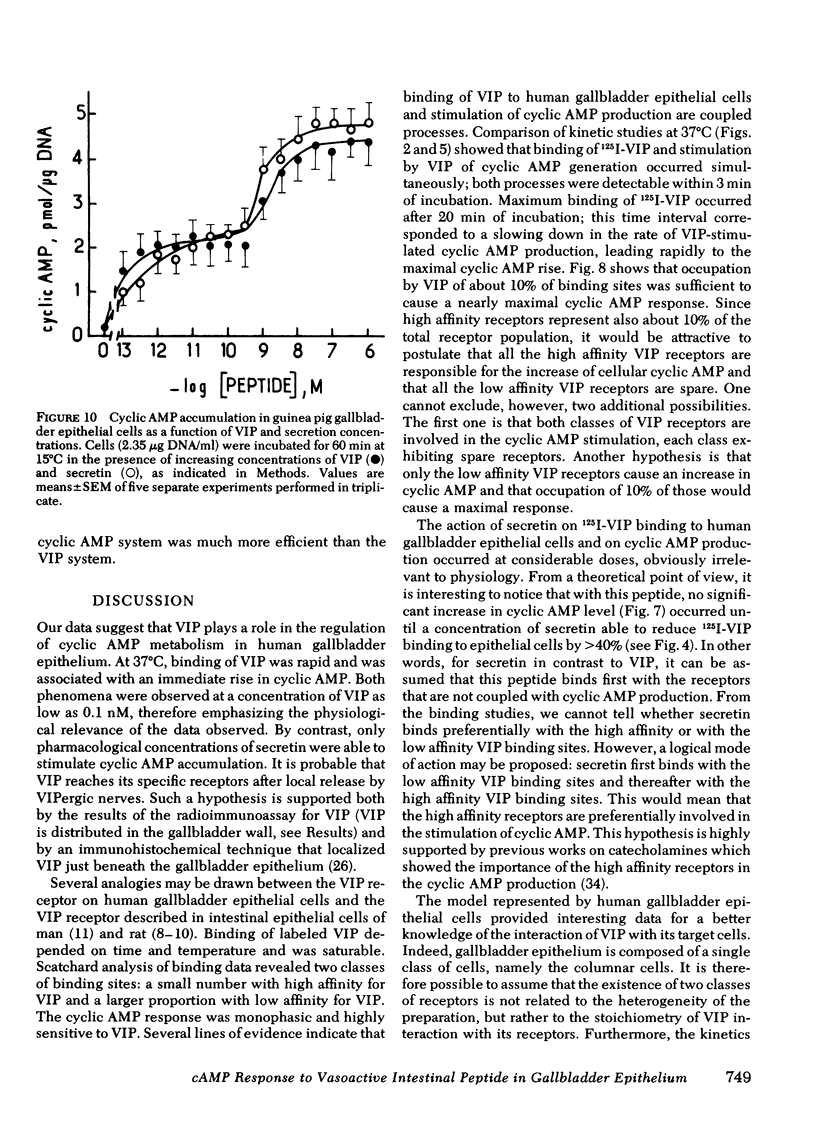
In the guinea pig, VIP and secretin were equipotent stimulators of cyclic AMP in gallbladder epithelial cells. This particular feature was shown to be due to receptors specific for each peptide that were present in these cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E. H., Sneyd J. G. An effect of glucagon on 3', 5'-cyclic AMP phosphodiesterase activity in isolated rat hepatocytes. Biochem Biophys Res Commun. 1975 Feb 3;62(3):594–601. doi: 10.1016/0006-291x(75)90440-4. [DOI] [PubMed] [Google Scholar]
- Amiranoff B., Laburthe M., Dupont C., Rosselin G. Characterization of a vasoactive intestinal peptide-sensitive adenylate cyclase in rat intestinal epithelial cell membranes. Biochim Biophys Acta. 1978 Dec 18;544(3):474–481. doi: 10.1016/0304-4165(78)90321-5. [DOI] [PubMed] [Google Scholar]
- Bataille D., Freychet P., Rosselin G. Interactions of glucagon, gut glucagon, vasoactive intestinal polypeptide and secretin with liver and fat cell plasma membranes: binding to specific sites and stimulation of adenylate cyclase. Endocrinology. 1974 Sep;95(3):713–721. doi: 10.1210/endo-95-3-713. [DOI] [PubMed] [Google Scholar]
- Bergstrand H., Lundquist B., Schurmann A. Cyclic nucleotide phosphodiesterase. Partial purification and characterization of a high affinity enzyme activity from human lung tissue. J Biol Chem. 1978 Mar 25;253(6):1881–1891. [PubMed] [Google Scholar]
- Besson J., Laburthe M., Bataille D., Dupont C., Rosselin G. Vasoactive intestinal peptide (VIP): tissue distribution in the rat as measured by radioimmunoassay and by radioreceptorassay. Acta Endocrinol (Copenh) 1978 Apr;87(4):799–810. doi: 10.1530/acta.0.0870799. [DOI] [PubMed] [Google Scholar]
- Boissard C., Jarrousse C., Rosselin G. Mesure de l'AMP cyclique dans les ilots de Langerhans du Rat nouveau-né. Effet des acides aminés. C R Acad Sci Hebd Seances Acad Sci D. 1977 Sep 19;285(5):567–570. [PubMed] [Google Scholar]
- Broer Y., Fouchereau M., Rosselin G. Dasoages radio-immunologiques de l'AMPc et du GMPc. Préparation, purification et contrôle du 2'0 succinyl AMP-3'5'-cyclique et du 2'0 succinyl-GMP-3'5'-cyclique. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jul 24;275(4):619–622. [PubMed] [Google Scholar]
- Broer Y., Lhiaubet-Grapin A. M., Rosselin G. Dosage radio-immunologique de l'AMP-3',5'-cyclique. Préparation, purification et contrôle du tyrosine-succinyl-AMPc et de son dérivé iodé. C R Acad Sci Hebd Seances Acad Sci D. 1972 Aug 16;275(7):883–886. [PubMed] [Google Scholar]
- Carr D., Friesen H. G. Growth hormone and insulin binding to human liver. J Clin Endocrinol Metab. 1976 Mar;42(3):484–493. doi: 10.1210/jcem-42-3-484. [DOI] [PubMed] [Google Scholar]
- Christophe J. P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. J Biol Chem. 1976 Aug 10;251(15):4629–4634. [PubMed] [Google Scholar]
- Davies T. F., Walsh P. C., Hodgen G. D., Dufau M. L., Catt K. J. Characterization of the primate luteinizing hormone receptor in testis homogenates and Leydig cells. J Clin Endocrinol Metab. 1979 Apr;48(4):680–685. doi: 10.1210/jcem-48-4-680. [DOI] [PubMed] [Google Scholar]
- Desbuguois B., Laudat M. H., Laudat P. Vasoactive intestinal polypeptide and glucagon: stimulation of adenylate cyclase activity via distinct receptors in liver and fat cell membranes. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1187–1194. doi: 10.1016/0006-291x(73)90590-1. [DOI] [PubMed] [Google Scholar]
- Desbuquois B. The interaction of vasoactive intestinal polypeptide and secretin with liver-cell membranes. Eur J Biochem. 1974 Aug 1;46(3):439–450. doi: 10.1111/j.1432-1033.1974.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., Christophe J. Characterization of VIP-sensitive adenylate cyclase in guinea pig brain. FEBS Lett. 1977 Nov 1;83(1):76–80. doi: 10.1016/0014-5793(77)80645-5. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Labrie F., Christophe J. In vitro interactions of gastrointestinal hormones on cyclic adenosine 3':5'-monophosphate levels and amylase output in the rat pancreas. Gastroenterology. 1975 Feb;68(2):318–325. [PubMed] [Google Scholar]
- Dupont C., Laburthe M., Broyart J. P., Bataille D., Rosselin G. Cyclic AMP production in isolated colonic epithelial crypts: a highly sensitive model for the evaluation of vasoactive intestinal peptide action in human intestine. Eur J Clin Invest. 1980 Feb;10(1):67–76. doi: 10.1111/j.1365-2362.1980.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Adams T. D. Cyclic AMP in pancreatic acinar cells: effects of gastrointestinal hormones. Gastroenterology. 1976 Jan;70(1):29–35. [PubMed] [Google Scholar]
- Götz R., Heintze K., Koerlings H. Unidirectional and net fluxes of 23Na, 42K and 36Cl by the guinea-pig gall-bladder during prostaglandin E1-induced secretion [proceedings]. J Physiol. 1976 Dec;263(1):227P–228P. [PubMed] [Google Scholar]
- Heintze K., Leinesser W., Petersen K. U., Heidenreich O. Triphasic effect of prostaglandins E1, E2 and F2alpha on the fluid transport of isolated gall-bladder of guinea-pigs. Prostaglandins. 1975 Feb;9(2):309–322. doi: 10.1016/0090-6980(75)90035-0. [DOI] [PubMed] [Google Scholar]
- Jansson R., Steen G., Svanvik J. A comparison of glucagon, gastric inhibitory peptide, and secretin on gallbladder function, formation of bile, and pancreatic secretion in the cat. Scand J Gastroenterol. 1978;13(8):919–925. doi: 10.3109/00365527809181369. [DOI] [PubMed] [Google Scholar]
- Jansson R., Steen G., Svanvik J. Effects of intravenous vasoactive intestinal peptide (VIP) on gallbladder function in the cat. Gastroenterology. 1978 Jul;75(1):47–50. [PubMed] [Google Scholar]
- Jansson R., Svanvik J. Effects of intravenous secretin and cholecystokinin on gallbladder net water absorption and motility in the cat. Gastroenterology. 1977 Apr;72(4 Pt 1):639–643. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Keltz T. N., Straus E., Yalow R. S. Degradation of vasoactive intestinal polypeptide by tissue homogenates. Biochem Biophys Res Commun. 1980 Jan 29;92(2):669–674. doi: 10.1016/0006-291x(80)90385-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laburthe M., Besson J., Bon Hoa D. H., Rosselin G. Récepteurs du peptide intestinal vasoactif (VIP) dans les entérocytes: liaison spécifique et stimulation de l'AMP cyclique. C R Acad Sci Hebd Seances Acad Sci D. 1977 Jun 6;284(21):2139–2142. [PubMed] [Google Scholar]
- Laburthe M., Prieto J. C., Amiranoff B., Dupont C., Hui Bon Hoa D., Rosselin G. Interaction of vasoactive intestinal peptide with isolated intestinal epithelial cells from rat. 2. Characterization and structural requirements of the stimulatory effect of vasoactive intestinal peptide on production of adenosine 3':5'-monophosphate. Eur J Biochem. 1979 May 15;96(2):239–248. doi: 10.1111/j.1432-1033.1979.tb13034.x. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Rousset M., Boissard C., Chevalier G., Zweibaum A., Rosselin G. Vasoactive intestinal peptide: a potent stimulator of adenosine 3':5'-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2772–2775. doi: 10.1073/pnas.75.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens R. B., Wheeler H. O., Mayer S. E. Effects of cholera toxin and phosphodiesterase inhibitors on fluid transport and cyclic adenosine 3',5'-monophosphate concentrations in rabbit gallbladder. Gastroenterology. 1974 Nov;67(5):898–906. [PubMed] [Google Scholar]
- Morton I. K., Phillips S. J., Saverymuttu S. H., Wood J. R. Secretin and vasoactive intestinal peptide inhibit fluid absorption and induce secretion in the isolated gall-bladder of the guinea-pig [proceedings]. J Physiol. 1977 Mar;266(1):65P–66P. [PMC free article] [PubMed] [Google Scholar]
- Prieto J. C., Laburthe M., Rosselin G. Interaction of vasoactive intestinal peptide with isolated intestinal epithelial cells from rat. 1. Characterization, quantitative aspects and structural requirements of binding sites. Eur J Biochem. 1979 May 15;96(2):229–237. doi: 10.1111/j.1432-1033.1979.tb13033.x. [DOI] [PubMed] [Google Scholar]
- Racusen L. C., Binder H. J. Alteration of large intestinal electrolyte transport by vasoactive intestinal polypeptide in the rat. Gastroenterology. 1977 Oct;73(4 Pt 1):790–796. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Russell T. R., Terasaki W. L., Appleman M. M. Separate phosphodiesterases for the hydrolysis of cyclic adenosine 3',5'-monophosphate and cyclic guanosine 3',5'-monophosphate in rat liver. J Biol Chem. 1973 Feb 25;248(4):1334–1340. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Sundler F., Alumets J., Håkanson R., Ingemansson S., Fahrenkrug J., Schaffalitzky de Muckadell O. VIP innervation of the gallbladder. Gastroenterology. 1977 Jun;72(6):1375–1377. [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]